Microbiota Modulates the Immunomodulatory Effects of Filifolinone on Atlantic Salmon
Abstract
1. Introduction
2. Materials and Methods
2.1. Extraction and Isolation of the Natural Compound Filifolinone
2.2. Fish and Maintenance
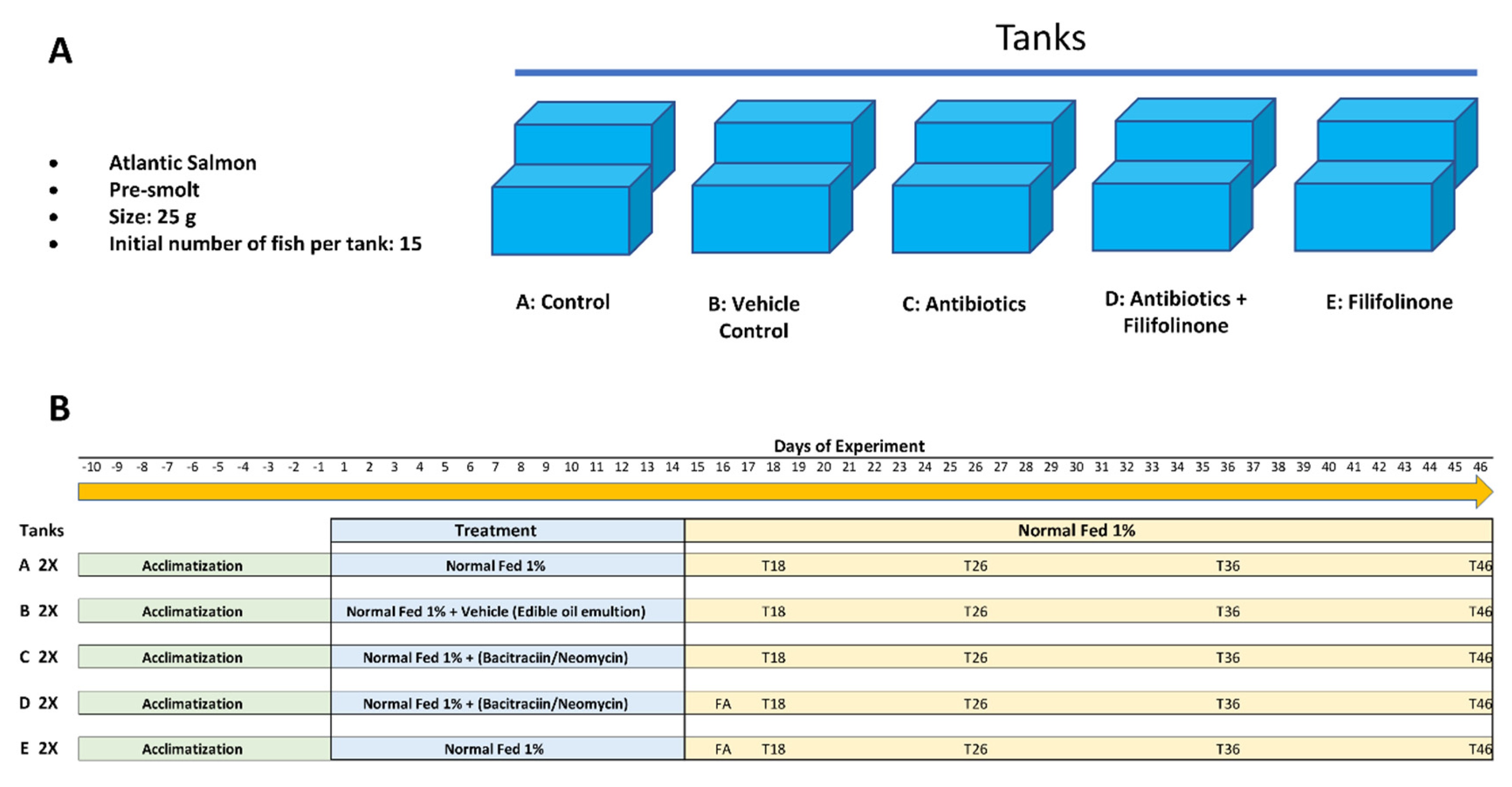
2.3. Experimental Design
2.4. Primary Macrophage Culture and Treatment with Filifolinone
2.5. RNA Extraction and cDNA Synthesis
2.6. Analysis of Cytokine Expression by qPCR
2.7. DNA Extraction
2.8. Bacterial Load
2.9. Metagenomic Analysis
3. Results
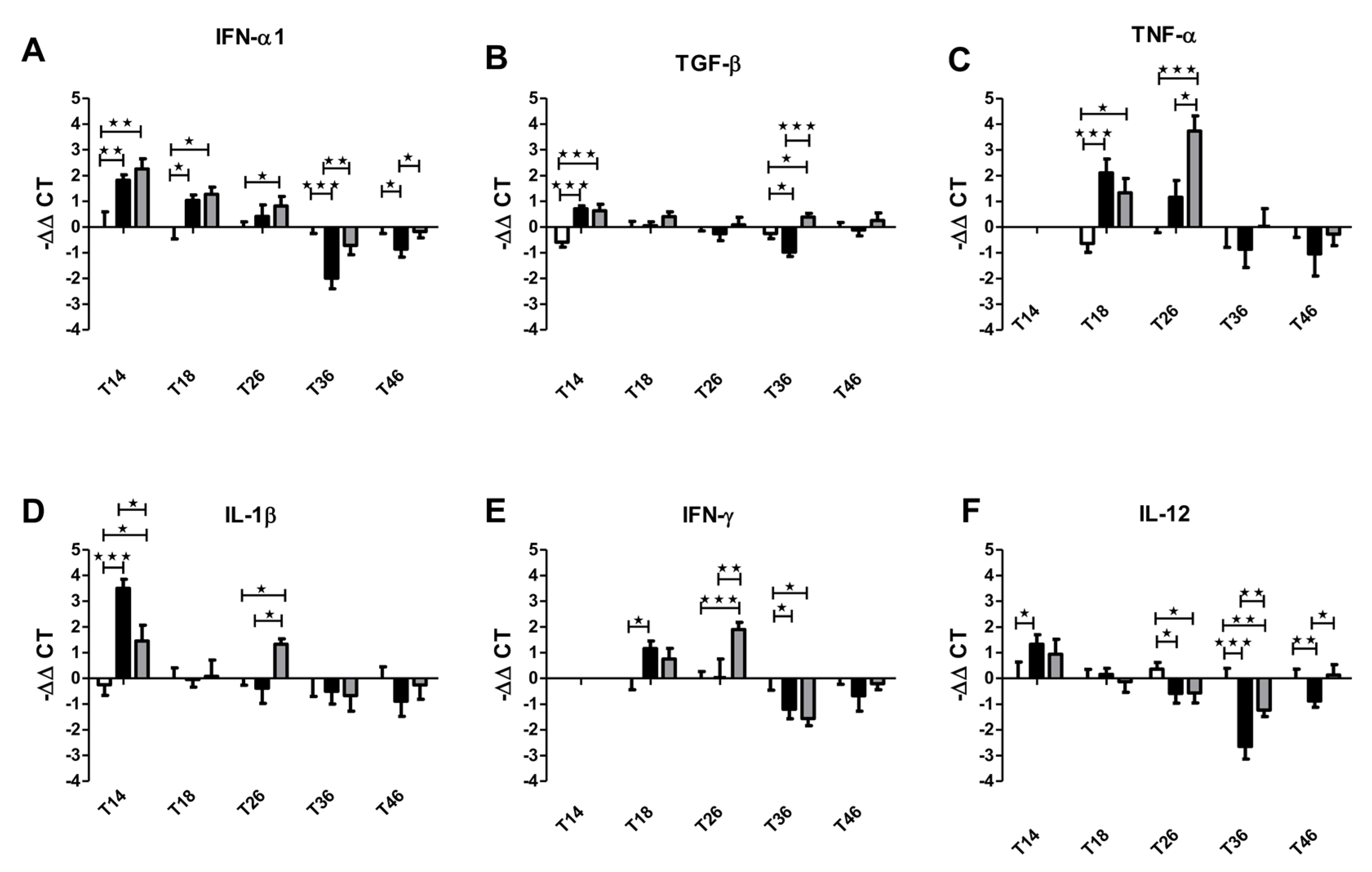
3.1. Effect of Filifolinone on the Immune Status of Atlantic Salmon
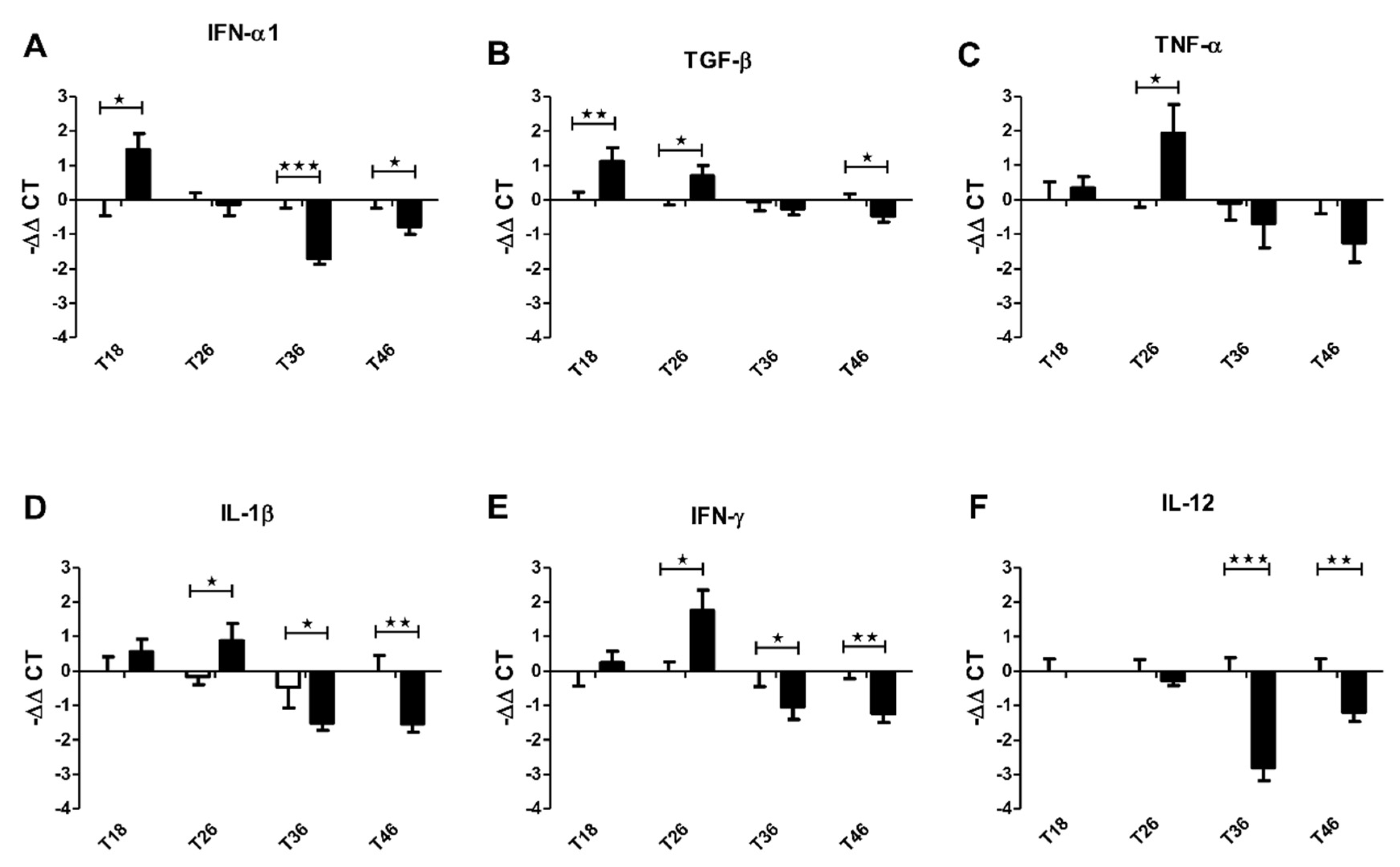
3.2. Effect of Antibiotics on the Immunomodulatory Capacity of Filifolinone
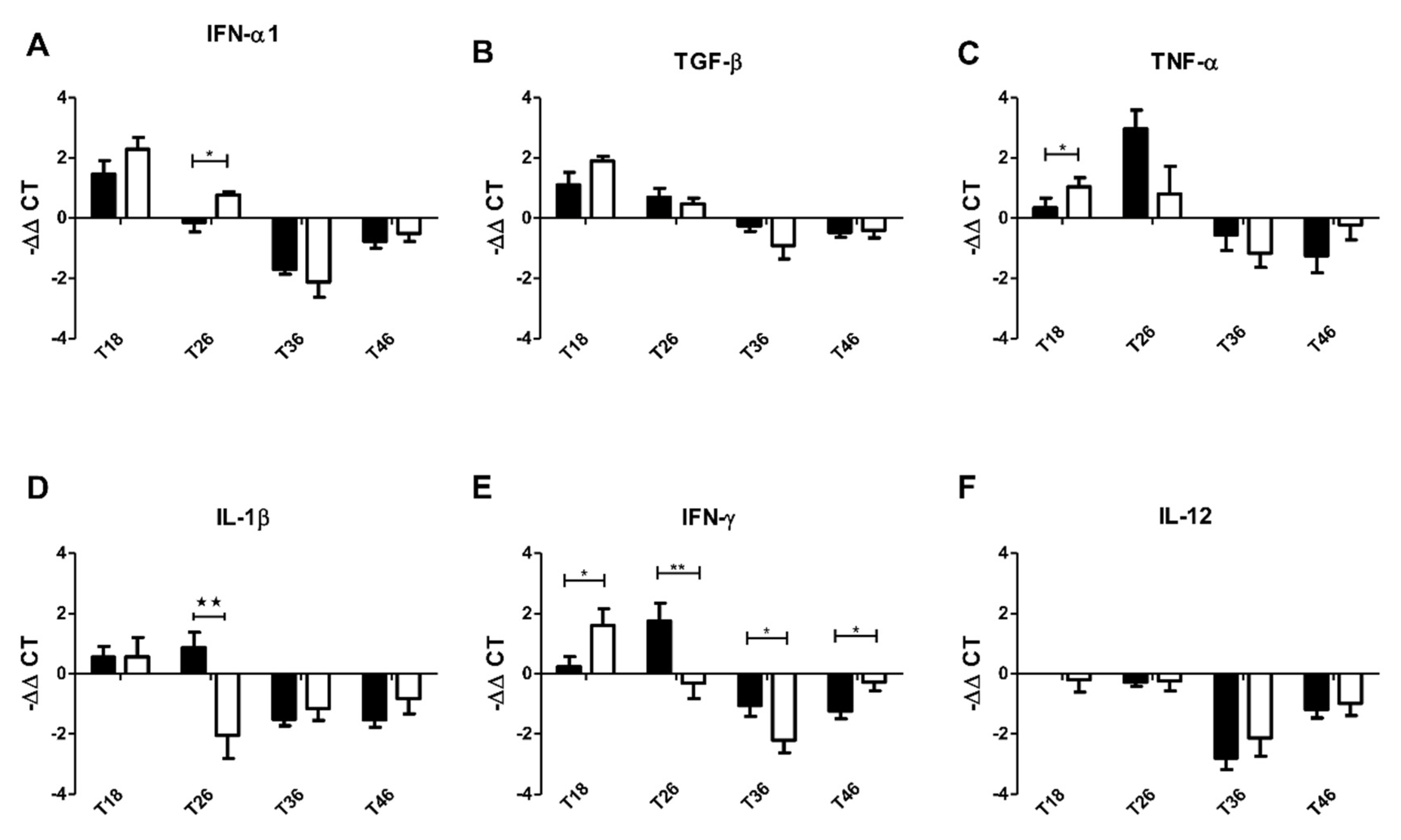
3.3. The Effect of Non-Absorbable Antibiotics on the Expression of Cytokine in Atlantic Salmon
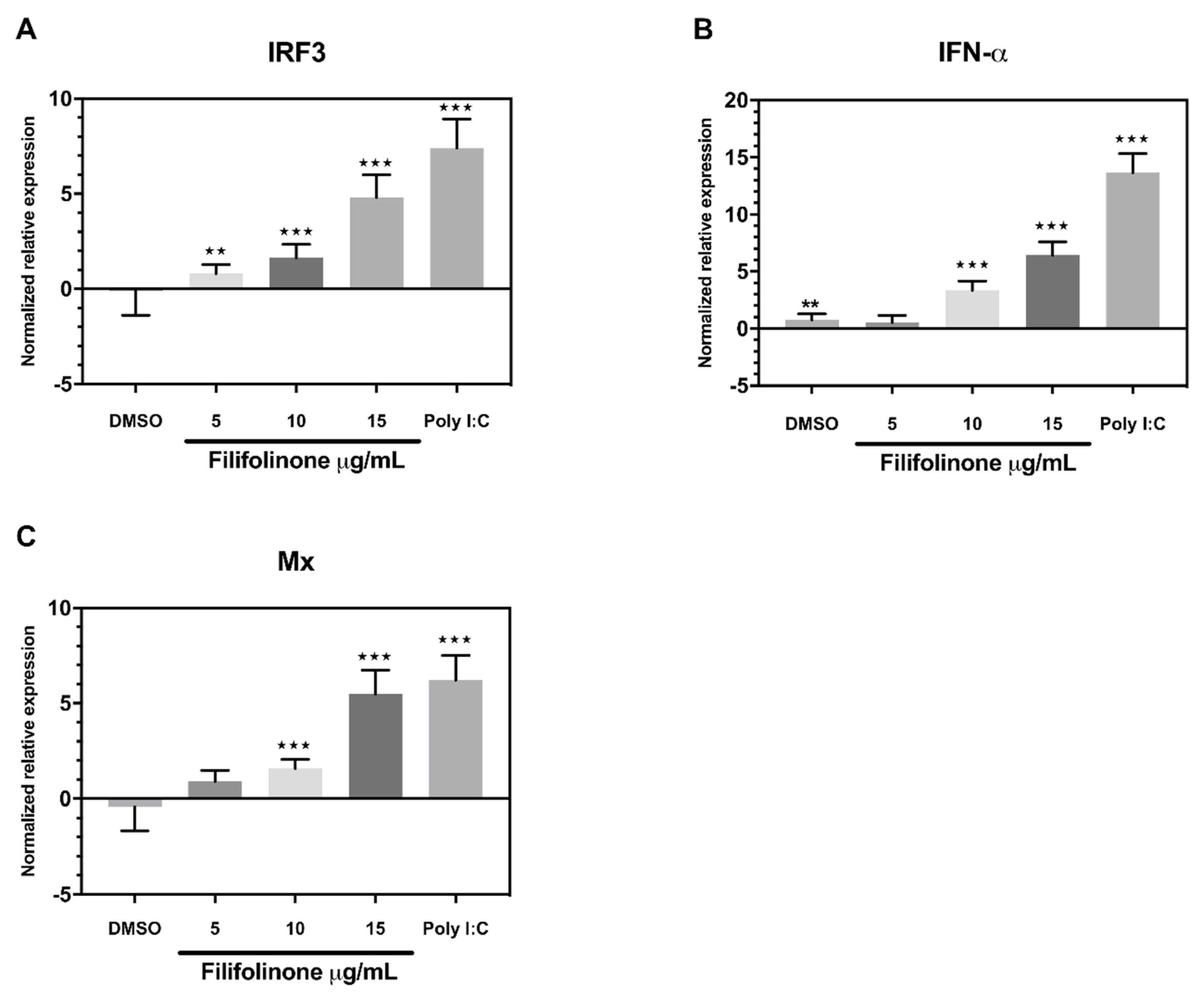
3.4. Induction of a Filifolinone-Dependent Antiviral Response is Independent of Microbiota
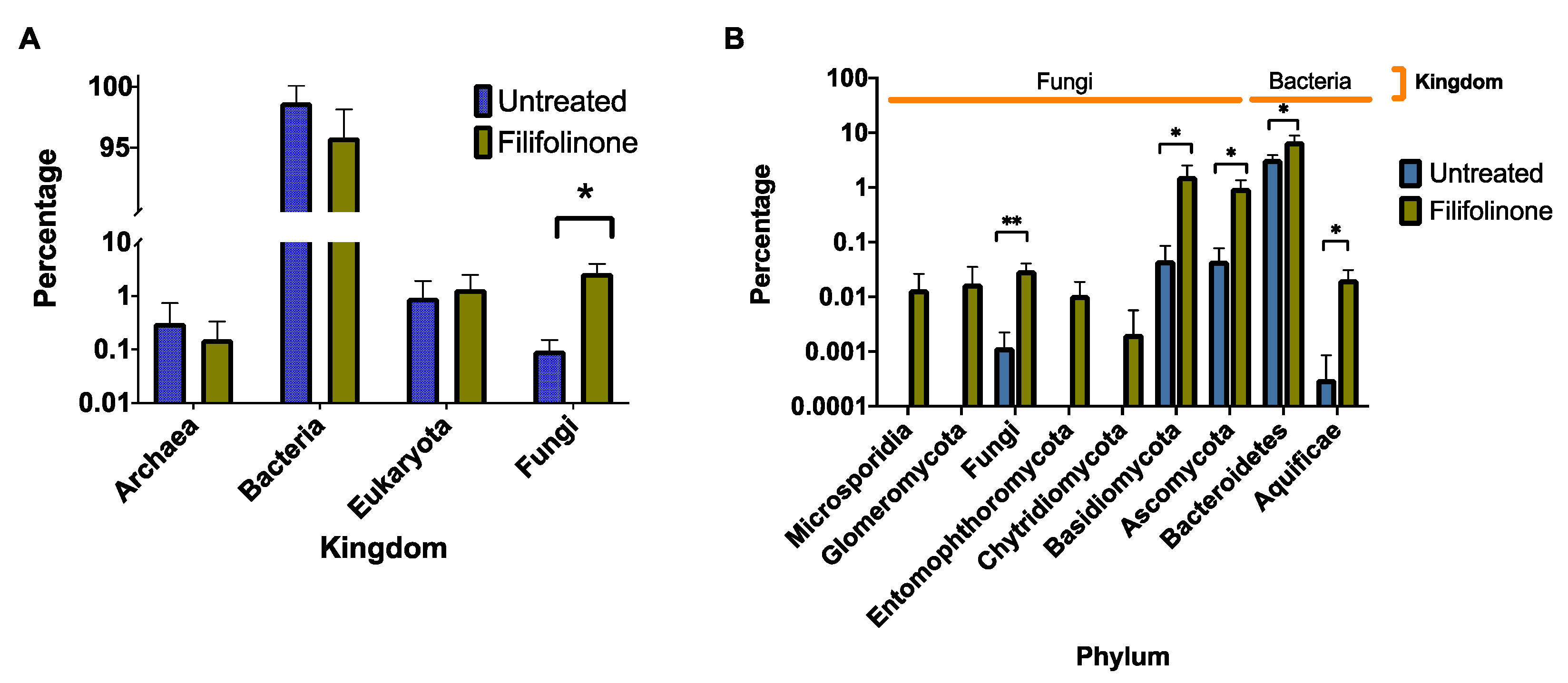
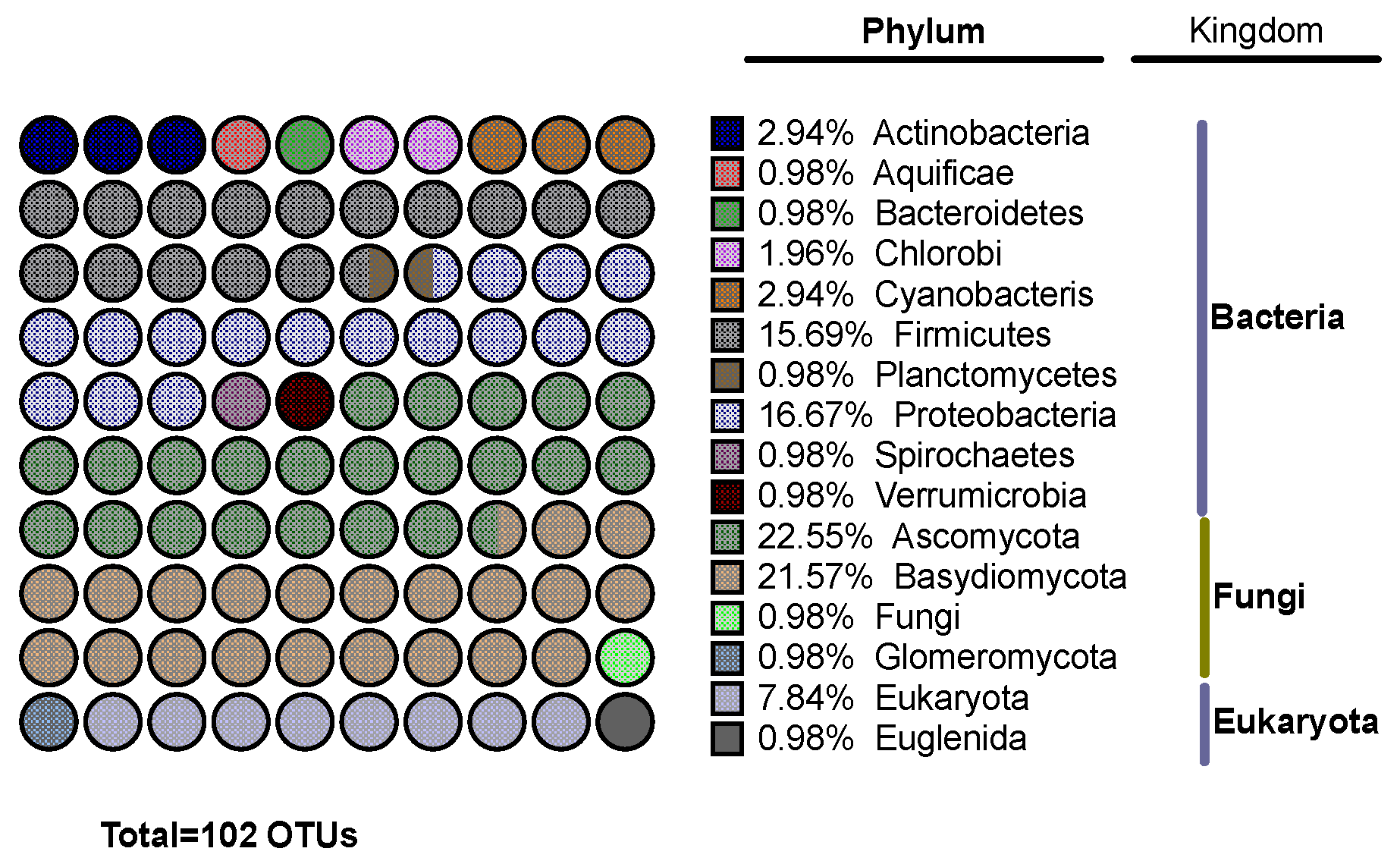
3.5. Effects of Filifolinone on Microbiotic Composition
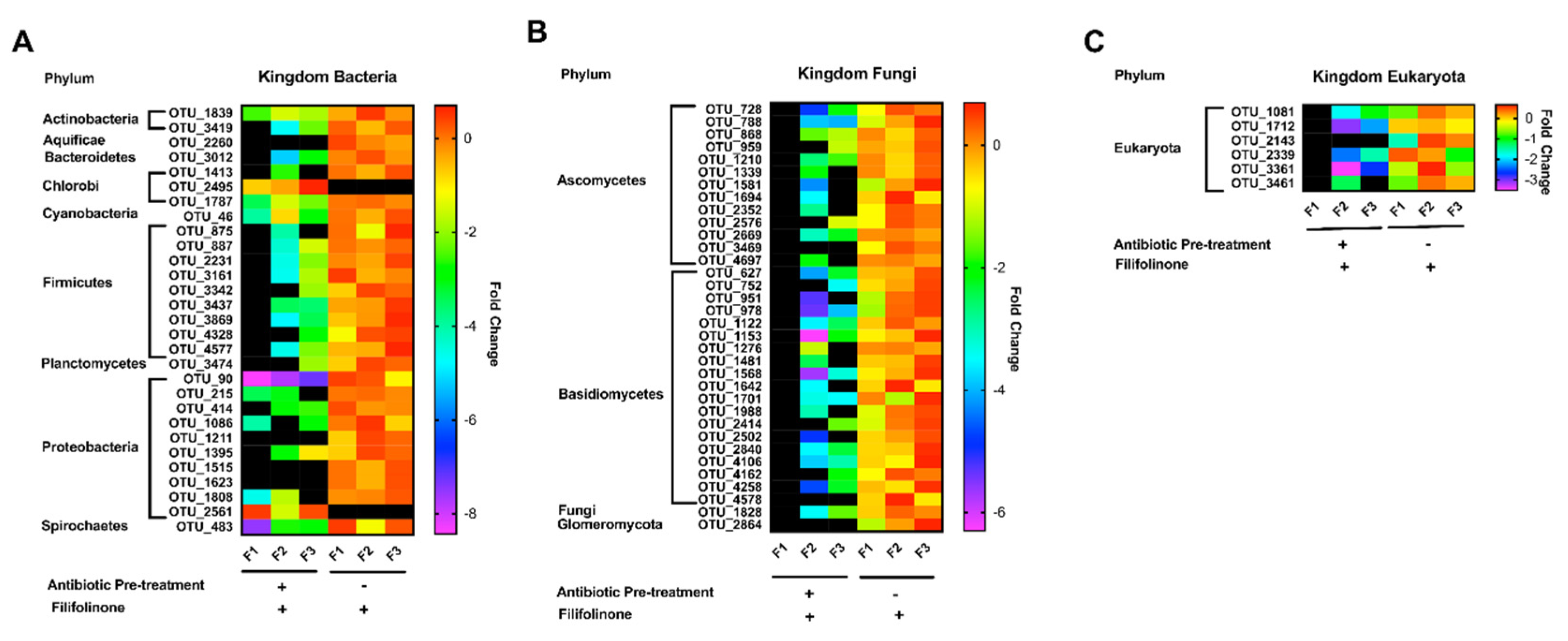
3.6. Effects of Antibiotics on the Microbial Composition of Filifolinone-Treated Fish
3.7. Effects of Filifolinone on the Class Clostridia
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAO SOFIA 2018—State of Fisheries and Aquaculture in the World. 2018. Available online: http://www.fao.org/state-of-fisheries-aquaculture (accessed on 25 September 2018).
- Béné, C.; Barange, M.; Subasinghe, R.; Pinstrup-Andersen, P.; Merino, G.; Hemre, G.-I.; Williams, M. Feeding 9 billion by 2050—Putting fish back on the menu. Food Secur. 2015, 7, 261–274. [Google Scholar] [CrossRef]
- Figueroa, J.; Cárcamo, J.; Yañez, A.; Olavarria, V.; Ruiz, P.; Manríquez, R.; Muñoz, C.; Romero, A.; Avendaño-Herrera, R. Addressing viral and bacterial threats to salmon farming in Chile: Historical contexts and perspectives for management and control. Rev. Aquac. 2019, 11, 299–324. [Google Scholar] [CrossRef]
- Miranda, C.D.; Godoy, F.A.; Lee, M.R. Current Status of the Use of Antibiotics and the Antimicrobial Resistance in the Chilean Salmon Farms. Front. Microbiol. 2018, 9, 1284. [Google Scholar] [CrossRef]
- Tobar, I.; Arancibia, S.; Torres, C.; Vera, V.; Soto, P.; Carrasco, C.; Alvarado, M.; Neira, E.; Arcos, S.; Tobar, J.A. Successive Oral Immunizations Against Piscirickettsia Salmonis and Infectious Salmon Anemia Virus are Required to Maintain a Long-Term Protection in Farmed Salmonids. Front. Immunol. 2015, 6, 244. [Google Scholar] [CrossRef]
