Lactic Acid Bacteria Adjunct Cultures Exert a Mitigation Effect against Spoilage Microbiota in Fresh Cheese
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Culture Conditions
2.2. Sampling and Microbiological Analyses
2.3. DNA Fingerprinting and Taxonomical Identification
2.4. Inhibition Assays
2.5. Industrial Trial
2.6. DNA Extraction
2.7. 16S rRNA Amplicon Library Construction, Sequencing, and Bioinformatics
Nucleotide Sequence Accession Numbers
3. Results
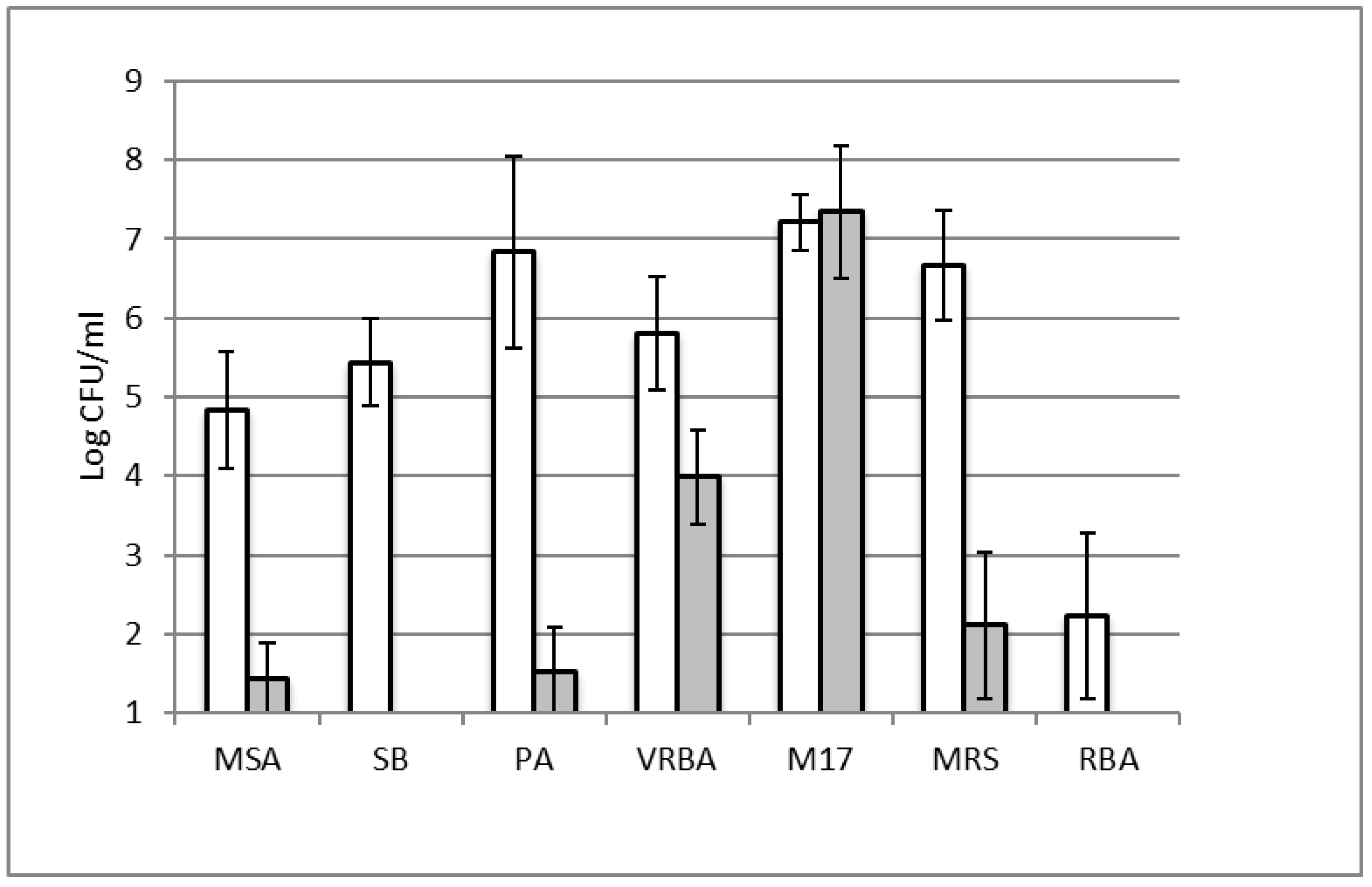
3.1. Characterization of Fresh Cheese Spoilage Microbiota by Culture-Dependent Approach
3.2. In Vitro Selection of Adjunct Cultures
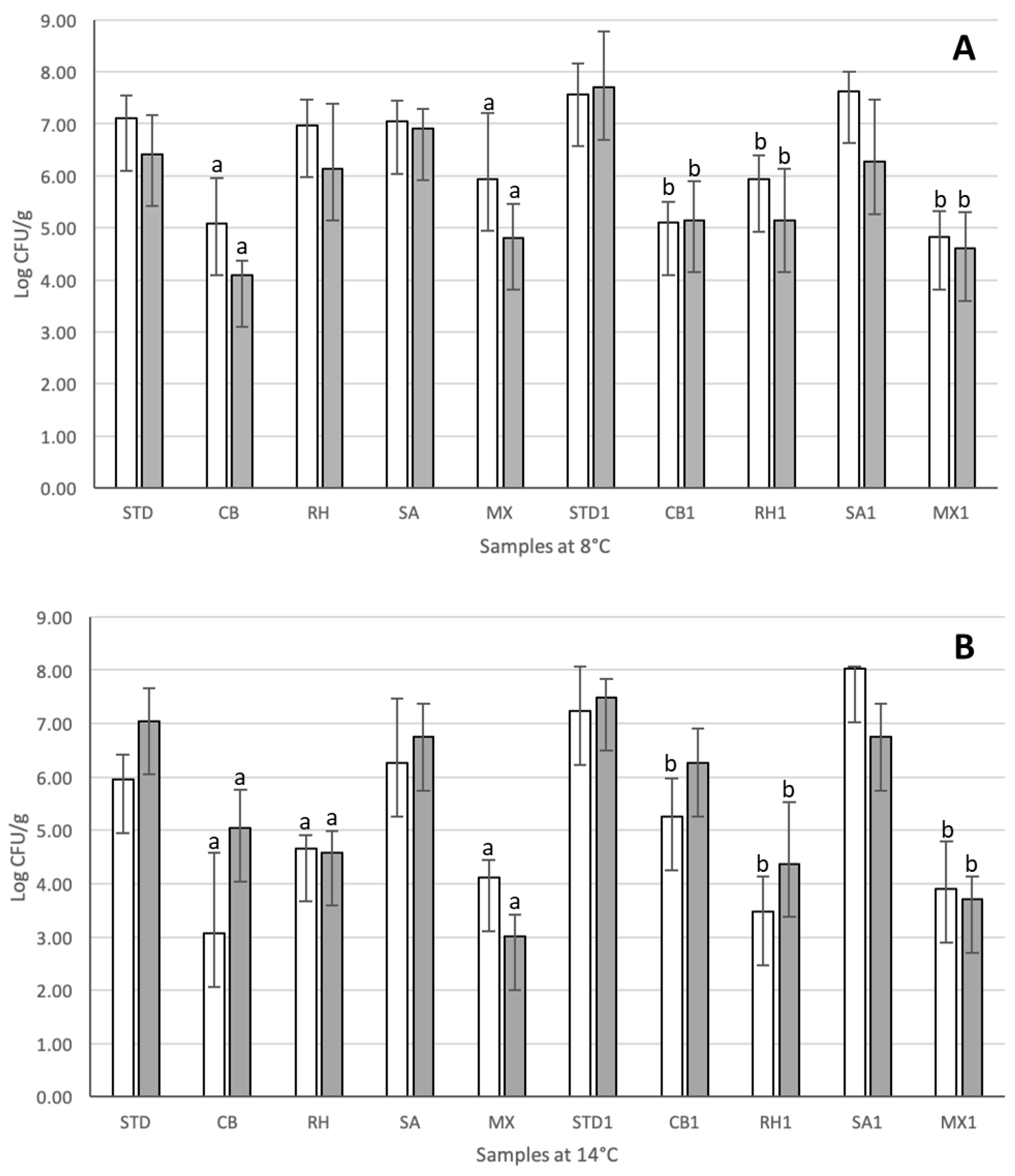
3.3. Efficacy Assessment of Adjunct Cultures in Industrial Conditions
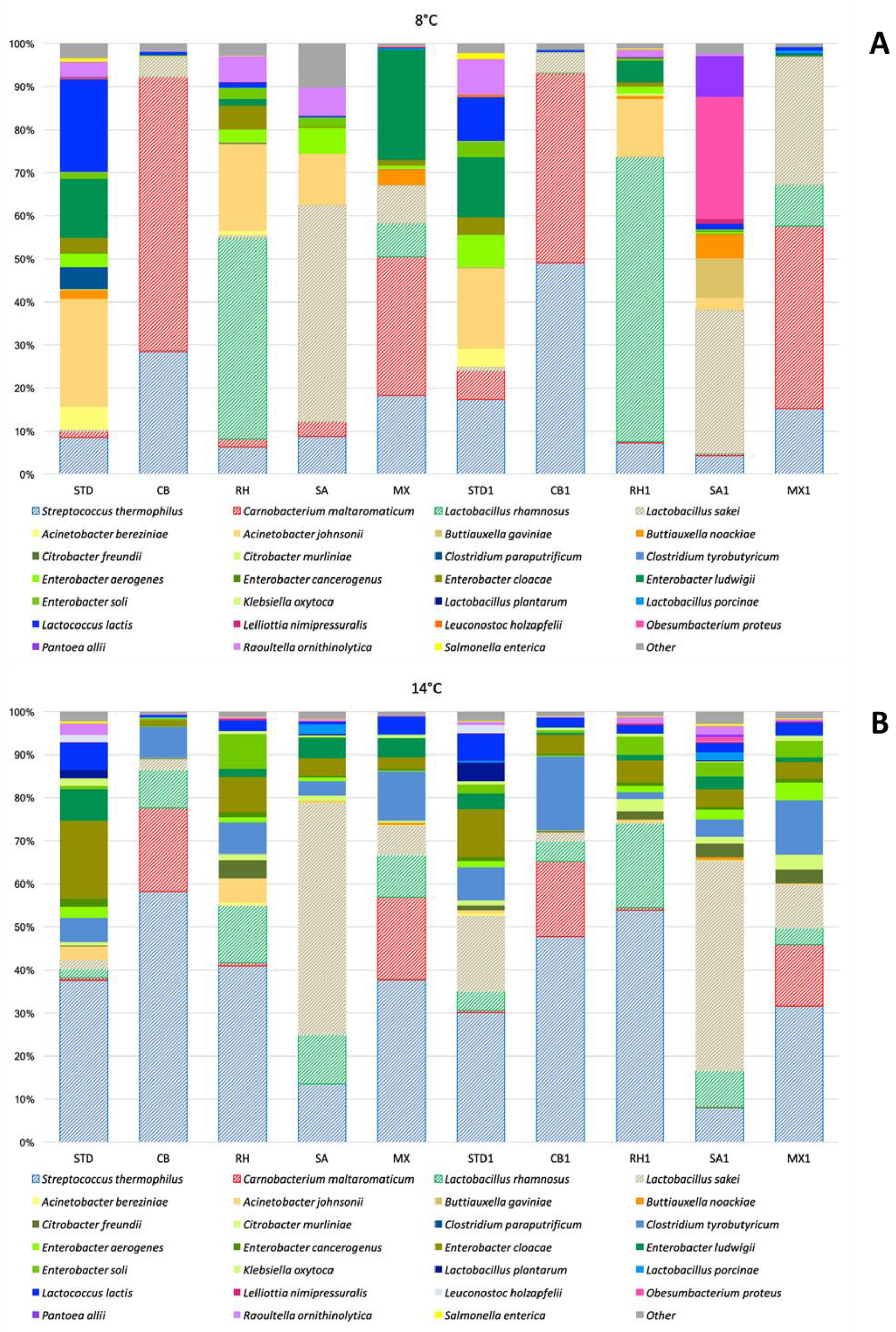
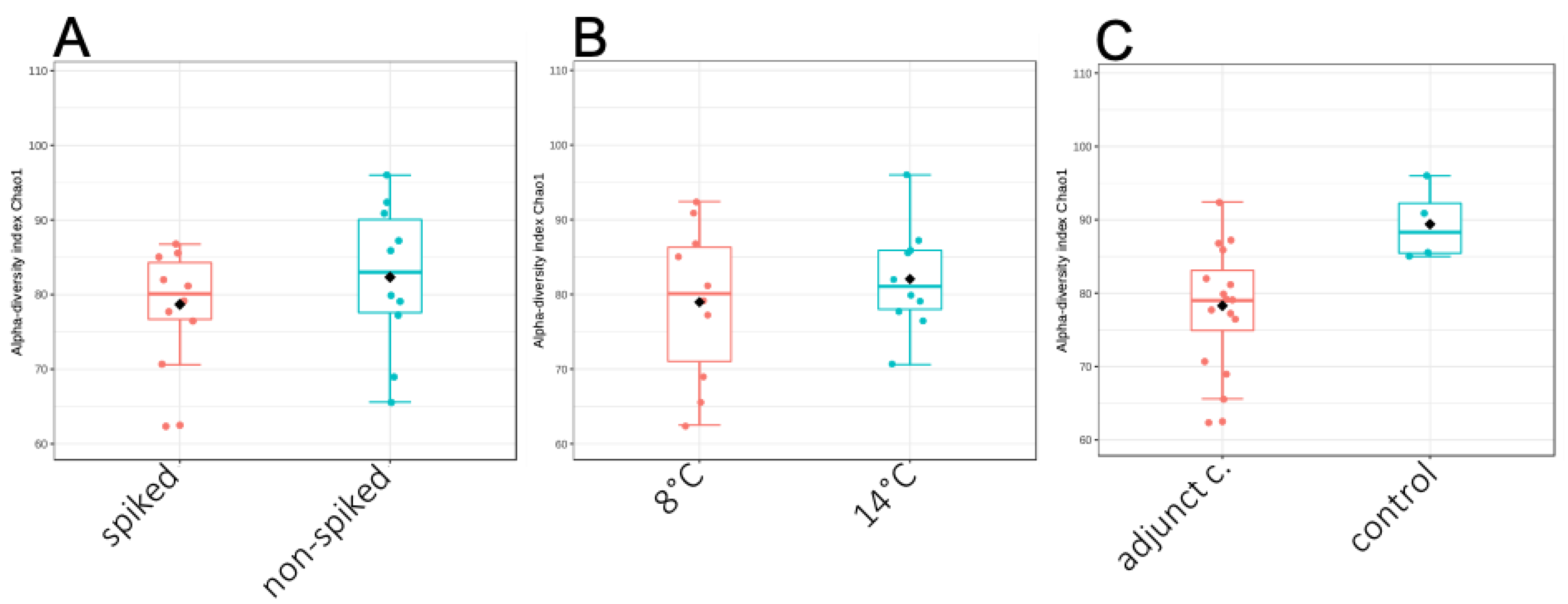
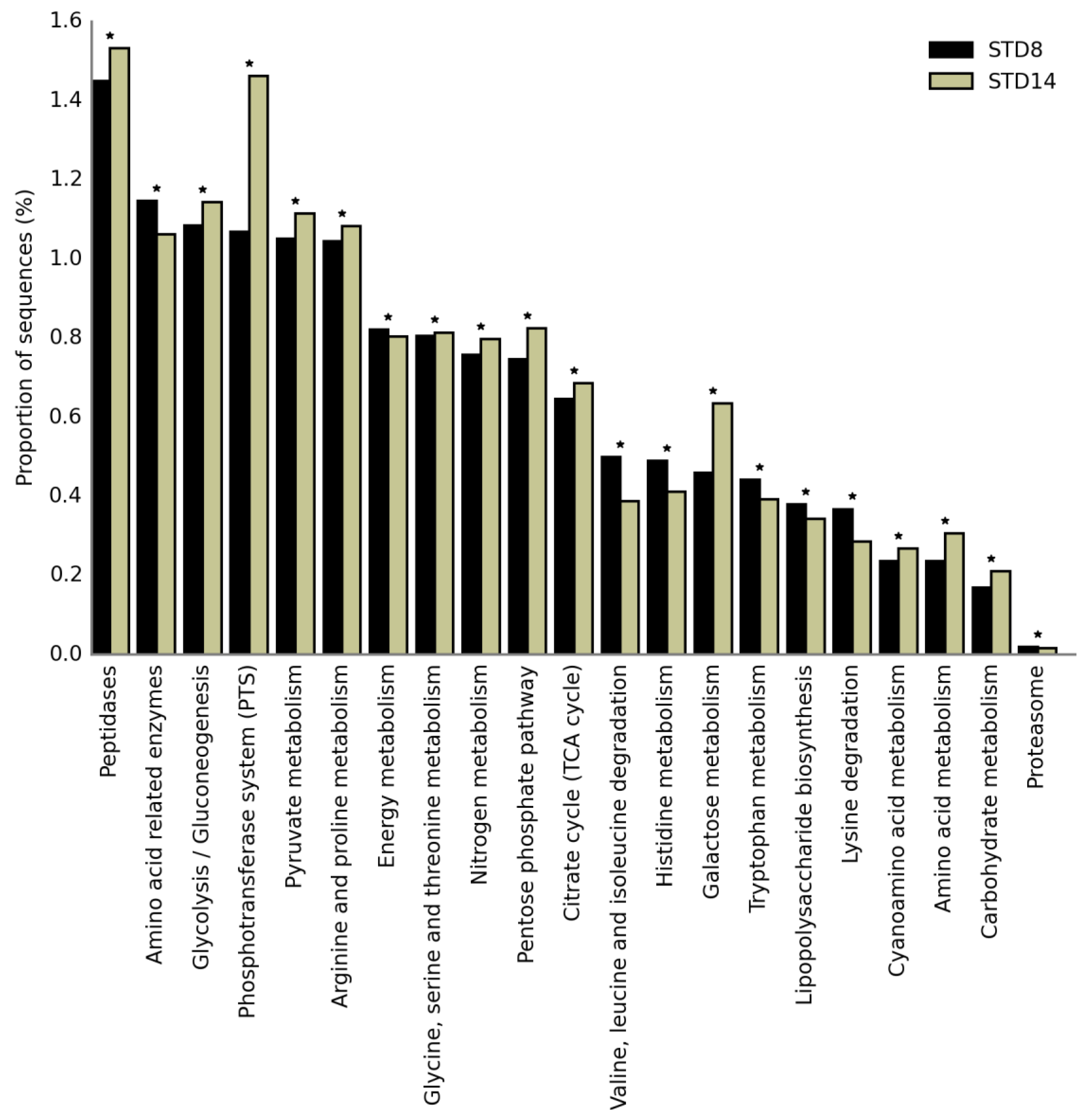
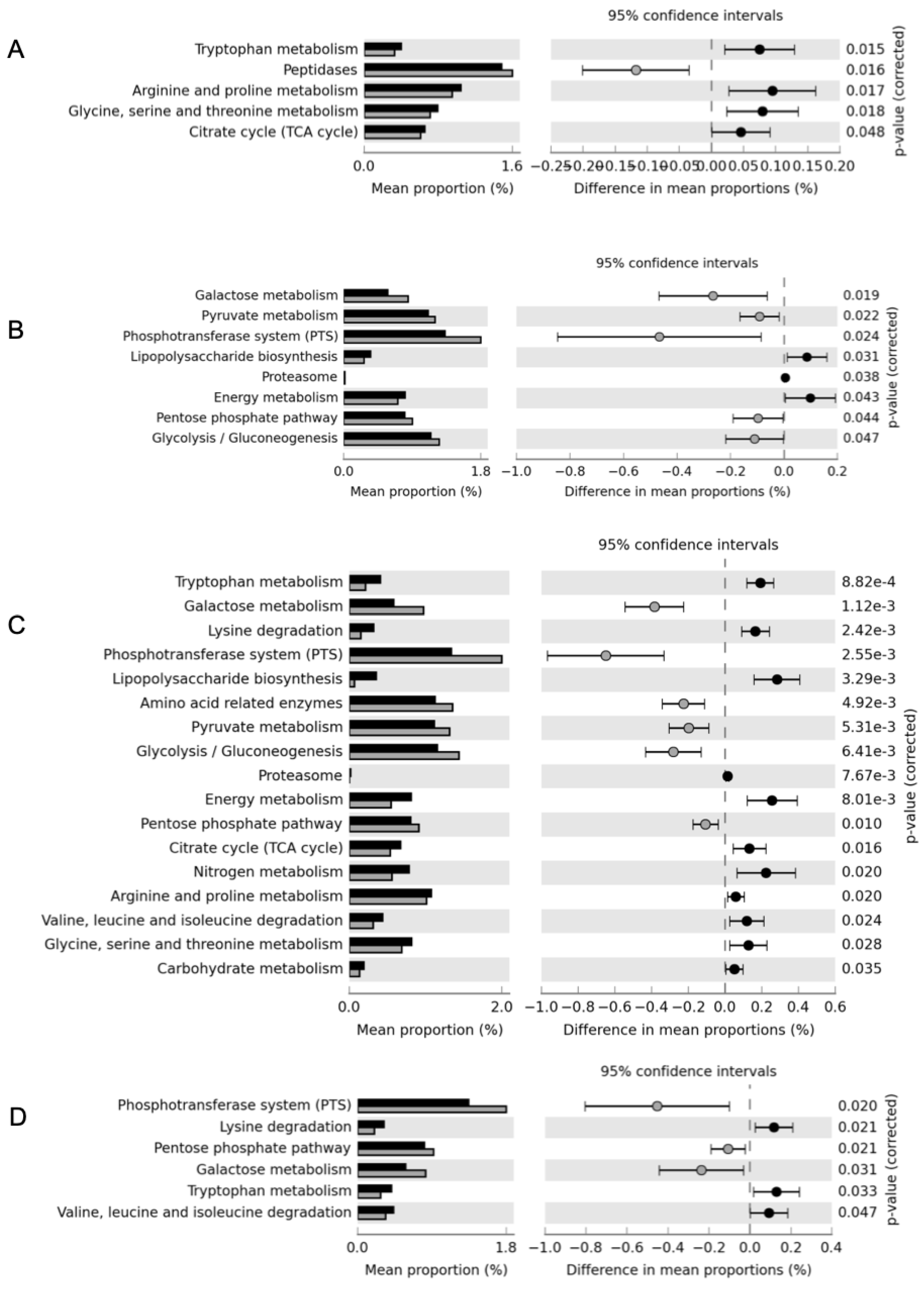
3.4. Effect of Adjunct Cultures on the Functional Profiling of Cheese Microbiome
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gustavsson, J.; Cederberg, C.; Sonesson, U.; van Otterdijk, R.; Meybeck, A. Global Food Losses and Food Waste—Extent, Causes and Prevention; Food and Agriculture Oganization of the United Nations: Rome, Italy, 2011. [Google Scholar]
- Raak, N.; Symmank, C.; Zahn, S.; Aschemann-Witzel, J.; Rohm, H. Processing- and product-related causes for food waste and implications for the food supply chain. Waste Manag. 2017, 61, 461–472. [Google Scholar] [CrossRef]
