Iron-Uptake Systems of Chicken-Associated Salmonella Serovars and Their Role in Colonizing the Avian Host
Abstract
:1. Introduction
1.1. Iron Homeostasis by Salmonella in a Nutshell: Regulation and Iron-Uptake Systems
1.2. Ferric Uptake Regulator (Fur)-Mediated Regulation of Iron Uptake, Storage and Utilization
1.3. Uptake of Ferric (Fe3+) Iron via Siderophores
1.4. Uptake of Ferrous Iron (Fe2+) via FeoABC, SitABCD and MntH
2. Emergence of Chicken-Associated Invasive NTS: The Iron Link
3. Iron Uptake in NTS Virulence: Chicken vs. Mammalian Models
3.1. Feo-Mediated Fe2+ Uptake Involved in Rapid Colonization of the Gut and Systemic Spread
- (i)
- Feo-mediated ferrous iron uptake is important for rapid colonization by and systemic spread of Salmonella Typhimurium in NRAMP+/+ mice. We predict the same in chicken–NTS interaction.
- (ii)
- Feo may not be essential for persistent infection in mouse models due to redundancy of various iron-uptake systems. This includes Mn2+ uptake via SitABCD and MntH, and uptake of siderophores.
- (iii)
- NTS predilects to iron-rich hemophagocytes during systemic infection.
3.2. Siderophore Synthesis Is Important During Persistent Infection and Bacteremia
- (i)
- Ferric iron uptake mechanisms are important for persistent infection.We predict similar results for chicken as those found in mouse models because bioavailability of iron is expected to be low in most compartments of the host.
- (ii)
- Aerobactin, salmochelin and yersiniabactin provide a serum resistance during bacteremia and systemic infection. This may explain the siderophore link towards chicken-associated virulent NTS serovars.
- (iii)
- The role of stealth siderophores of NTS in adult chickens during colonization may be nonessential due to tolerogenic response.
4. Opening the Pandora’s Box of Gallus-Iron-Salmonella Interaction
4.1. Nutritional Immunity Status in Chicken during Salmonella Infection
4.2. Non-Canonical Function of Siderophores: Defense against Respiratory Burst and Immunomodulatory Function
5. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ryan, M.P.; O’Dwyer, J.; Adley, C.C. Evaluation of the complex nomenclature of the clinically and veterinary significant pathogen Salmonella. Biomed Res. Int. 2017, 2017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tindall, B.; Grimont, P.; Garrity, G.; Euzeby, J. Nomenclature and taxonomy of the genus Salmonella. Int. J. Syst. Evol. Microbiol. 2005, 55, 521–524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grimont, P.A.; Weill, F.-X. Antigenic formulae of the Salmonella serovars. Who Collab. Cent. Ref. Res. Salmonella 2007, 9, 1–166. [Google Scholar]
- Giammanco, G.M.; Pignato, S.; Mammina, C.; Grimont, F.; Grimont, P.A.; Nastasi, A.; Giammanco, G. Persistent endemicity of Salmonella bongori 48: z35: In southern Italy: Molecular characterization of human, animal, and environmental isolates. J. Clin. Microbiol. 2002, 40, 3502–3505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aleksic, S.; Heinzerling, F.; Bockemühl, J. Human infection caused by salmonellae of subspecies II to VI in Germany, 1977–1992. Z. Bakteriol. 1996, 283, 391–398. [Google Scholar] [CrossRef]
- Desai, P.T.; Porwollik, S.; Long, F.; Cheng, P.; Wollam, A.; Clifton, S.W.; Weinstock, G.M.; McClelland, M. Evolutionary genomics of Salmonella enterica subspecies. MBio 2013, 4, e00579-12. [Google Scholar] [CrossRef] [Green Version]
- Porwollik, S.; Wong, R.M.-Y.; McClelland, M. Evolutionary genomics of Salmonella: Gene acquisitions revealed by microarray analysis. Proc. Natl. Acad. Sci. USA 2002, 99, 8956–8961. [Google Scholar] [CrossRef] [Green Version]
- Fricke, W.F.; Mammel, M.K.; McDermott, P.F.; Tartera, C.; White, D.G.; LeClerc, J.E.; Ravel, J.; Cebula, T.A. Comparative genomics of 28 Salmonella enterica isolates: Evidence for CRISPR-mediated adaptive sublineage evolution. J. Bacteriol. 2011, 193, 3556–3568. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Havelaar, A.H.; Hoffmann, S.; Hald, T.; Kirk, M.D.; Torgerson, P.R.; Devleesschauwer, B. Global disease burden of pathogens in animal source foods, 2010. PLoS ONE 2019, 14, e0216545. [Google Scholar] [CrossRef]
- Ferrari, R.G.; Rosario, D.K.; Cunha-Neto, A.; Mano, S.B.; Figueiredo, E.E.; Conte-Junior, C.A. Worldwide epidemiology of Salmonella serovars in animal-based foods: A meta-analysis. Appl. Environ. Microbiol. 2019, 85, e00591-19. [Google Scholar] [CrossRef] [Green Version]
- Borges, K.A.; Furian, T.Q.; De Souza, S.N.; Tondo, E.C.; Streck, A.F.; Salle, C.T.P.; de Souza Moraes, H.L.; Do Nascimento, V.P. Spread of a major clone of Salmonella enterica serotype Enteritidis in poultry and in salmonellosis outbreaks in Southern Brazil. J. Food Prot. 2017, 80, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.-H.; Huang, A.S.; Liao, Y.-S.; Liu, Y.-L.; Chiou, C.-S. A large outbreak of salmonellosis associated with sandwiches contaminated with multiple bacterial pathogens purchased via an online shopping service. Foodborne Pathog. Dis. 2014, 11, 230–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kinross, P.; Van Alphen, L.; Urtaza, J.M.; Struelens, M.; Takkinen, J.; Coulombier, D.; Mäkelä, P.; Bertrand, S.; Mattheus, W.; Schmid, D. Multidisciplinary investigation of a multicountry outbreak of Salmonella Stanley infections associated with turkey meat in the European Union, August 2011 to January 2013. Eurosurveillance 2014, 19, 20801. [Google Scholar] [CrossRef] [PubMed]
- Hörmansdorfer, S.; Messelhäußer, U.; Rampp, A.; Schönberger, K.; Dallman, T.; Allerberger, F.; Kornschober, C.; Sing, A.; Wallner, P.; Zapf, A. Re-evaluation of a 2014 multi-country European outbreak of Salmonella Enteritidis phage type 14b using recent epidemiological and molecular data. Euro Surveill. 2017, 22, 17-00196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ford, L.; Wang, Q.; Stafford, R.; Ressler, K.-A.; Norton, S.; Shadbolt, C.; Hope, K.; Franklin, N.; Krsteski, R.; Carswell, A. Seven Salmonella Typhimurium outbreaks in Australia linked by trace-back and whole genome sequencing. Foodborne Pathog. Dis. 2018, 15, 285–292. [Google Scholar] [CrossRef]
- Andrews, S.C.; Robinson, A.K.; Rodríguez-Quiñones, F. Bacterial iron homeostasis. FEMS Microbiol. Rev. 2003, 27, 215–237. [Google Scholar] [CrossRef]
- Wooldridge, K.G.; Williams, P.H. Iron uptake mechanisms of pathogenic bacteria. FEMS Microbiol. Rev. 1993, 12, 325–348. [Google Scholar] [CrossRef]
- Krewulak, K.D.; Vogel, H.J. Structural biology of bacterial iron uptake. Biochim. Biophys. Acta Biomembr. 2008, 1778, 1781–1804. [Google Scholar] [CrossRef] [Green Version]
- Ratledge, C.; Dover, L.G. Iron metabolism in pathogenic bacteria. Ann. Rev. Microbiol. 2000, 54, 881–941. [Google Scholar] [CrossRef]
- Miethke, M.; Marahiel, M.A. Siderophore-based iron acquisition and pathogen control. Microbiol. Mol. Biol. Rev. 2007, 71, 413–451. [Google Scholar] [CrossRef] [Green Version]