- Raida, M.K.; Buchmann, K. Bath vaccination of rainbow trout (Oncorhynchus mykiss Walbaum) against Yersinia ruckeri: Effects of temperature on protection and gene expression. Vaccine 2008, 26, 1050–1062. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Quillet, E.; Boudinot, P.; Fischer, U. What could be the mechanisms of immunological memory in fish? Fish Shellfish Immunol. 2019, 85, 3–8. [Google Scholar] [CrossRef]
- Awate, S.; Babiuk, L.A.; Mutwiri, G. Mechanisms of Action of Adjuvants. Front. Immunol. 2013, 4, 114. [Google Scholar] [CrossRef]
- Thim, H.; Villoing, S.; McLoughlin, M.; Christie, K.; Grove, S.; Frost, P.; Jørgensen, J. Vaccine Adjuvants in Fish Vaccines Make a Difference: Comparing Three Adjuvants (Montanide ISA763A Oil, CpG/Poly I:C Combo and VHSV Glycoprotein) Alone or in Combination Formulated with an Inactivated Whole Salmonid Alphavirus Antigen. Vaccines 2014, 2, 228–251. [Google Scholar] [CrossRef]
- Tafalla, C.; Bøgwald, J.; Dalmo, R.A. Adjuvants and immunostimulants in fish vaccines: Current knowledge and future perspectives. Fish Shellfish Immunol. 2013, 35, 1740–1750. [Google Scholar] [CrossRef]
- Talmadge, J.E. Natural product derived immune-regulatory agents. Int. Immunopharmacol. 2016, 37, 5–15. [Google Scholar] [CrossRef]
- Chen, L.; Yu, J. Modulation of Toll-like receptor signaling in innate immunity by natural products. Int. Immunopharmacol. 2016, 37, 65–70. [Google Scholar] [CrossRef]
- Chakraborty, S.B.; Hancz, C. Application of phytochemicals as immunostimulant, antipathogenic and antistress agents in finfish culture. Rev. Aquac. 2011, 3, 103–119. [Google Scholar] [CrossRef]
- O’Hagan, D.T.; Fox, C.B. New generation adjuvants—From empiricism to rational design. Vaccine 2015, 33, B14–B20. [Google Scholar] [CrossRef]
- Modak, B.; Valenzuela, B.; Imarai, M.; Torres, R. Actividad inmunoestimulante in vitro del derivado aromático geranilado Filifolinona. Bol. Latinoam. Caribe plantas Med. Aromát 2012, 11, 285–290. [Google Scholar]
- Valenzuela, B.; Imarai, M.; Torres, R.; Modak, B. Immunomodulatory effects of the aromatic geranyl derivative filifolinone tested by the induction of cytokine expression. Dev. Comp. Immunol. 2013, 41, 675–682. [Google Scholar] [CrossRef]
- Parra, M.; Valenzuela, B.; Imarai, M.; Modak, B. Obtainment and evaluation of adjuvant effect of the aromatic geranyl derivative Filifolinone in bacterin of Piscirickettsia salmonis. J. Fish Dis. 2018, 41, 157–159. [Google Scholar] [CrossRef]
- Lazar, V.; Ditu, L.-M.; Pircalabioru, G.G.; Gheorghe, I.; Curutiu, C.; Holban, A.M.; Picu, A.; Petcu, L.; Chifiriuc, M.C. Aspects of Gut Microbiota and Immune System Interactions in Infectious Diseases, Immunopathology, and Cancer. Front. Immunol. 2018, 9, 1830. [Google Scholar] [CrossRef]
- Tomkovich, S.; Jobin, C. Microbiota and host immune responses: A love-hate relationship. Immunology 2016, 147, 1–10. [Google Scholar] [CrossRef]