- Bassi, D.; Puglisi, E.; Cocconcelli, P.S. Understanding the bacterial communities of hard cheese with blowing defect. Food Microbiol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Ávila, M.; Gómez-Torres, N.; Delgado, D.; Gaya, P.; Garde, S. Industrial-scale application of Lactobacillus reuteri coupled with glycerol as a biopreservation system for inhibiting Clostridium tyrobutyricum in semi-hard ewe milk cheese. Food Microbiol. 2017, 66, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Ledenbach, L.; Marshall, R. Microbiological Spoilage of Dairy Products. In Compendium of the Microbiological Spoilage of Foods and Beverages; Springer: Cham, Switzerland, 2009; pp. 41–67. [Google Scholar]
- Martin, N.H.; Murphy, S.C.; Ralyea, R.D.; Wiedmann, M.; Boor, K.J. When cheese gets the blues: Pseudomonas fluorescens as the causative agent of cheese spoilage. J. Dairy Sci. 2011, 94, 3176–3183. [Google Scholar] [CrossRef] [PubMed]
- Fanelli, F.; Liuzzi, V.C.; Quintieri, L.; Mulè, G.; Baruzzi, F.; Logrieco, A.F.; Caputo, L. Draft Genome Sequence of Pseudomonas fluorescens Strain ITEM 17298, Associated with Cheese Spoilage. Genome Announc. 2017, 5, e01141-17. [Google Scholar] [CrossRef] [PubMed]
- Machado, S.G.; Baglinière, F.; Marchand, S.; Van Coillie, E.; Vanetti, M.C.D.; De Block, J.; Heyndrickx, M. The Biodiversity of the Microbiota Producing Heat-Resistant Enzymes Responsible for Spoilage in Processed Bovine Milk and Dairy Products. Front. Microbiol. 2017, 8, 302. [Google Scholar] [CrossRef] [PubMed]
- Stellato, G.; De Filippis, F.; La Storia, A.; Ercolini, D. Coexistence of lactic acid bacteria and potential spoilage microbiota in a dairy processing environment. Appl. Environ. Microbiol. 2015, 81, 7893–7904. [Google Scholar] [CrossRef]