- Sheldon, J.R.; Heinrichs, D.E. Recent developments in understanding the iron acquisition strategies of gram positive pathogens. FEMS Microbiol. Rev. 2015, 39, 592–630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imlay, J.A. Oxidative Stress. Ecosal Plus 2009, 3. [Google Scholar] [CrossRef] [PubMed]
- Liochev, S.I.; Fridovich, I. The Haber-Weiss cycle—70 years later: An alternative view. Redox Rep. 2002, 7, 55–57. [Google Scholar] [CrossRef] [PubMed]
- Stojiljkovic, I.; Hantke, K. Functional domains of theescherichia coli ferric uptake regulator protein (fur). Mol. Gen. Genet. 1995, 247, 199–205. [Google Scholar] [CrossRef]
- Bagg, A.; Neilands, J. Ferric uptake regulation protein acts as a repressor, employing iron (II) as a cofactor to bind the operator of an iron transport operon in Escherichia coli. Biochemistry 1987, 26, 5471–5477. [Google Scholar] [CrossRef]
- Tsolis, R.M.; Bäumler, A.J.; Stojiljkovic, I.; Heffron, F. Fur regulon of Salmonella typhimurium: Identification of new iron-regulated genes. J. Bacteriol. 1995, 177, 4628–4637. [Google Scholar] [CrossRef] [Green Version]
- Foster, J.W.; Hall, H.K. Effect of Salmonella typhimurium ferric uptake regulator (fur) mutations on iron-and pH-regulated protein synthesis. J. Bacteriol. 1992, 174, 4317–4323. [Google Scholar] [CrossRef] [Green Version]
- Carpenter, B.M.; Whitmire, J.M.; Merrell, D.S. This is not your mother’s repressor: The complex role of fur in pathogenesis. Infect. Immun. 2009, 77, 2590–2601. [Google Scholar] [CrossRef] [Green Version]
- Oglesby-Sherrouse, A.G.; Murphy, E.R. Iron-responsive bacterial small RNAs: Variations on a theme. Metallomics 2013, 5, 276–286. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.N.; Kwon, Y.M. Genetic and phenotypic characterization of the RyhB regulon in Salmonella Typhimurium. Microbiol. Res. 2013, 168, 41–49. [Google Scholar] [CrossRef]
- Massé, E.; Gottesman, S. A small RNA regulates the expression of genes involved in iron metabolism in Escherichia coli. Proc. Natl. Acad. Sci. USA 2002, 99, 4620–4625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Figueroa-Bossi, N.; Bossi, L. Sponges and predators in the small RNA world. Regulating with RNA in Bacteria and Archaea. microbiolspec 2018, 6. [Google Scholar] [CrossRef]
- Scarrow, R.C.; Ecker, D.J.; Ng, C.; Liu, S.; Raymond, K.N. Iron (III) coordination chemistry of linear dihydroxyserine compounds derived from enterobactin. Inorg. Chem. 1991, 30, 900–906. [Google Scholar] [CrossRef]
- Hantke, K. Dihydroxybenzolyserine—a siderophore for E. coli. FEMS Microbiol. Lett. 1990, 67, 5–8. [Google Scholar]
- Raymond, K.N.; Dertz, E.A.; Kim, S.S. Enterobactin: An archetype for microbial iron transport. Proc. Natl. Acad. Sci. USA 2003, 100, 3584–3588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langridge, G.C.; Fookes, M.; Connor, T.R.; Feltwell, T.; Feasey, N.; Parsons, B.N.; Seth-Smith, H.M.; Barquist, L.; Stedman, A.; Humphrey, T. Patterns of genome evolution that have accompanied host adaptation in Salmonella. Proc. Natl. Acad. Sci. USA 2015, 112, 863–868. [Google Scholar] [CrossRef] [Green Version]
- Rabsch, W.; Methner, U.; Voigt, W.; Tschäpe, H.; Reissbrodt, R.; Williams, P.H. Role of receptor proteins for enterobactin and 2, 3-dihydroxybenzoylserine in virulence of Salmonella enterica. Infect. Immun. 2003, 71, 6953–6961. [Google Scholar] [CrossRef] [Green Version]
- Hantke, K.; Nicholson, G.; Rabsch, W.; Winkelmann, G. Salmochelins, siderophores of Salmonella enterica and uropathogenic Escherichia coli strains, are recognized by the outer membrane receptor IroN. Proc. Natl. Acad. Sci. USA 2003, 100, 3677–3682. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.; Fischbach, M.A.; Liu, D.R.; Walsh, C.T. In vitro characterization of salmochelin and enterobactin trilactone hydrolases IroD, IroE, and Fes. J. Am. Chem. Soc. 2005, 127, 11075–11084. [Google Scholar] [CrossRef] [Green Version]
- Bister, B.; Bischoff, D.; Nicholson, G.J.; Valdebenito, M.; Schneider, K.; Winkelmann, G.; Hantke, K.; Süssmuth, R.D. The structure of salmochelins: C-glucosylated enterobactins of Salmonella enterica §. Biometals 2004, 17, 471–481. [Google Scholar] [CrossRef]
- Oves-Costales, D.; Kadi, N.; Challis, G.L. The long-overlooked enzymology of a nonribosomal peptide synthetase-independent pathway for virulence-conferring siderophore biosynthesis. Chem. Commun. 2009, 43, 6530–6541. [Google Scholar] [CrossRef] [PubMed]
- De Lorenzo, V.; Martinez, J. Aerobactin production as a virulence factor: A reevaluation. Eur. J. Clin. Microbiol. Infect. Dis. 1988, 7, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Köster, W. ABC transporter-mediated uptake of iron, siderophores, heme and vitamin B12. Res. Microbiol. 2001, 152, 291–301. [Google Scholar] [CrossRef]
- Oelschlaeger, T.; Zhang, D.; Schubert, S.; Carniel, E.; Rabsch, W.; Karch, H.; Hacker, J. The high-pathogenicity island is absent in human pathogens of Salmonella enterica subspecies I but present in isolates of subspecies III and VI. J. Bacteriol. 2003, 185, 1107–1111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lau, C.K.; Krewulak, K.D.; Vogel, H.J. Bacterial ferrous iron transport: The Feo system. FEMS Microbiol. Rev. 2016, 40, 273–298. [Google Scholar] [CrossRef]
- Sestok, A.E.; Linkous, R.O.; Smith, A.T. Toward a mechanistic understanding of Feo-mediated ferrous iron uptake. Metallomics 2018, 10, 887–898. [Google Scholar] [CrossRef]
- Kehres, D.G.; Janakiraman, A.; Slauch, J.M.; Maguire, M.E. SitABCD is the alkaline Mn2+ transporter of Salmonella enterica serovar Typhimurium. J. Bacteriol. 2002, 184, 3159–3166. [Google Scholar] [CrossRef] [Green Version]
- Cartron, M.L.; Maddocks, S.; Gillingham, P.; Craven, C.J.; Andrews, S.C. Feo–transport of ferrous iron into bacteria. Biometals 2006, 19, 143–157. [Google Scholar] [CrossRef]
- Dhillon, A.; Alisantosa, B.; Shivaprasad, H.; Jack, O.; Schaberg, D.; Bandli, D. Pathogenicity of Salmonella enteritidis phage types 4, 8, and 23 in broiler chicks. Avian Dis. 1999, 43, 506–515. [Google Scholar] [CrossRef]
- Roy, P.; Dhillon, A.; Shivaprasad, H.; Schaberg, D.; Bandli, D.; Johnson, S. Pathogenicity of different serogroups of avian salmonellae in specific-pathogen-free chickens. Avian Dis. 2001, 45, 922–937. [Google Scholar] [CrossRef]
- Hazards, E.P.o.B.; Koutsoumanis, K.; Allende, A.; Alvarez-Ordóñez, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; De Cesare, A.; Herman, L.; Hilbert, F. Salmonella control in poultry flocks and its public health impact. EFSA J. 2019, 17, e05596. [Google Scholar]
- Shah, D.H.; Paul, N.C.; Sischo, W.C.; Crespo, R.; Guard, J. Population dynamics and antimicrobial resistance of the most prevalent poultry-associated Salmonella serotypes. Poult. Sci. 2017, 96, 687–702. [Google Scholar] [CrossRef] [PubMed]
- Suez, J.; Porwollik, S.; Dagan, A.; Marzel, A.; Schorr, Y.I.; Desai, P.T.; Agmon, V.; McClelland, M.; Rahav, G.; Gal-Mor, O. Virulence gene profiling and pathogenicity characterization of non-typhoidal Salmonella accounted for invasive disease in humans. PLoS ONE 2013, 8, e58449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waters, V.L.; Crosa, J.H. Colicin V virulence plasmids. Microbiol. Mol. Biol. Rev. 1991, 55, 437–450. [Google Scholar] [CrossRef]