- Koppel, N.; Rekdal, V.M.; Balskus, E.P. Chemical transformation of xenobiotics by the human gut microbiota. Science 2017, 356, 1246–1257. [Google Scholar] [CrossRef]
- Panebianco, C.; Andriulli, A.; Pazienza, V. Pharmacomicrobiomics: Exploiting the drug-microbiota interactions in anticancer therapies. Microbiome 2018, 6, 92. [Google Scholar] [CrossRef]
- Iida, N.; Dzutsev, A.; Stewart, C.A.; Smith, L.; Bouladoux, N.; Weingarten, R.A.; Molina, D.A.; Salcedo, R.; Back, T.; Cramer, S.; et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science 2013, 342, 967–970. [Google Scholar] [CrossRef] [PubMed]
- Vétizou, M.; Pitt, J.M.; Daillère, R.; Lepage, P.; Waldschmitt, N.; Flament, C.; Rusakiewicz, S.; Routy, B.; Roberti, M.P.; Duong, C.P.M.; et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 2015, 350, 1079–1084. [Google Scholar] [CrossRef] [PubMed]
- Viaud, S.; Saccheri, F.; Mignot, G.; Yamazaki, T.; Daillère, R.; Hannani, D.; Enot, D.P.; Pfirschke, C.; Engblom, C.; Pittet, M.J.; et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science 2013, 342, 971–976. [Google Scholar] [CrossRef] [PubMed]
- Routy, B.; Le Chatelier, E.; Derosa, L.; Duong, C.P.M.; Alou, M.T.; Daillère, R.; Fluckiger, A.; Messaoudene, M.; Rauber, C.; Roberti, M.P.; et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 2018, 359, 91–97. [Google Scholar] [CrossRef]
- Daillère, R.; Vétizou, M.; Waldschmitt, N.; Yamazaki, T.; Isnard, C.; Poirier-Colame, V.; Duong, C.P.M.; Flament, C.; Lepage, P.; Roberti, M.P.; et al. Enterococcus hirae and Barnesiella intestinihominis Facilitate Cyclophosphamide-Induced Therapeutic Immunomodulatory Effects. Immunity 2016, 45, 931–943. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Reyes-Cerpa, S.; Reyes-López, F.; Toro-Ascuy, D.; Montero, R.; Maisey, K.; Acuña-Castillo, C.; Sunyer, J.O.; Parra, D.; Sandino, A.M.; Imarai, M. Induction of anti-inflammatory cytokine expression by IPNV in persistent infection. Fish Shellfish Immunol. 2014, 41, 172–182. [Google Scholar] [CrossRef]
- Sun, B.; Skjaeveland, I.; Svingerud, T.; Zou, J.; Jorgensen, J.; Robertsen, B. Antiviral Activity of Salmonid Gamma Interferon against Infectious Pancreatic Necrosis Virus and Salmonid Alphavirus and Its Dependency on Type I Interferon. J. Virol. 2011, 85, 9188–9198. [Google Scholar] [CrossRef]
- Rouxel, R.N.; Tafalla, C.; Mérour, E.; Leal, E.; Biacchesi, S.; Brémont, M. Attenuated Infectious Hematopoietic Necrosis Virus with Rearranged Gene Order as Potential Vaccine. J. Virol. 2016, 90, 10857–10866. [Google Scholar] [CrossRef]
- del Mar Ortega-Villaizan, M.; Nombela, I.; Carrion, A.; Puente-Marin, S.; Chico, V.; Mercado, L.; Perez, L.; Coll, J. Infectious pancreatic necrosis virus triggers antiviral immune response in rainbow trout red blood cells, despite not being infective. F1000Research 2017, 6. [Google Scholar] [CrossRef]