- Stellato, G.; Utter, D.R.; Voorhis, A.; De Angelis, M.; Eren, A.M.; Ercolini, D. A Few Pseudomonas Oligotypes Dominate in the Meat and Dairy Processing Environment. Front. Microbiol. 2017, 8, 264. [Google Scholar] [CrossRef]
- Dugat-Bony, E.; Sarthou, A.-S.; Perello, M.-C.; de Revel, G.; Bonnarme, P.; Helinck, S. The effect of reduced sodium chloride content on the microbiological and biochemical properties of a soft surface-ripened cheese. J. Dairy Sci. 2016, 99, 2502–2511. [Google Scholar] [CrossRef]
- Reis, J.A.; Paula, A.T.; Casarotti, S.N.; Penna, A.L.B. Lactic Acid Bacteria Antimicrobial Compounds: Characteristics and Applications. Food Eng. Rev. 2012, 4, 124–140. [Google Scholar] [CrossRef]
- Alvarez-Sieiro, P.; Montalbán-López, M.; Mu, D.; Kuipers, O.P. Bacteriocins of lactic acid bacteria: Extending the family. Appl. Microbiol. Biotechnol. 2016, 100, 2939–2951. [Google Scholar] [CrossRef] [PubMed]
- Al-Gamal, M.S.; Ibrahim, G.A.; Sharaf, O.M.; Radwan, A.A.; Dabiza, N.M.; Youssef, A.M.; El-Ssayad, M.F. The protective potential of selected lactic acid bacteria against the most common contaminants in various types of cheese in Egypt. Heliyon 2019, 5, e01362. [Google Scholar] [CrossRef] [PubMed]
- Coelho, M.C.; Silva, C.C.G.; Ribeiro, S.C.; Dapkevicius, M.L.N.E.; Rosa, H.J.D. Control of Listeria monocytogenes in fresh cheese using protective lactic acid bacteria. Int. J. Food Microbiol. 2014, 191, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Mills, S.; Griffin, C.; O’Connor, P.M.; Serrano, L.M.; Meijer, W.C.; Hill, C.; Ross, R.P. A multibacteriocin cheese starter system, comprising nisin and lacticin 3147 in Lactococcus lactis, in combination with plantaricin from Lactobacillus plantarum. Appl. Environ. Microbiol. 2017, 83, e00799-17. [Google Scholar] [CrossRef] [PubMed]