- Johnson, T.J.; Thorsness, J.L.; Anderson, C.P.; Lynne, A.M.; Foley, S.L.; Han, J.; Fricke, W.F.; McDermott, P.F.; White, D.G.; Khatri, M. Horizontal gene transfer of a ColV plasmid has resulted in a dominant avian clonal type of Salmonella enterica serovar Kentucky. PLoS ONE 2010, 5, e15524. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Pedroso, A.A.; Porwollik, S.; McClelland, M.; Lee, M.D.; Kwan, T.; Zamperini, K.; Soni, V.; Sellers, H.S.; Russell, S.M. rpoS-Regulated core genes involved in the competitive fitness of Salmonella enterica serovar Kentucky in the intestines of chickens. Appl. Environ. Microbiol. 2015, 81, 502–514. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, M.; Zhao, S.; Pettengill, J.; Luo, Y.; Monday, S.R.; Abbott, J.; Ayers, S.L.; Cinar, H.N.; Muruvanda, T.; Li, C. Comparative genomic analysis and virulence differences in closely related Salmonella enterica serotype Heidelberg isolates from humans, retail meats, and animals. Genome Biol. Evol. 2014, 6, 1046–1068. [Google Scholar] [CrossRef] [Green Version]
- Dhanani, A.S.; Block, G.; Dewar, K.; Forgetta, V.; Topp, E.; Beiko, R.G.; Diarra, M.S. Genomic comparison of non-typhoidal Salmonella enterica serovars Typhimurium, Enteritidis, Heidelberg, Hadar and Kentucky isolates from broiler chickens. PLoS ONE 2015, 10, e0128773. [Google Scholar] [CrossRef]
- Branchu, P.; Bawn, M.; Kingsley, R.A. Genome variation and molecular epidemiology of Salmonella enterica serovar typhimurium pathovariants. Infect. Immun. 2018, 86, e00079-18. [Google Scholar] [CrossRef] [Green Version]
- Mulvey, M.R.; Boyd, D.A.; Olson, A.B.; Doublet, B.; Cloeckaert, A. The genetics of Salmonella genomic island 1. Microbes Infect. 2006, 8, 1915–1922. [Google Scholar] [CrossRef]
- Guiney, D.G.; Fierer, J. The role of the spv genes in Salmonella pathogenesis. Front. Microbiol. 2011, 2, 129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khajanchi, B.K.; Hasan, N.A.; Choi, S.Y.; Han, J.; Zhao, S.; Colwell, R.R.; Cerniglia, C.E.; Foley, S.L. Comparative genomic analysis and characterization of incompatibility group FIB plasmid encoded virulence factors of Salmonella enterica isolated from food sources. BMC Genom. 2017, 18, 570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrovska, L.; Mather, A.E.; AbuOun, M.; Branchu, P.; Harris, S.R.; Connor, T.; Hopkins, K.L.; Underwood, A.; Lettini, A.A.; Page, A. Microevolution of monophasic Salmonella typhimurium during epidemic, United Kingdom, 2005–2010. Emerg. Infect. Dis. 2016, 22, 617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huehn, S.; Bunge, C.; Junker, E.; Helmuth, R.; Malorny, B. Poultry-associated Salmonella enterica subsp. enterica serovar 4, 12: d:− reveals high clonality and a distinct pathogenicity gene repertoire. Appl. Environ. Microbiol. 2009, 75, 1011–1020. [Google Scholar] [CrossRef] [Green Version]
- Thomson, N.R.; Clayton, D.J.; Windhorst, D.; Vernikos, G.; Davidson, S.; Churcher, C.; Quail, M.A.; Stevens, M.; Jones, M.A.; Watson, M. Comparative genome analysis of Salmonella Enteritidis PT4 and Salmonella Gallinarum 287/91 provides insights into evolutionary and host adaptation pathways. Genome Res. 2008, 18, 1624–1637. [Google Scholar] [CrossRef] [Green Version]
- Wuyts, V.; Denayer, S.; Roosens, N.H.; Mattheus, W.; Bertrand, S.; Marchal, K.; Dierick, K.; De Keersmaecker, S.C. Whole genome sequence analysis of Salmonella Enteritidis PT4 outbreaks from a national reference laboratory’s viewpoint. PLoS Curr. 2015. [Google Scholar] [CrossRef] [Green Version]
- Silva, C.A.; Blondel, C.J.; Quezada, C.P.; Porwollik, S.; Andrews-Polymenis, H.L.; Toro, C.S.; Zaldívar, M.; Contreras, I.; McClelland, M.; Santiviago, C.A. Infection of mice by Salmonella enterica serovar Enteritidis involves additional genes that are absent in the genome of serovar Typhimurium. Infect. Immun. 2012, 80, 839–849. [Google Scholar] [CrossRef] [Green Version]
- Clayton, D.J.; Bowen, A.J.; Hulme, S.D.; Buckley, A.M.; Deacon, V.L.; Thomson, N.R.; Barrow, P.A.; Morgan, E.; Jones, M.A.; Watson, M. Analysis of the role of 13 major fimbrial subunits in colonisation of the chicken intestines by Salmonella enterica serovar Enteritidis reveals a role for a novel locus. BMC Microbiol. 2008, 8, 228. [Google Scholar] [CrossRef] [Green Version]
- Bachmann, N.L.; Petty, N.K.; Zakour, N.L.B.; Szubert, J.M.; Savill, J.; Beatson, S.A. Genome analysis and CRISPR typing of Salmonella enterica serovar Virchow. BMC Genom. 2014, 15, 389. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, S.V.; Harhay, D.M.; Bono, J.L.; Smith, T.P.; Fields, P.I.; Dinsmore, B.A.; Santovenia, M.; Wang, R.; Bosilevac, J.M.; Harhay, G.P. Comparative genomics of Salmonella enterica serovar Montevideo reveals lineage-specific gene differences that may influence ecological niche association. Microb. Genom. 2018, 4, e000202. [Google Scholar] [CrossRef]
- Franco, A.; Leekitcharoenphon, P.; Feltrin, F.; Alba, P.; Cordaro, G.; Iurescia, M.; Tolli, R.; D’Incau, M.; Staffolani, M.; Di Giannatale, E. Emergence of a clonal lineage of multidrug-resistant ESBL-producing Salmonella Infantis transmitted from broilers and broiler meat to humans in Italy between 2011 and 2014. PLoS ONE 2015, 10, e0144802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bogomazova, A.N.; Gordeeva, V.D.; Krylova, E.V.; Soltynskaya, I.V.; Davydova, E.E.; Ivanova, O.E.; Komarov, A.A. Mega-plasmid found worldwide confers multiple antimicrobial resistance in Salmonella Infantis of broiler origin in Russia. Int. J. Food Microbiol. 2019, 319, 108497. [Google Scholar] [CrossRef] [PubMed]
- Aviv, G.; Elpers, L.; Mikhlin, S.; Cohen, H.; Vitman Zilber, S.; Grassl, G.A.; Rahav, G.; Hensel, M.; Gal-Mor, O. The plasmid-encoded Ipf and Klf fimbriae display different expression and varying roles in the virulence of Salmonella enterica serovar Infantis in mouse vs. avian hosts. PLoS Pathog. 2017, 13, e1006559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alba, P.; Leekitcharoenphon, P.; Carfora, V.; Amoruso, R.; Cordaro, G.; Di Matteo, P.; Ianzano, A.; Iurescia, M.; Diaconu, E.L.; Study Group, E.-E.-A.N. Molecular epidemiology of Salmonella Infantis in Europe: Insights into the success of the bacterial host and its parasitic pESI-like megaplasmid. Microb. Genom. 2020, 6, e000365. [Google Scholar] [CrossRef] [PubMed]
- Foley, S.; Lynne, A.; Nayak, R. Salmonella challenges: Prevalence in swine and poultry and potential pathogenicity of such isolates. J. Anim. Sci. 2008, 86, E149–E162. [Google Scholar] [CrossRef] [PubMed]
- Salisbury, A.-M.; Leeming, G.; Nikolaou, G.; Kipar, A.; Wigley, P. Salmonella Virchow infection of the chicken elicits cellular and humoral systemic and mucosal responses, but limited protection to homologous or heterologous re-challenge. Front. Vet. Sci. 2014, 1, 6. [Google Scholar] [CrossRef] [Green Version]