- Salazar, C.; Haussmann, D.; Kausel, G.; Figueroa, J. Molecular cloning of Salmo salar Toll-like receptors (TLR1, TLR22, TLR5M and TLR5S) and expression analysis in SHK-1 cells during Piscirickettsia salmonis infection. J. Fish Dis. 2016, 39, 239–248. [Google Scholar] [CrossRef]
- Furet, J.P.; Firmesse, O.; Gourmelon, M.; Bridonneau, C.; Tap, J.; Mondot, S.; Doré, J.; Corthier, G. Comparative assessment of human and farm animal faecal microbiota using real-time quantitative PCR. FEMS Microbiol. Ecol. 2009, 68, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Logares, R.; Sunagawa, S.; Salazar, G.; Cornejo-Castillo, F.M.; Ferrera, I.; Sarmento, H.; Hingamp, P.; Ogata, H.; de Vargas, C.; Lima-Mendez, G.; et al. Metagenomic 16S rDNA Illumina tags are a powerful alternative to amplicon sequencing to explore diversity and structure of microbial communities. Environ. Microbiol. 2014, 16, 2659–2671. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Dillon, M.; Bokulich, N.; Abnet, C.; Al-Ghalith, G.; Alexander, H.; Alm, E.; Arumugam, M.; Asnicar, F.; Bai, Y.; et al. QIIME 2: Reproducible, interactive, scalable, and extensible microbiome data science. PeerJ Prepr. 2018. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High resolution sample inference from Illumina amplicon data Benjamin. Nat. Methods. 2016, 13, 4–5. [Google Scholar] [CrossRef]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-Source, Platform-Independent, Community-Supported Software for Describing and Comparing Microbial Communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef]
- Kozich, J.J.; Westcott, S.L.; Baxter, N.T.; Highlander, S.K.; Schloss, P.D. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl. Environ. Microbiol. 2013, 79, 5112–5120. [Google Scholar] [CrossRef]
- Pruesse, E.; Quast, C.; Knittel, K.; Fuchs, B.M.; Ludwig, W.; Peplies, J.; Glöckner, F.O. SILVA: A comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB. Nucleic Acids Res. 2007, 35, 7188–7196. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Torres, R.; Modak, B.; Urzúa, A.; Delle Monache, F.; Pujol, E.D.Y.C.A. Propiedades antivirales de compuestos naturales y semi-sinteticos de la resina de Heliotropium filifolium. Bol. Soc. Chil. Quim. 2002, 47, 259–263. [Google Scholar] [CrossRef]
- Valenzuela, B.; Obreque, J.; Soto-Aguilera, S.; Maisey, K.; Imarai, M.; Modak, B. Key cytokines of adaptive immunity are differentially induced in rainbow trout kidney by a group of structurally related geranyl aromatic derivatives. Fish Shellfish Immunol. 2016, 49, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.N.; Zou, P.F.; Nie, P. Retinoic acid-inducible gene I (RIG-I)-like receptors (RLRs) in fish: Current knowledge and future perspectives. Immunology 2017, 151, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Loo, Y.M.; Gale, M. Immune Signaling by RIG-I-like Receptors. Immunity 2011, 34, 680–692. [Google Scholar] [CrossRef] [PubMed]