- Callon, C.; Arliguie, C.; Montel, M.-C. Control of Shigatoxin-producing Escherichia coli in cheese by dairy bacterial strains. Food Microbiol. 2016, 53, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, I.d.S.; de Souza, J.V.; Ramos, C.L.; da Costa, M.M.; Schwan, R.F.; Dias, F.S. Selection of autochthonous lactic acid bacteria from goat dairies and their addition to evaluate the inhibition of Salmonella typhi in artisanal cheese. Food Microbiol. 2016, 60, 29–38. [Google Scholar] [CrossRef]
- Silva, C.C.G.; Silva, S.P.M.; Ribeiro, S.C. Application of Bacteriocins and Protective Cultures in Dairy Food Preservation. Front. Microbiol. 2018, 9, 594. [Google Scholar] [CrossRef]
- Fernandez, B.; Vimont, A.; Desfossés-Foucault, É.; Daga, M.; Arora, G.; Fliss, I. Antifungal activity of lactic and propionic acid bacteria and their potential as protective culture in cottage cheese. Food Control. 2017, 78, 350–356. [Google Scholar] [CrossRef]
- Cosentino, S.; Viale, S.; Deplano, M.; Fadda, M.E.; Pisano, M.B. Application of Autochthonous Lactobacillus Strains as Biopreservatives to Control Fungal Spoilage in Caciotta Cheese. Biomed. Res. Int. 2018, 2018, 3915615. [Google Scholar] [CrossRef]
- Cocconcelli, P.S.; Porro, D.; Galandini, S.; Senini, L. Development of RAPD protocol for typing of strains of lactic acid bacteria and enterococci. Lett. Appl. Microbiol. 1995, 21, 376–379. [Google Scholar] [CrossRef]