- Stanaway, J.D.; Parisi, A.; Sarkar, K.; Blacker, B.F.; Reiner, R.C.; Hay, S.I.; Nixon, M.R.; Dolecek, C.; James, S.L.; Mokdad, A.H. The global burden of non-typhoidal salmonella invasive disease: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Infect. Dis. 2019, 19, 1312–1324. [Google Scholar] [CrossRef] [Green Version]
- Jones, T.F.; Ingram, L.A.; Cieslak, P.R.; Vugia, D.J.; Tobin-D’Angelo, M.; Hurd, S.; Medus, C.; Cronquist, A.; Angulo, F.J. Salmonellosis outcomes differ substantially by serotype. J. Infect. Dis. 2008, 198, 109–114. [Google Scholar] [CrossRef]
- Rabsch, W.; Tschäpe, H.; Bäumler, A.J. Non-typhoidal salmonellosis: Emerging problems. Microbes Infect. 2001, 3, 237–247. [Google Scholar] [CrossRef]
- Foley, S.L.; Nayak, R.; Hanning, I.B.; Johnson, T.J.; Han, J.; Ricke, S.C. Population dynamics of Salmonella enterica serotypes in commercial egg and poultry production. Appl. Environ. Microbiol. 2011, 77, 4273–4279. [Google Scholar] [CrossRef] [Green Version]
- den Bakker, H.C.; Switt, A.I.M.; Govoni, G.; Cummings, C.A.; Ranieri, M.L.; Degoricija, L.; Hoelzer, K.; Rodriguez-Rivera, L.D.; Brown, S.; Bolchacova, E. Genome sequencing reveals diversification of virulence factor content and possible host adaptation in distinct subpopulations of Salmonella enterica. BMC Genom. 2011, 12, 425. [Google Scholar] [CrossRef] [Green Version]
- Lo-Ten-Foe, J.; Van Oers, J.; Kotsopoulos, A.; Buiting, A. Pulmonary colonization with Salmonella enterica serovar Kentucky in an intensive care unit. J. Hosp. Infect. 2007, 67, 105–107. [Google Scholar] [CrossRef] [PubMed]
- Neelambike, S.; Shivappa, S.G. A Fatal Case of Acute Gastroenteritis with Sepsis due to Salmonella enterica Serovar Kentucky in an Immunocompetent Patient: A Case Report. J. Clin. Diagn. Res. 2019, 13, 1–2. [Google Scholar] [CrossRef]
- Collard, J.-M.; Place, S.; Denis, O.; Rodriguez-Villalobos, H.; Vrints, M.; Weill, F.-X.; Baucheron, S.; Cloeckaert, A.; Struelens, M.; Bertrand, S. Travel-acquired salmonellosis due to Salmonella Kentucky resistant to ciprofloxacin, ceftriaxone and co-trimoxazole and associated with treatment failure. J. Antimicrob. Chemother. 2007, 60, 190–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carramiñana, J.J.; Yangüela, J.; Blanco, D.; Rota, C.; Agustín, A.I.; Herrera, A. Potential virulence determinants of Salmonella serovars from poultry and human sources in Spain. Vet. Microbiol. 1997, 54, 375–383. [Google Scholar] [CrossRef]
- Visca, P.; Filetici, E.; Anastasio, M.; Vetriani, C.; Fantasia, M.; Orsi, N. Siderophore production by Salmonella species isolated from different sources. FEMS Microbiol. Lett. 1991, 79, 225–232. [Google Scholar] [CrossRef]
- Rabsch, W.; Reissbrodt, R. Investigations of Salmonella strains from different clinical-epidemiological origin with phenolate and hydroxamate (aerobactin)--siderophore bioassays. J. Hyg. Epidemiol. Microbiol. Immunol. 1988, 32, 353–360. [Google Scholar] [PubMed]
- Baker, E.N.; Anderson, B.F.; Baker, H.M.; Day, C.L.; Haridas, M.; Norris, G.E.; Rumball, S.V.; Smith, C.A.; Thomas, D.H. Three-dimensional structure of lactoferrin in various functional states. Adv. Exp. Med. Biol. 1994, 357, 1–12. [Google Scholar] [CrossRef]
- Kell, D.B.; Heyden, E.L.; Pretorius, E. The Biology of Lactoferrin, an Iron-Binding Protein That Can Help Defend Against Viruses and Bacteria. Front. Immunol. 2020, 11, 1221. [Google Scholar] [CrossRef]
- Dho-Moulin, M.; Fairbrother, J.M. Avian pathogenic Escherichia coli (APEC). Vet. Res. BioMed Central 1999, 30, 299–316. [Google Scholar]
- Dozois, C.M.; Daigle, F.; Curtiss, R. Identification of pathogen-specific and conserved genes expressed in vivo by an avian pathogenic Escherichia coli strain. Proc. Natl. Acad. Sci. USA 2003, 100, 247–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ling, J.; Pan, H.; Gao, Q.; Xiong, L.; Zhou, Y.; Zhang, D.; Gao, S.; Liu, X. Aerobactin synthesis genes iucA and iucC contribute to the pathogenicity of avian pathogenic Escherichia coli O2 strain E058. PLoS ONE 2013, 8, e57794. [Google Scholar] [CrossRef] [PubMed]
- Russo, T.A.; Olson, R.; MacDonald, U.; Beanan, J.; Davidson, B.A. Aerobactin, but not yersiniabactin, salmochelin, or enterobactin, enables the growth/survival of hypervirulent (hypermucoviscous) Klebsiella pneumoniae ex vivo and in vivo. Infect. Immun. 2015, 83, 3325–3333. [Google Scholar] [CrossRef] [Green Version]
- Nolan, L.K.; Horne, S.; Giddings, C.; Foley, S.; Johnson, T.; Lynne, A.; Skyberg, J. Resistance to serum complement, iss, and virulence of avian Escherichia coli. Vet. Res. Commun. 2003, 27, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Aviv, G.; Tsyba, K.; Steck, N.; Salmon-Divon, M.; Cornelius, A.; Rahav, G.; Grassl, G.A.; Gal-Mor, O. A unique megaplasmid contributes to stress tolerance and pathogenicity of an emergent S almonella enterica serovar Infantis strain. Environ. Microbiol. 2014, 16, 977–994. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, K.S.; Hung, C.S.; Giblin, D.E.; Urushidani, S.; Austin, A.M.; Dinauer, M.C.; Henderson, J.P. Cupric yersiniabactin is a virulence-associated superoxide dismutase mimic. ACS Chem. Biol. 2014, 9, 551–561. [Google Scholar] [CrossRef]
- Chaturvedi, K.S.; Hung, C.S.; Crowley, J.R.; Stapleton, A.E.; Henderson, J.P. The siderophore yersiniabactin binds copper to protect pathogens during infection. Nat. Chem. Biol. 2012, 8, 731–736. [Google Scholar] [CrossRef] [Green Version]
- Osman, D.; Waldron, K.J.; Denton, H.; Taylor, C.M.; Grant, A.J.; Mastroeni, P.; Robinson, N.J.; Cavet, J.S. Copper homeostasis in Salmonella is atypical and copper-CueP is a major periplasmic metal complex. J. Biol. Chem. 2010, 285, 25259–25268. [Google Scholar] [CrossRef] [Green Version]
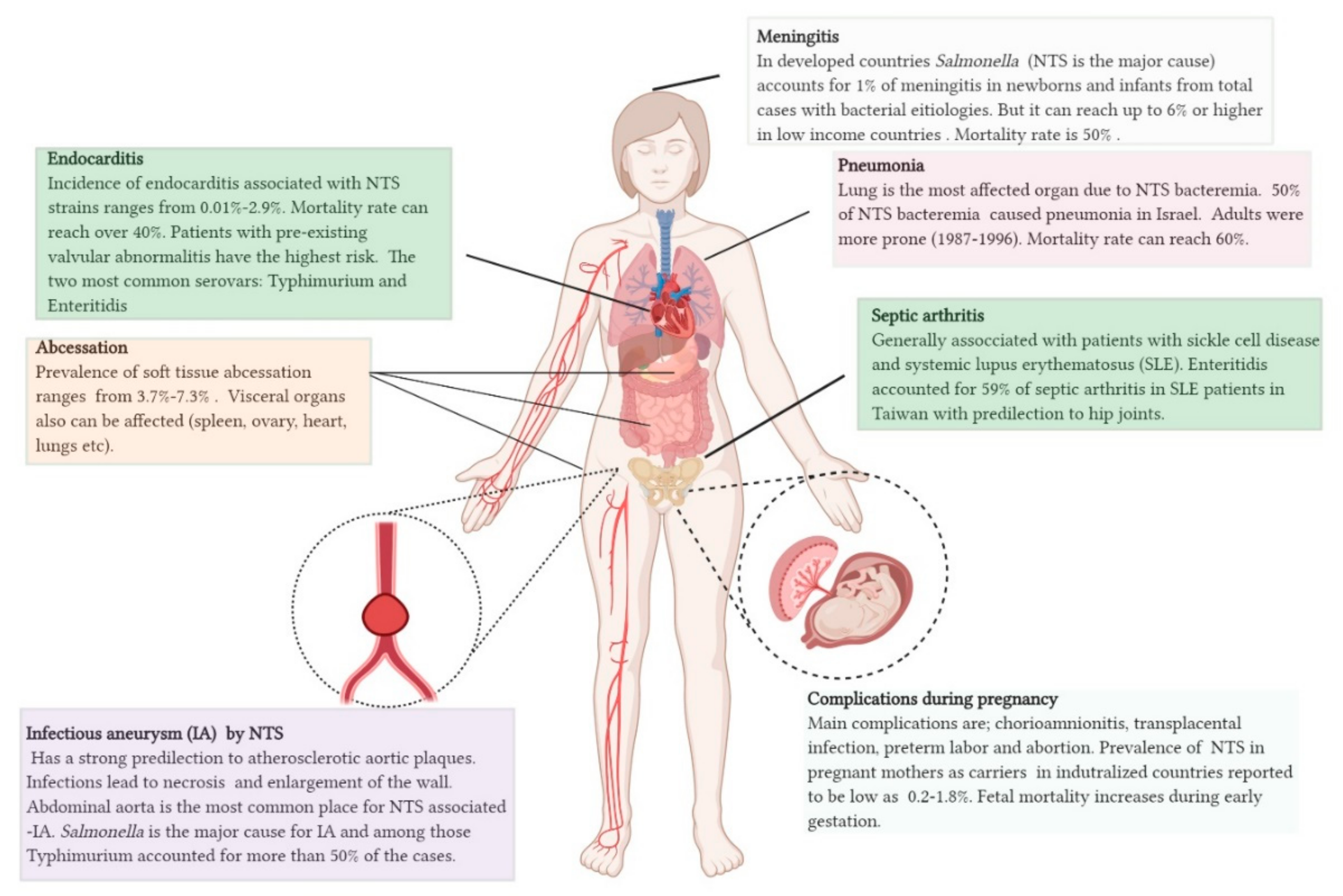
- Guerrero, M.L.F.; Aguado, J.M.; Arribas, A.; Lumbreras, C.; de Gorgolas, M. The spectrum of cardiovascular infections due to Salmonella enterica: A review of clinical features and factors determining outcome. Medicine 2004, 83, 123–138. [Google Scholar] [CrossRef]
- Shimoni, Z.; Pitlik, S.; Leibovici, L.; Samra, Z.; Konigsberger, H.; Drucker, M.; Agmon, V.; Ashkenazi, S.; Weinberger, M. Nontyphoid Salmonella bacteremia: Age-related differences in clinical presentation, bacteriology, and outcome. Clin. Infect. Dis. 1999, 28, 822–827. [Google Scholar] [CrossRef]
- Acheson, D.; Hohmann, E.L. Nontyphoidal salmonellosis. Clin. Infect. Dis. 2001, 32, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Hall, R.L.; Partridge, R.; Venkatraman, N.; Wiselka, M. Invasive non-typhoidal Salmonella infection with multifocal seeding in an immunocompetent host: An emerging disease in the developed world. Case Rep. 2013, 2013, bcr2012008230. [Google Scholar] [CrossRef] [PubMed]
- Brnčić, N.; Gorup, L.; Strčić, M.; Abram, M.; Mustač, E. Breast abscess in a man due to Salmonella enterica serotype Enteritidis. J. Clin. Microbiol. 2012, 50, 192–193. [Google Scholar] [CrossRef] [Green Version]