- Rebl, A.; Goldammer, T.; Seyfert, H.M. Toll-like receptor signaling in bony fish. Vet. Immunol. Immunopathol. 2010, 134, 139–150. [Google Scholar] [CrossRef]
- Vercammen, E.; Staal, J.; Beyaert, R. Sensing of viral infection and activation of innate immunity by toll-like receptor 3. Clin. Microbiol. Rev. 2008, 21, 13–25. [Google Scholar] [CrossRef]
- Müller, T.; Hamm, S.; Bauer, S. TLR9-Mediated Recognition of DNA; Springer: Berlin/Heidelberg, Germany, 2008; pp. 51–70. [Google Scholar]
- Liu, H.; Wang, J.; He, T.; Becker, S.; Zhang, G.; Li, D.; Ma, X. Butyrate: A Double-Edged Sword for Health? Adv. Nutr. 2018, 9, 21–29. [Google Scholar] [CrossRef]
- Vital, M.; Howe, A.C.; Tiedje, J.M. Revealing the bacterial butyrate synthesis pathways by analyzing (meta)genomic data. MBio 2014, 5. [Google Scholar] [CrossRef]
- Doestzada, M.; Vila, A.V.; Zhernakova, A.; Koonen, D.P.Y.; Weersma, R.K.; Touw, D.J.; Kuipers, F.; Wijmenga, C.; Fu, J. Pharmacomicrobiomics: A novel route towards personalized medicine? Protein Cell 2018, 9, 432–445. [Google Scholar] [CrossRef]
- Visconti, A.; Le Roy, C.I.; Rosa, F.; Rossi, N.; Martin, T.C.; Mohney, R.P.; Li, W.; de Rinaldis, E.; Bell, J.T.; Venter, J.C.; et al. Interplay between the human gut microbiome and host metabolism. Nat. Commun. 2019, 10. [Google Scholar] [CrossRef]
- Geuking, M.B.; Köller, Y.; Rupp, S.; McCoy, K.D. The interplay between the gut microbiota and the immune system. Gut Microbes 2014, 5, 411–418. [Google Scholar] [CrossRef]
- Sun, L.; Xie, C.; Wang, G.; Wu, Y.; Wu, Q.; Wang, X.; Liu, J.; Deng, Y.; Xia, J.; Chen, B.; et al. Gut microbiota and intestinal FXR mediate the clinical benefits of metformin. Nat. Med. 2018, 24, 1919–1929. [Google Scholar] [CrossRef] [PubMed]
- Wallace, B.D.; Wang, H.; Lane, K.T.; Scott, J.E.; Orans, J.; Koo, J.S.; Venkatesh, M.; Jobin, C.; Yeh, L.A.; Mani, S.; et al. Alleviating cancer drug toxicity by inhibiting a bacterial enzyme. Science 2010, 330, 831–835. [Google Scholar] [CrossRef]
- Xu, Y.; Villalona-Calero, M.A. Irinotecan: Mechanisms of tumor resistance and novel strategies for modulating its activity. Ann. Oncol. 2002, 13, 1841–1851. [Google Scholar] [CrossRef]
- Frank, M.; Hennenberg, E.M.; Eyking, A.; Rünzi, M.; Gerken, G.; Scott, P.; Parkhill, J.; Walker, A.W.; Cario, E. TLR Signaling Modulates Side Effects of Anticancer Therapy in the Small Intestine. J. Immunol. 2015, 194, 1983–1995. [Google Scholar] [CrossRef] [PubMed]