- Woods, C.R.; Versalovic, J.; Koeuth, T.; Lupski, J.R. Whole-cell repetitive element sequence-based polymerase chain reaction allows rapid assessment of clonal relationships of bacterial isolates. J. Clin. Microbiol. 1993, 31, 1927–1931. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [PubMed]
- Maidak, B.L.; Cole, J.R.; Lilburn, T.G.; Parker, C.T., Jr.; Saxman, P.R.; Farris, R.J.; Garrity, G.M.; Olsen, G.J.; Schmidt, T.M.; Tiedje, J.M. The RDP-II (Ribosomal Database Project). Nucleic Acids Res. 2001, 29, 173–174. [Google Scholar] [CrossRef] [PubMed]
- Nadkarni, M.A.; Martin, F.E.; Jacques, N.A.; Hunter, N. Determination of bacterial load by real-time PCR using a broad-range (universal) probe and primers set. Microbiology 2002, 148, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Subramanian, S.; Faith, J.J.; Gevers, D.; Gordon, J.I.; Knight, R.; Mills, D.A.; Caporaso, J.G. Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat. Methods 2013, 10, 57–59. [Google Scholar] [CrossRef]
- Dhariwal, A.; Chong, J.; Habib, S.; King, I.L.; Agellon, L.B.; Xia, J. MicrobiomeAnalyst: A web-based tool for comprehensive statistical, visual and meta-analysis of microbiome data. Nucleic Acids Res. 2017, 45, W180–W188. [Google Scholar] [CrossRef]
- Parks, D.H.; Beiko, R.G. Identifying biologically relevant differences between metagenomic communities. Bioinformatics 2010, 26, 715–721. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Request for updating the former SCVPH opinion on Listeria monocytogenes risk related to ready-to-eat foods and scientific advice on different levels of Listeria monocytogenes in ready-to-eat foods and the related risk for human illness-Scientific Opinion of the Panel on Biological Hazards. EFSA J. 2008, 6, 599. [Google Scholar] [CrossRef]