- Hibbert, B.; Costiniuk, C.; Hibbert, R.; Joseph, P.; Alanazi, H.; Simard, T.; Dennie, C.; Angel, J.B.; O’Brien, E.R. Cardiovascular complications of Salmonella enteritidis infection. Can. J. Cardiol. 2010, 26, e323–e325. [Google Scholar] [CrossRef] [Green Version]
- Schloesser, R.L.; Schaefer, V.; Groll, A.H. Fatal transplacental infection with non-typhoidal Salmonella. Scand. J. Infect. Dis. 2004, 36, 773–774. [Google Scholar] [CrossRef] [PubMed]
- Ispahani, P.; Slack, R. Enteric fever and other extraintestinal salmonellosis in University Hospital, Nottingham, UK, between 1980 and 1997. Eur. J. Clin. Microbiol. Infect. Dis. 2000, 19, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.-L.; Hung, J.-J.; Wu, K.-C.; Lee, W.-I.; Chan, C.-K.; Ou, L.-S. Septic arthritis in patients with systemic lupus erythematosus: Salmonella and nonsalmonella infections compared. Semin Arthritis Rheum. 2006, 36, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Hakim, S.; Davila, F.; Amin, M.; Hader, I.; Cappell, M.S. Infectious aortitis: A life-threatening endovascular complication of nontyphoidal salmonella bacteremia. Case Rep. Med. 2018, 2018, 6845617. [Google Scholar] [CrossRef] [Green Version]
- Laohapensang, K.; Rutherford, R.B.; Arworn, S. Infected aneurysm. Ann. Vasc. Dis. 2010, 3, 16–23. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.-M.; Huang, W.-Y.; Lee, M.-L.; Yang, A.D.; Chaou, K.-P.; Hsieh, L.-Y. Clinical features, acute complications, and outcome of Salmonella meningitis in children under one year of age in Taiwan. BMC Infect. Dis. 2011, 11, 30. [Google Scholar] [CrossRef] [Green Version]
- Kaiser, P. Advances in avian immunology—prospects for disease control: A review. Avian Pathol. 2010, 39, 309–324. [Google Scholar] [CrossRef]
- Davison, F. The importance of the avian immune system and its unique features. Avian Immunol. 2014, 1–9. [Google Scholar] [CrossRef]
- Agoro, R.; Mura, C. Iron Supplementation Therapy, A Friend and Foe of Mycobacterial Infections? Pharmaceuticals 2019, 12, 75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Penniall, R.; Spitznagel, J.K. Chicken neutrophils: Oxidative metabolism in phagocytic cells devoid of myeloperoxidase. Proc. Natl. Acad. Sci. USA 1975, 72, 5012–5015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
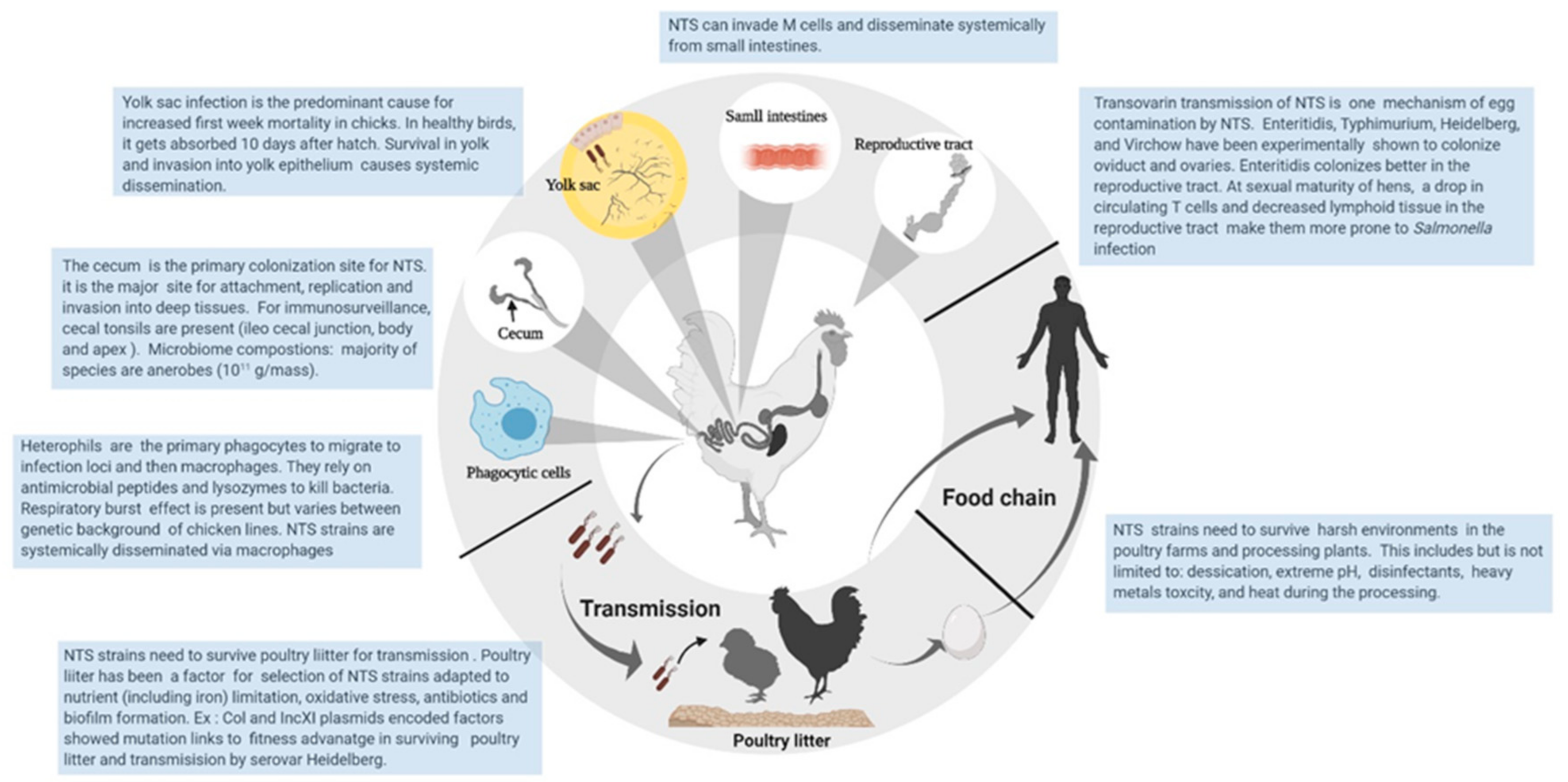
- Clench, M.H.; Mathias, J.R. The avian cecum: A review. Wilson Bull. 1995, 107, 93–121. [Google Scholar]
- Foley, S.L.; Johnson, T.J.; Ricke, S.C.; Nayak, R.; Danzeisen, J. Salmonella pathogenicity and host adaptation in chicken-associated serovars. Microbiol. Mol. Biol. Rev. 2013, 77, 582–607. [Google Scholar] [CrossRef] [Green Version]
- Gantois, I.; Eeckhaut, V.; Pasmans, F.; Haesebrouck, F.; Ducatelle, R.; Van Immerseel, F. A comparative study on the pathogenesis of egg contamination by different serotypes of Salmonella. Avian Pathol. 2008, 37, 399–406. [Google Scholar] [CrossRef] [Green Version]
- Desmidt, M.; Ducatelle, R.; Haesebrouck, F. Pathogenesis of Salmonella enteritidis phage type four after experimental infection of young chickens. Vet. Microbiol. 1997, 56, 99–109. [Google Scholar] [CrossRef]
- Johnston, C.E.; Hartley, C.; Salisbury, A.-M.; Wigley, P. Immunological changes at point-of-lay increase susceptibility to Salmonella enterica Serovar enteritidis infection in vaccinated chickens. PLoS ONE 2012, 7, e48195. [Google Scholar] [CrossRef] [Green Version]
- Oladeinde, A.; Cook, K.; Orlek, A.; Zock, G.; Herrington, K.; Cox, N.; Plumblee Lawrence, J.; Hall, C. Hotspot mutations and ColE1 plasmids contribute to the fitness of Salmonella Heidelberg in poultry litter. PLoS ONE 2018, 13, e0202286. [Google Scholar] [CrossRef] [Green Version]
- Wigley, P.; Hulme, S.; Rothwell, L.; Bumstead, N.; Kaiser, P.; Barrow, P. Macrophages isolated from chickens genetically resistant or susceptible to systemic salmonellosis show magnitudinal and temporal differential expression of cytokines and chemokines following Salmonella enterica challenge. Infect. Immun. 2006, 74, 1425–1430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andino, A.; Hanning, I. Salmonella enterica: Survival, colonization, and virulence differences among serovars. Sci. World J. 2015, 2015, 520179. [Google Scholar] [CrossRef] [Green Version]
- Harvey, P.; Watson, M.; Hulme, S.; Jones, M.; Lovell, M.; Berchieri, A.; Young, J.; Bumstead, N.; Barrow, P. Salmonella enterica serovar typhimurium colonizing the lumen of the chicken intestine grows slowly and upregulates a unique set of virulence and metabolism genes. Infect. Immun. 2011, 79, 4105–4121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabri, M.; Caza, M.; Proulx, J.; Lymberopoulos, M.H.; Brée, A.; Moulin-Schouleur, M.; Curtiss, R.; Dozois, C.M. Contribution of the SitABCD, MntH, and FeoB metal transporters to the virulence of avian pathogenic Escherichia coli O78 strain χ7122. Infect. Immun. 2008, 76, 601–611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boyer, E.; Bergevin, I.; Malo, D.; Gros, P.; Cellier, M. Acquisition of Mn (II) in addition to Fe (II) is required for full virulence of Salmonella enterica serovar Typhimurium. Infect. Immun. 2002, 70, 6032–6042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Portillo, F.G.d.; Foster, J.W.; Maguire, M.E.; Finlay, B.B. Characterization of the micro-environment of Salmonella typhimurium–containing vacuoles within MDCK epithelial cells. Mol. Microbiol. 1992, 6, 3289–3297. [Google Scholar] [CrossRef]
- Tsolis, R.M.; Bäumler, A.J.; Heffron, F.; Stojiljkovic, I. Contribution of TonB-and Feo-mediated iron uptake to growth of Salmonella typhimurium in the mouse. Infect. Immun. 1996, 64, 4549–4556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, L.F.; Mol, J.P.; Silva, A.P.C.; Macêdo, A.A.; Silva, T.M.; Alves, G.E.; Winter, S.; Winter, M.G.; Velazquez, E.M.; Byndloss, M.X. Iron acquisition pathways and colonization of the inflamed intestine by Salmonella enterica serovar Typhimurium. Int. J. Med Microbiol. 2016, 306, 604–610. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Kaiser, M.; Lamont, S. Natural resistance-associated macrophage protein 1 gene polymorphisms and response to vaccine against or challenge with Salmonella enteritidis in young chicks. Poult. Sci. 2003, 82, 259–266. [Google Scholar] [CrossRef]
- Forbes, J.R.; Gros, P. Divalent-metal transport by NRAMP proteins at the interface of host–pathogen interactions. Trends Microbiol. 2001, 9, 397–403. [Google Scholar] [CrossRef]
- Hu, J.; Bumstead, N.; Barrow, P.; Sebastiani, G.; Olien, L.; Morgan, K.; Malo, D. Resistance to salmonellosis in the chicken is linked to NRAMP1 and TNC. Genome Res. 1997, 7, 693–704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagy, T.A.; Moreland, S.M.; Detweiler, C.S. S almonella acquires ferrous iron from haemophagocytic macrophages. Mol. Microbiol. 2014, 93, 1314–1326. [Google Scholar]