- Modak, B.; Torres, P.; Mascayano, C.; Torres, R.; Urzua, A.; Valenzuela, B. Derivatives of 3H-spiro1-benzofuran-2, 1′-cyclohexanes: Immunostimulants for veterinary use. Bol. Latinoam. Caribe Plantas Med. Aromat. 2014, 14, 375–380. [Google Scholar]
- Zheng, Y.; Wang, T.; Tu, X.; Huang, Y.; Zhang, H.; Tan, D.; Jiang, W.; Cai, S.; Zhao, P.; Song, R.; et al. Gut microbiome affects the response to anti-PD-1 immunotherapy in patients with hepatocellular carcinoma. J. Immunother. Cancer 2019, 7. [Google Scholar] [CrossRef]
- Sivan, A.; Corrales, L.; Hubert, N.; Williams, J.B.; Aquino-Michaels, K.; Earley, Z.M.; Benyamin, F.W.; Lei, Y.M.; Jabri, B.; Alegre, M.L.; et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science 2015, 350, 1084–1089. [Google Scholar] [CrossRef]
- Matson, V.; Fessler, J.; Bao, R.; Chongsuwat, T.; Zha, Y.; Alegre, M.L.; Luke, J.J.; Gajewski, T.F. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science 2018, 359, 104–108. [Google Scholar] [CrossRef]
- Von Bültzingslöwen, I.; Adlerberth, I.; Wold, A.E.; Dahlén, G.; Jontell, M. Oral and intestinal microflora in 5-fluorouracil treated rats, translocation to cervical and mesenteric lymph nodes and effects of probiotic bacteria. Oral Microbiol. Immunol. 2003, 18, 278–284. [Google Scholar] [CrossRef]
- Pezo, R.C.; Wong, M.; Martin, A. Impact of the gut microbiota on immune checkpoint inhibitor-associated toxicities. Therap. Adv. Gastroenterol. 2019, 12, 175628481987091. [Google Scholar] [CrossRef]
- Rawls, J.F.; Samuel, B.S.; Gordon, J.I. Gnotobiotic zebrafish reveal evolutionarily conserved responses to the gut microbiota. Proc. Natl. Acad. Sci. USA 2004, 101, 4596–4601. [Google Scholar] [CrossRef] [PubMed]
- Murdoch, C.C.; Rawls, J.F. Commensal Microbiota Regulate Vertebrate Innate Immunity-Insights From the Zebrafish. Front. Immunol. 2019, 10, 2100. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo-Torres, A.; Rawling, M.D.; Rodiles, A.; Mikalsen, H.E.; Johansen, L.H.; Tinsley, J.; Forberg, T.; Aasum, E.; Castex, M.; Merrifield, D.L. Influence of dietary supplementation of probiotic Pediococcus acidilactici MA18/5M during the transition from freshwater to seawater on intestinal health and microbiota of atlantic salmon (Salmo salar L.). Front. Microbiol. 2019, 10, 2243. [Google Scholar] [CrossRef] [PubMed]
- Refstie, S.; Baeverfjord, G.; Ripman Seim, R.; Elvebø, O. Effects of dietary yeast cell wall β-glucans and MOS on performance, gut health, and salmon lice resistance in Atlantic salmon (Salmo salar) fed sunflower and soybean meal. Aquaculture 2010, 305, 109–116. [Google Scholar] [CrossRef]