- Spanu, C.; Scarano, C.; Piras, F.; Spanu, V.; Pala, C.; Casti, D.; Lamon, S.; Cossu, F.; Ibba, M.; Nieddu, G.; et al. Testing commercial biopreservative against spoilage microorganisms in MAP packed Ricotta fresca cheese. Food Microbiol. 2017, 66, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.R.B.; Forauer, E.C.; D’Amico, D.J. Effect of modified atmosphere packaging on the growth of spoilage microorganisms and Listeria monocytogenes on fresh cheese. J. Dairy Sci. 2018, 101, 7768–7779. [Google Scholar] [CrossRef] [PubMed]
- Quintieri, L.; Fanelli, F.; Caputo, L. Antibiotic Resistant Pseudomonas Spp. Spoilers in Fresh Dairy Products: An Underestimated Risk and the Control Strategies. Foods 2019, 8, 372. [Google Scholar] [CrossRef] [PubMed]
- Koivula, T.T.; Juvonen, R.; Haikara, A.; Suihko, M.-L. Characterization of the brewery spoilage bacterium Obesumbacterium proteus by automated ribotyping and development of PCR methods for its biotype 1. J. Appl. Microbiol. 2006, 100, 398–406. [Google Scholar] [CrossRef]
- Abriouel, H.; Martín-Platero, A.; Maqueda, M.; Valdivia, E.; Martínez-Bueno, M. Biodiversity of the microbial community in a Spanish farmhouse cheese as revealed by culture-dependent and culture-independent methods. Int. J. Food Microbiol. 2008, 127, 200–208. [Google Scholar] [CrossRef]