- Pilonieta, M.C.; Moreland, S.M.; English, C.N.; Detweiler, C.S. Salmonella enterica infection stimulates macrophages to hemophagocytose. mBio 2014, 5, e02211-14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nix, R.N.; Altschuler, S.E.; Henson, P.M.; Detweiler, C.S. Hemophagocytic macrophages harbor Salmonella enterica during persistent infection. PLoS Pathog 2007, 3, e193. [Google Scholar] [CrossRef]
- Sánchez-Moreno, P.; Olbrich, P.; Falcón-Neyra, L.; Lucena, J.M.; Aznar, J.; Neth, O. Typhoid fever causing haemophagocytic lymphohistiocytosis in a non-endemic country–first case report and review of the current literature. Enferm. Infecc. Microbiol. Clin. 2019, 37, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.; Rabsch, W.; Methner, U.; Voigt, W.; Tschäpe, H.; Reissbrodt, R. Catecholate receptor proteins in Salmonella enterica: Role in virulence and implications for vaccine development. Vaccine 2006, 24, 3840–3844. [Google Scholar] [CrossRef]
- Wellman-Labadie, O.; Picman, J.; Hincke, M. Comparative antibacterial activity of avian egg white protein extracts. Br. Poult. Sci. 2008, 49, 125–132. [Google Scholar] [CrossRef]
- Baron, F.; Gautier, M.; Brule, G. Factors involved in the inhibition of growth of Salmonella enteritidis in liquid egg white. J. Food Prot. 1997, 60, 1318–1323. [Google Scholar] [CrossRef]
- Kang, H.; Loui, C.; Clavijo, R.; Riley, L.; Lu, S. Survival characteristics of Salmonella enterica serovar Enteritidis in chicken egg albumen. Epidemiol. Infect. 2006, 134, 967–976. [Google Scholar] [CrossRef]
- Réhault-Godbert, S.; Guyot, N.; Nys, Y. The golden egg: Nutritional value, bioactivities, and emerging benefits for human health. Nutrients 2019, 11, 684. [Google Scholar] [CrossRef] [Green Version]
- Qin, X.; He, S.; Zhou, X.; Cheng, X.; Huang, X.; Wang, Y.; Wang, S.; Cui, Y.; Shi, C.; Shi, X. Quantitative proteomics reveals the crucial role of YbgC for Salmonella enterica serovar Enteritidis survival in egg white. Int. J. Food Microbiol. 2019, 289, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Julien, L.A.; Baron, F.; Bonnassie, S.; Nau, F.; Guérin, C.; Jan, S.; Andrews, S.C. The anti-bacterial iron-restriction defence mechanisms of egg white; the potential role of three lipocalin-like proteins in resistance against Salmonella. BioMetals 2019, 32, 453–467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sattar Khan, M.; Nakamura, S.; Ogawa, M.; Akita, E.; Azakami, H.; Kato, A. Bactericidal action of egg yolk phosvitin against Escherichia coli under thermal stress. J. Agric. Food Chem. 2000, 48, 1503–1506. [Google Scholar] [CrossRef] [PubMed]
- Van Immerseel, F. Stress-induced survival strategies enable Salmonella Enteritidis to persistently colonize the chicken oviduct tissue and cope with antimicrobial factors in egg white: A hypothesis to explain a pandemic. Gut Pathog. 2010, 2, 23. [Google Scholar] [CrossRef] [Green Version]
- Raspoet, R.; Appia-Ayme, C.; Shearer, N.; Martel, A.; Pasmans, F.; Haesebrouck, F.; Ducatelle, R.; Thompson, A.; Van Immerseel, F. Microarray-based detection of Salmonella enterica serovar Enteritidis genes involved in chicken reproductive tract colonization. Appl. Environ. Microbiol. 2014, 80, 7710–7716. [Google Scholar] [CrossRef] [Green Version]
- Gantois, I.; Ducatelle, R.; Pasmans, F.; Haesebrouck, F.; Van Immerseel, F. Salmonella enterica serovar Enteritidis genes induced during oviduct colonization and egg contamination in laying hens. Appl. Environ. Microbiol. 2008, 74, 6616–6622. [Google Scholar] [CrossRef] [Green Version]
- Luo, M.; Lin, H.; Fischbach, M.A.; Liu, D.R.; Walsh, C.T.; Groves, J.T. Enzymatic tailoring of enterobactin alters membrane partitioning and iron acquisition. ACS Chem. Biol. 2006, 1, 29–32. [Google Scholar] [CrossRef]
- Moore, D.G.; Yancey, R.; Lankford, C.; Earhart, C. Bacteriostatic enterochelin-specific immunoglobulin from normal human serum. Infect. Immun. 1980, 27, 418–423. [Google Scholar] [CrossRef] [Green Version]
- Benjamin, W.; Turnbough, C.; Posey, B.; Briles, D. The ability of Salmonella typhimurium to produce the siderophore enterobactin is not a virulence factor in mouse typhoid. Infect. Immun. 1985, 50, 392–397. [Google Scholar] [CrossRef] [Green Version]
- Yancey, R.J.; Breeding, S.; Lankford, C. Enterochelin (enterobactin): Virulence factor for Salmonella typhimurium. Infect. Immun. 1979, 24, 174–180. [Google Scholar] [CrossRef] [Green Version]
- Nagy, T.A.; Moreland, S.M.; Andrews-Polymenis, H.; Detweiler, C.S. The ferric enterobactin transporter Fep is required for persistent Salmonella enterica serovar typhimurium infection. Infect. Immun. 2013, 81, 4063–4070. [Google Scholar] [CrossRef] [Green Version]
- Crouch, M.L.V.; Castor, M.; Karlinsey, J.E.; Kalhorn, T.; Fang, F.C. Biosynthesis and IroC-dependent export of the siderophore salmochelin are essential for virulence of Salmonella enterica serovar Typhimurium. Mol. Microbiol. 2008, 67, 971–983. [Google Scholar] [CrossRef] [PubMed]
- Oshota, O.; Conway, M.; Fookes, M.; Schreiber, F.; Chaudhuri, R.R.; Yu, L.; Morgan, F.J.; Clare, S.; Choudhary, J.; Thomson, N.R. Transcriptome and proteome analysis of Salmonella enterica serovar Typhimurium systemic infection of wild type and immune-deficient mice. PLoS ONE 2017, 12, e0181365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raffatellu, M.; George, M.D.; Akiyama, Y.; Hornsby, M.J.; Nuccio, S.-P.; Paixao, T.A.; Butler, B.P.; Chu, H.; Santos, R.L.; Berger, T. Lipocalin-2 resistance confers an advantage to Salmonella enterica serotype Typhimurium for growth and survival in the inflamed intestine. Cell Host Microbe 2009, 5, 476–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, G.J.; Frazer, D.M. Current understanding of iron homeostasis. Am. J. Clin. Nutr. 2017, 106, 1559S–1566S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konopka, K.; Bindereif, A.; Neilands, J. Aerobactin-mediated utilization of transferrin iron. Biochemistry 1982, 21, 6503–6508. [Google Scholar] [CrossRef]
- Montgomerie, J.; Bindereif, A.; Neilands, J.; Kalmanson, G.; Guze, L. Association of hydroxamate siderophore (aerobactin) with Escherichia coli isolated from patients with bacteremia. Infect. Immun. 1984, 46, 835–838. [Google Scholar] [CrossRef] [Green Version]
- Correnti, C.; Clifton, M.C.; Abergel, R.J.; Allred, B.; Hoette, T.M.; Ruiz, M.; Cancedda, R.; Raymond, K.N.; Descalzi, F.; Strong, R.K. Galline Ex-FABP is an antibacterial siderocalin and a lysophosphatidic acid sensor functioning through dual ligand specificities. Structure 2011, 19, 1796–1806. [Google Scholar] [CrossRef] [Green Version]
- Matulova, M.; Varmuzova, K.; Sisak, F.; Havlickova, H.; Babak, V.; Stejskal, K.; Zdrahal, Z.; Rychlik, I. Chicken innate immune response to oral infection with Salmonella enterica serovar Enteritidis. Vet. Res. 2013, 44, 37. [Google Scholar] [CrossRef] [Green Version]
- Rychlik, I.; Elsheimer-Matulova, M.; Kyrova, K. Gene expression in the chicken caecum in response to infections with non-typhoid Salmonella. Vet. Res. 2014, 45, 119. [Google Scholar] [CrossRef] [Green Version]