- Reyes-Cerpa, S.; Vallejos-Vidal, E.; Gonzalez-Bown, M.J.; Morales-Reyes, J.; Pérez-Stuardo, D.; Vargas, D.; Imarai, M.; Cifuentes, V.; Spencer, E.; Sandino, A.M.; et al. Effect of yeast (Xanthophyllomyces dendrorhous) and plant (Saint John’s wort, lemon balm, and rosemary) extract based functional diets on antioxidant and immune status of Atlantic salmon (Salmo salar) subjected to crowding stress. Fish Shellfish Immunol. 2018, 74, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Hoseinifar, S.H.; Sun, Y.Z.; Wang, A.; Zhou, Z. Probiotics as means of diseases control in aquaculture, a review of current knowledge and future perspectives. Front. Microbiol. 2018, 9, 2429. [Google Scholar] [CrossRef] [PubMed]
- Parra, M.; Valenzuela, B.; Soto, S.; Modak, B. Antibacterial activity of cuticular components from Heliotropium species, against Staphylococcus aureus and Salmonella tiphymurium | Revista Blacpma. Bol. Latinoam. Caribe Plantas Med. Aromat. 2016, 15, 422–428. [Google Scholar]
- Banu, M.R.; Akter, S.; Islam, M.R.; Mondol, M.N.; Hossain, M.A. Probiotic yeast enhanced growth performance and disease resistance in freshwater catfish gulsa tengra, Mystus cavasius. Aquac. Rep. 2020, 16, 100237. [Google Scholar] [CrossRef]
- Schmidt, V.; Gomez-Chiarri, M.; Roy, C.; Smith, K.; Amaral-Zettler, L. Subtle Microbiome Manipulation Using Probiotics Reduces Antibiotic-Associated Mortality in Fish. mSystems 2017, 2. [Google Scholar] [CrossRef]
- Zhou, L.; Limbu, S.M.; Shen, M.; Zhai, W.; Qiao, F.; He, A.; Du, Z.Y.; Zhang, M. Environmental concentrations of antibiotics impair zebrafish gut health. Environ. Pollut. 2018, 235, 245–254. [Google Scholar] [CrossRef]
- Greer, R.; Dong, X.; Morgun, A.; Shulzhenko, N. Investigating a holobiont: Microbiota perturbations and transkingdom networks. Gut Microbes 2016, 7, 126–135. [Google Scholar] [CrossRef]
- Frankel, A.E.; Coughlin, L.A.; Kim, J.; Froehlich, T.W.; Xie, Y.; Frenkel, E.P.; Koh, A.Y. Metagenomic Shotgun Sequencing and Unbiased Metabolomic Profiling Identify Specific Human Gut Microbiota and Metabolites Associated with Immune Checkpoint Therapy Efficacy in Melanoma Patients. Neoplasia (USA) 2017, 19, 848–855. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, V.; Spencer, C.N.; Nezi, L.; Reuben, A.; Andrews, M.C.; Karpinets, T.V.; Prieto, P.A.; Vicente, D.; Hoffman, K.; Wei, S.C.; et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 2018, 359, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Arpaia, N.; Campbell, C.; Fan, X.; Dikiy, S.; van der Veeken, J.; Deroos, P.; Liu, H.; Cross, J.R.; Pfeffer, K.; Coffer, P.J.; et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 2013, 504, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Martin-Gallausiaux, C.; Béguet-Crespel, F.; Marinelli, L.; Jamet, A.; Ledue, F.; Blottière, H.M.; Lapaque, N. Butyrate produced by gut commensal bacteria activates TGF-beta1 expression through the transcription factor SP1 in human intestinal epithelial cells. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Atarashi, K.; Tanoue, T.; Shima, T.; Imaoka, A.; Kuwahara, T.; Momose, Y.; Cheng, G.; Yamasaki, S.; Saito, T.; Ohba, Y.; et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science 2011, 331, 337–341. [Google Scholar] [CrossRef]