- Pham, N.-P.; Landaud, S.; Lieben, P.; Bonnarme, P.; Monnet, C. Transcription Profiling Reveals Cooperative Metabolic Interactions in a Microbial Cheese-Ripening Community Composed of Debaryomyces hansenii, Brevibacterium aurantiacum, and Hafnia alvei. Front. Microbiol. 2019, 10, 1901. [Google Scholar] [CrossRef]
- Gammariello, D.; Conte, A.; Di Giulio, S.; Attanasio, M.; Del Nobile, M.A. Shelf life of Stracciatella cheese under modified-atmosphere packaging. J. Dairy Sci. 2009, 92, 483–490. [Google Scholar] [CrossRef]
- Wang, G.; Li, M.; Ma, F.; Wang, H.; Xu, X.; Zhou, G. Physicochemical properties of Pseudomonas fragi isolates response to modified atmosphere packaging. FEMS Microbiol. Lett. 2017, 364. [Google Scholar] [CrossRef][Green Version]
- Duan, C.; Li, S.; Zhao, Z.; Wang, C.; Zhao, Y.; Yang, G.E.; Niu, C.; Gao, L.; Liu, X.; Zhao, L. Proteolytic Activity of Lactobacillus plantarum Strains in Cheddar Cheese as Adjunct Cultures. J. Food Prot. 2019, 82, 2108–2118. [Google Scholar] [CrossRef]
- Spanu, C.; Piras, F.; Mocci, A.M.; Nieddu, G.; De Santis, E.P.L.; Scarano, C. Use of Carnobacterium spp. protective culture in MAP packed Ricotta fresca cheese to control Pseudomonas spp. Food Microbiol. 2018, 74, 50–56. [Google Scholar] [CrossRef]
- Solomakos, N.; Govari, M.; Botsoglou, E.; Pexara, A. Effect of modified atmosphere packaging on physicochemical and microbiological characteristics of Graviera Agraphon cheese during refrigerated storage. J. Dairy Res. 2019, 86, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Leisner, J.J.; Laursen, B.G.; Prévost, H.; Drider, D.; Dalgaard, P. Carnobacterium: Positive and negative effects in the environment and in foods. FEMS Microbiol. Rev. 2007, 31, 592–613. [Google Scholar] [CrossRef] [PubMed]