- Withanage, G.; Wigley, P.; Kaiser, P.; Mastroeni, P.; Brooks, H.; Powers, C.; Beal, R.; Barrow, P.; Maskell, D.; McConnell, I. Cytokine and chemokine responses associated with clearance of a primary Salmonella enterica serovar Typhimurium infection in the chicken and in protective immunity to rechallenge. Infect. Immun. 2005, 73, 5173–5182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Setta, A.; Barrow, P.; Kaiser, P.; Jones, M. Early immune dynamics following infection with Salmonella enterica serovars Enteritidis, Infantis, Pullorum and Gallinarum: Cytokine and chemokine gene expression profile and cellular changes of chicken cecal tonsils. Comp. Immunol. Microbiol. Infect. Dis. 2012, 35, 397–410. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, E.D. Nutritional immunity: Host’s attempt to withhold iron from microbial invaders. JAMA 1975, 231, 39–41. [Google Scholar] [CrossRef] [PubMed]
- Julien, L.A.; Fau, C.; Baron, F.; Bonnassie, S.; Guérin-Dubiard, C.; Nau, F.; Gautier, M.; Karatzas, K.A.; Jan, S.; Andrews, S.C. The three lipocalins of egg-white: Only Ex-FABP inhibits siderophore-dependent iron sequestration by Salmonella Enteritidis. Front. Microbiol. 2020, 11, 913. [Google Scholar] [CrossRef]
- Giansanti, F.; Leboffe, L.; Pitari, G.; Ippoliti, R.; Antonini, G. Physiological roles of ovotransferrin. Biochim. Biophys. Acta Gen. Subj. 2012, 1820, 218–225. [Google Scholar] [CrossRef]
- Hegenauer, J.; Saltman, P.; Nace, G. Iron (III)-phosphoprotein chelates: Stoichiometric equilibrium constant for interaction of iron (III) and phosphorylserine residues of phosvitin and casein. Biochemistry 1979, 18, 3865–3879. [Google Scholar] [CrossRef]
- Denic, S.; Agarwal, M.M. Nutritional iron deficiency: An evolutionary perspective. Nutrition 2007, 23, 603–614. [Google Scholar] [CrossRef]
- Muckenthaler, M.U.; Rivella, S.; Hentze, M.W.; Galy, B. A red carpet for iron metabolism. Cell 2017, 168, 344–361. [Google Scholar] [CrossRef] [Green Version]
- Drakesmith, H.; Prentice, A.M. Hepcidin and the iron-infection axis. Science 2012, 338, 768–772. [Google Scholar] [CrossRef] [Green Version]
- Hilton, K.B.; Lambert, L.A. Molecular evolution and characterization of hepcidin gene products in vertebrates. Gene 2008, 415, 40–48. [Google Scholar] [CrossRef]
- Wessling-Resnick, M. Iron homeostasis and the inflammatory response. Ann. Rev. Nutr. 2010, 30, 105–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganz, T. Hepcidin—a regulator of intestinal iron absorption and iron recycling by macrophages. Best Pract. Res. Clin. Haematol. 2005, 18, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.C.; Neves, J.V.; Silva, T.; Oliveira, P.; Gomes, M.S.; Rodrigues, P.N. Hepcidin-(in) dependent mechanisms of iron metabolism regulation during infection by Listeria and Salmonella. Infect. Immun. 2017, 85, e00353-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.-K.; Jeong, J.-H.; Lee, J.-M.; Kim, K.S.; Park, S.-H.; Kim, Y.D.; Koh, M.; Shin, M.; Jung, Y.S.; Kim, H.-S. Inverse agonist of estrogen-related receptor γ controls Salmonella typhimurium infection by modulating host iron homeostasis. Nat. Med. 2014, 20, 419–424. [Google Scholar] [CrossRef]
- Willemetz, A.; Beatty, S.; Richer, E.; Rubio, A.; Auriac, A.; Milkereit, R.J.; Thibaudeau, O.; Vaulont, S.; Malo, D.; Canonne-Hergaux, F. Iron-and hepcidin-independent downregulation of the iron exporter ferroportin in macrophages during Salmonella infection. Front. Immunol. 2017, 8, 498. [Google Scholar] [CrossRef]
- Hill, R.; Smith, I.; Mohammadi, H.; Licence, S. Altered absorption and regulation of iron in chicks with acute Salmonella gallinarum infection. Res. Vet. Sci. 1977, 22, 371–375. [Google Scholar] [CrossRef]
- Ganz, T. Anemia of inflammation. N. Engl. J. Med. 2019, 381, 1148–1157. [Google Scholar] [CrossRef]
- Canals, R.; Hammarlöf, D.L.; Kröger, C.; Owen, S.V.; Fong, W.Y.; Lacharme-Lora, L.; Zhu, X.; Wenner, N.; Carden, S.E.; Honeycutt, J. Adding function to the genome of African Salmonella Typhimurium ST313 strain D23580. PLoS Biol. 2019, 17, e3000059. [Google Scholar] [CrossRef] [Green Version]
- Lokken, K.L.; Stull-Lane, A.R.; Poels, K.; Tsolis, R.M. Malaria parasite-mediated alteration of macrophage function and increased iron availability predispose to disseminated nontyphoidal Salmonella infection. Infect. Immun. 2018, 86, e00301-18. [Google Scholar] [CrossRef] [Green Version]
- Allan, B.; Wheler, C.; Köster, W.; Sarfraz, M.; Potter, A.; Gerdts, V.; Dar, A. In Ovo Administration of Innate Immune Stimulants and Protection from Early Chick Mortalities due to Yolk Sac Infection. Avian Dis. 2018, 62, 316–321. [Google Scholar] [CrossRef]
- Johnstone, T.C.; Nolan, E.M. Beyond iron: Non-classical biological functions of bacterial siderophores. Dalton Trans. 2015, 44, 6320–6339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adler, C.; Corbalán, N.S.; Seyedsayamdost, M.R.; Pomares, M.F.; de Cristóbal, R.E.; Clardy, J.; Kolter, R.; Vincent, P.A. Catecholate siderophores protect bacteria from pyochelin toxicity. PLoS ONE 2012, 7, e46754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adler, C.; Corbalan, N.S.; Peralta, D.R.; Pomares, M.F.; de Cristóbal, R.E.; Vincent, P.A. The alternative role of enterobactin as an oxidative stress protector allows Escherichia coli colony development. PLoS ONE 2014, 9, e84734. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; San Yeoh, B.; Xiao, X.; Kumar, M.; Bachman, M.; Borregaard, N.; Joe, B.; Vijay-Kumar, M. Interplay between enterobactin, myeloperoxidase and lipocalin 2 regulates E. coli survival in the inflamed gut. Nat. Commun. 2015, 6, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peralta, D.R.; Adler, C.; Corbalán, N.S.; Paz García, E.C.; Pomares, M.F.; Vincent, P.A. Enterobactin as part of the oxidative stress response repertoire. PLoS ONE 2016, 11, e0157799. [Google Scholar] [CrossRef] [Green Version]
- Vazquez-Torres, A.; Jones-Carson, J.; Mastroeni, P.; Ischiropoulos, H.; Fang, F.C. Antimicrobial actions of the NADPH phagocyte oxidase and inducible nitric oxide synthase in experimental salmonellosis. I. Effects on microbial killing by activated peritoneal macrophages in vitro. J. Exp. Med. 2000, 192, 227–236. [Google Scholar] [CrossRef] [Green Version]
- Stabler, J.; McCormick, T.; Powell, K.; Kogut, M. Avian heterophils and monocytes: Phagocytic and bactericidal activities against Salmonella enteritidis. Vet. Microbiol. 1994, 38, 293–305. [Google Scholar] [CrossRef]
- Brooks, R.; Bounous, D.; Andreasen, C. Functional comparison of avian heterophils with human and canine neutrophils. Comp. Haematol. Int. 1996, 6, 153–159. [Google Scholar] [CrossRef]
- Wigley, P.; Hulme, S.D.; Bumstead, N.; Barrow, P.A. In vivo and in vitro studies of genetic resistance to systemic salmonellosis in the chicken encoded by the SAL1 locus. Microbes Infect. 2002, 4, 1111–1120. [Google Scholar] [CrossRef]