- Kespohl, M.; Vachharajani, N.; Luu, M.; Harb, H.; Pautz, S.; Wolff, S.; Sillner, N.; Walker, A.; Schmitt-Kopplin, P.; Boettger, T.; et al. The microbial metabolite butyrate induces expression of Th1- associated factors in cD4+ T cells. Front. Immunol. 2017, 8, 1036. [Google Scholar] [CrossRef]



| Gene | Sequence | Ref |
|---|---|---|
| ef1a | F: 5′-GGGTGAGTTTGAGGCTGGTA-3′ R: 5′-TTCTGGATCTCCTCAAACCG-3′ | [28] |
| IL-12 | F: 5′-AATCAGCTGTCGAGCCAA-3′ R: 5′-GAAGGGACCAGGGGGTCT-3′ | Imarai M. Laboratory |
| IFN-γ | F: 5′-CCGTACACCGATTGAGGACT-3′ R: 5′-GCGGCATTACTCCATCCTAA-3′ | [28] |
| TGF-β | F: 5′-AGCTCTCGGAAGAAACGACA-3′ R: 5′-AGTAGCCAGTGGGTTCATGG-3′ | [28] |
| IL-1β | F: 5′-CCCCATTGAGACTAAAGCCA-3′ R: 5′-GCAACCTCCTCTAGGTGCAG-3′ | [28] |
| TNF-α | F: 5′-AGGCTTTTTCCCAGGGC-3′ R: 5′-GACTCCGAATAGCGCCAA-3′ | [16] |
| IFN-α1 | F: 5′-GGACAAGAAAAACCTGGACG-3′ R: 5′-CGTTGATGTCAAACGGTTTCT-3′ | [28] |
| MX | F: 5′-TGTAACACGATGCCCTCTCG-3′ R: 5′-GACGTCAGGGGAGCCAATC-3′ | Imarai M. Laboratory |
| IRF3 | F: 5′-TGGACCAATCAGGAGCGAAC-3′ R: 5′-AGCCCACGCCTTGAAAATAA-3′ | [29] |
| RIG-1 | F: 5′-GTCAGCAGCCCAGGTGTTTCTA-3′ R: 5′-ATAGTCTTCTGCGTCCAGGGC-3′ | This Work |
| MDA-5 | F: 5′-AGAGCCCGTCCAAAGTGAAGT-3′ R: 5′-AACATCTTCCCCAGAGCAGACT-3′ | [30] |
| TLR3 | F: 5′-ACTCGGTGGTGCTGGTCTTC-3′ R: 5′-GAGGAGGCAATTTGGACGAA-3′ | [31] |
| TLR9 | F: 5′-TGGGCGTTTGCCAATCTGA-3′ R: 5′-TGTTGAAGCAGGGGAAGCAG-3′ | [32] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parra, M.; Espinoza, D.; Valdes, N.; Vargas, R.; Gonzalez, A.; Modak, B.; Tello, M. Microbiota Modulates the Immunomodulatory Effects of Filifolinone on Atlantic Salmon. Microorganisms 2020, 8, 1320. https://doi.org/10.3390/microorganisms8091320
Parra M, Espinoza D, Valdes N, Vargas R, Gonzalez A, Modak B, Tello M. Microbiota Modulates the Immunomodulatory Effects of Filifolinone on Atlantic Salmon. Microorganisms. 2020; 8(9):1320. https://doi.org/10.3390/microorganisms8091320
Chicago/Turabian StyleParra, Mick, Daniela Espinoza, Natalia Valdes, Rodrigo Vargas, Alex Gonzalez, Brenda Modak, and Mario Tello. 2020. "Microbiota Modulates the Immunomodulatory Effects of Filifolinone on Atlantic Salmon" Microorganisms 8, no. 9: 1320. https://doi.org/10.3390/microorganisms8091320
APA StyleParra, M., Espinoza, D., Valdes, N., Vargas, R., Gonzalez, A., Modak, B., & Tello, M. (2020). Microbiota Modulates the Immunomodulatory Effects of Filifolinone on Atlantic Salmon. Microorganisms, 8(9), 1320. https://doi.org/10.3390/microorganisms8091320