- Holden, V.I.; Breen, P.; Houle, S.; Dozois, C.M.; Bachman, M.A. Klebsiella pneumoniae siderophores induce inflammation, bacterial dissemination, and HIF-1α stabilization during pneumonia. MBio 2016, 7, e01397-16. [Google Scholar] [CrossRef] [Green Version]
- Holden, V.I.; Lenio, S.; Kuick, R.; Ramakrishnan, S.K.; Shah, Y.M.; Bachman, M.A. Bacterial siderophores that evade or overwhelm lipocalin 2 induce hypoxia inducible factor 1α and proinflammatory cytokine secretion in cultured respiratory epithelial cells. Infect. Immun. 2014, 82, 3826–3836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saha, P.; Xiao, X.; Yeoh, B.S.; Chen, Q.; Katkere, B.; Kirimanjeswara, G.S.; Vijay-Kumar, M. The bacterial siderophore enterobactin confers survival advantage to Salmonella in macrophages. Gut Microbes 2019, 10, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Lathrop, S.K.; Binder, K.A.; Starr, T.; Cooper, K.G.; Chong, A.; Carmody, A.B.; Steele-Mortimer, O. Replication of Salmonella enterica serovar Typhimurium in human monocyte-derived macrophages. Infect. Immun. 2015, 83, 2661–2671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benoit, M.; Desnues, B.; Mege, J.-L. Macrophage polarization in bacterial infections. J. Immunol. 2008, 181, 3733–3739. [Google Scholar] [CrossRef] [Green Version]
- Galván-Peña, S.; O’Neill, L.A. Metabolic reprograming in macrophage polarization. Front. Immunol. 2014, 5, 420. [Google Scholar]

| Serotype | Source | Year(s) | Geographical Region | No of Cases a | References |
|---|---|---|---|---|---|
| Enteritidis | Chicken (shell eggs) | 2010 | USA | 1939 b | CDC 2020 d |
| Enteritidis Virchow | Chicken | 2010 (from 2007) | Brazil | >260 | [11] |
| Enteritidis Virchow | Chicken | 2010 | Taiwan | >1000 | [12] |
| Stanley | Turkey (meat) | 2011–2013 | EU | 710 | [13] |
| Heidelberg | Chicken (meat) | 2011 | USA | 190 | CDC 2020 d |
| Infantis Newport Lille | Chicks, ducklings (live) | 2012 | USA | 195 | CDC 2020 d |
| Heidelberg | Chicken (meat) | 2013 | USA | 634 | CDC 2020 d |
| Typhimurium | Chicks, ducklings (live) | 2013 | USA | 356 | CDC 2020 d |
| Enteritidis | Chicken (eggs) | 2014 | EU | >400 | [14] |
| Multiple NTS c | Chicks, ducklings (live) | 2014 | USA | 363 | CDC 2020 d |
| Multiple NTS | Chicks, ducklings (live) | 2015 | USA | 252 | CDC 2020 d |
| Multiple NTS | Chicks, ducklings (live) | 2016 | USA | 895 | CDC 2020 d |
| Typhimurium | Chicken (egg) | 2015–2016 | Australia | 272 | [15] |
| Enteritidis | Chicken (eggs) | 2016–present | EU | 1656 | ECDC 2020 e |
| Multiple NTS | Chicks, ducklings (live) | 2017 | USA | 1120 | CDC 2020 d |
| Typhimurium | Chicken (salad) | 2018 | USA | 265 | CDC 2020 d |
| Reading | Turkey | 2018 | USA | 358 | CDC 2020 d |
| Enteritidis | Chicken (processed meat) | 2017–2019 | Canada | 584 | Public Health Service 2020 f |
| Enteritidis | Chicks, ducklings (live) | 2019 | USA | 1134 | CDC 2020 d |
| Salmonella Serovar | Genetic/Phenotypic Signatures | Role Related to Virulence in Chicken or Human | References |
|---|---|---|---|
| Kentucky (SKn) | (1) Colicin production (pColV) (2) Salmonella genomic island 1 (SGI1) (3) RpoS regulated gene cluster: csg (curli), prpBCDE (propionate catabolism) (4) Lack of Saf and Sef fimbria (5) Additional iron uptake carried in pColV; siderophores- aerobactin & salmochelin, sit operon (Mn2+, Fe2+ uptake) | (1) Increased colonization in chicken gut (2) Multidrug resistant (MDR) including 3rd generation cephalosporin, ciprofloxacin resistant *, (3) Upregulated in chicken cecal explants (4) Decreased invasiveness in humans compared to other NTS (5) NDA (no data available) | [54,55,56] |
| Heidelberg (SHb) | (6) Type IV secretion (T4SS) (7) SopE (T3SS1 effector) duplication in the chromosome (8) Salmonella atypical fimbria (safABCD) (9) Additional iron uptake carried in pColV; siderophore-aerobactin | (6) Dissemination of antibiotics resistance and efficient survival in macrophages (7) Invasion into epithelial cells and induce inflammation (8) Only presented in the outbreak strain linked to human salmonellosis. (9) NDA | [55,57,58] |
| Typhimurium (STm) | (10) Salmonella genomic island 1 (SGI1) (11) Salmonella genomic island 4 (SGI4) (12) Plasmid encoded factors; mig-5, rck, spv (Salmonella plasmid virulence), pef , (13) Additional iron uptake in pColV; aerobactin, salmochelin sit operon (Mn2+, Fe2+) | (10) Sequence type DT104 showed Increased egg contamination compared to SEn phage type 4, contains ACSSuT # drug-resistant phenotype (11) Heavy metal resistant in DT104 (12) colonization in chicken gut, systemic spread (13) NDA | [59,60,61,62] |
| Typhimurium mono phasic variant (STmv) (DT193/DT120) | (14) Phase 2 flagellin not expressed (fljBA operon) (15) SGI-4 (16) Lack of Salmonella plasmid virulence locus, (17) Lack of Gifsy prophages | (14) Predicted to be an adaptation related to the expansion of reservoir host (15) resistant to heavy metals copper and zinc (16) less invasive in humans (17) NDA | [59,63,64] |
| Enteritidis (SEn) | (18) pSLA5 plasmid (19) Plasmid encoded factors; mig-5, rck, spv (Salmonella plasmid virulence), pef (20) Peg fimbria | (18) Associated with recent outbreaks in the EU. (19) Colonization in chicken gut, systemic spread (20) Generally unique to Enteritidis. Facilitates cecal colonization in chickens | [65,66,67,68] |
| Virchow (SVr) | (21) Salmonella Typhi colonization factor: TcfA (22) Novel SopE effector | (21) TcfA fimbriae provides tissue tropism in invasion into human cells. Role in chickens unknown (22) Associated with invasive nature of SVQ1 strain linked to outbreaks in Australia | [53,69] |
| Montevideo (SMv) | (23) Typhoid-associated virulence factors; TcfA fimbria, cytolethal toxin B etc. | (23) Predicted to increase tissue tropism and invasions in humans. Role in chickens unknown | [53,70] |
| Infantis (SIn) | (24) Plasmid-encoded factors;(pESI like);
| (24) Associated in human outbreaks. Also, plasmid-encoded fimbria were contributed a colonization in the gastrointestinal tract of chicks | [71,72,73,74] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wellawa, D.H.; Allan, B.; White, A.P.; Köster, W. Iron-Uptake Systems of Chicken-Associated Salmonella Serovars and Their Role in Colonizing the Avian Host. Microorganisms 2020, 8, 1203. https://doi.org/10.3390/microorganisms8081203
Wellawa DH, Allan B, White AP, Köster W. Iron-Uptake Systems of Chicken-Associated Salmonella Serovars and Their Role in Colonizing the Avian Host. Microorganisms. 2020; 8(8):1203. https://doi.org/10.3390/microorganisms8081203
Chicago/Turabian StyleWellawa, Dinesh H., Brenda Allan, Aaron P. White, and Wolfgang Köster. 2020. "Iron-Uptake Systems of Chicken-Associated Salmonella Serovars and Their Role in Colonizing the Avian Host" Microorganisms 8, no. 8: 1203. https://doi.org/10.3390/microorganisms8081203
APA StyleWellawa, D. H., Allan, B., White, A. P., & Köster, W. (2020). Iron-Uptake Systems of Chicken-Associated Salmonella Serovars and Their Role in Colonizing the Avian Host. Microorganisms, 8(8), 1203. https://doi.org/10.3390/microorganisms8081203