- Pilchová, T.; Pilet, M.-F.; Cappelier, J.-M.; Pazlarová, J.; Tresse, O. Protective Effect of Carnobacterium spp. against Listeria monocytogenes during Host Cell Invasion Using in vitro HT29 Model. Front. Cell. Infect. Microbiol. 2016, 6, 88. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Afzal, M.I.; Gonzalez Ariceaga, C.C.; Lhomme, E.; Ali, N.K.; Payot, S.; Burgain, J.; Gaiani, C.; Borges, F.; Revol-Junelles, A.-M.; Delaunay, S.; et al. Characterization of Carnobacterium maltaromaticum LMA 28 for its positive technological role in soft cheese making. Food Microbiol. 2013, 36, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Voget, S.; Klippel, B.; Daniel, R.; Antranikian, G. Complete genome sequence of Carnobacterium sp. 17-4. J. Bacteriol. 2011, 193, 3403–3404. [Google Scholar] [CrossRef]
- Zhang, P.; Gänzle, M.; Yang, X. Complementary antibacterial effects of bacteriocins and organic acids as revealed by comparative analysis of Carnobacterium spp. from meat. Appl. Environ. Microbiol. 2019, 85, e01227-19. [Google Scholar] [CrossRef]
- Minervini, F.; Conte, A.; Alessandro, M.; Nobile, D.; Gobbetti, M.; De Angelis, M. Dietary Fibers and Protective Lactobacilli Drive Burrata Cheese Microbiome. Appl. Environ. Microbiol. 2017, 83, e01494-17. [Google Scholar] [CrossRef]
- Rolim, F.R.L.; dos Santos, K.M.O.; de Barcelos, S.C.; do Egito, A.S.; Ribeiro, T.S.; da Conceição, M.L.; Magnani, M.; de Oliveira, M.E.G.; Queiroga, R.d.C.R.d.E. Survival of Lactobacillus rhamnosus EM1107 in simulated gastrointestinal conditions and its inhibitory effect against pathogenic bacteria in semi-hard goat cheese. LWT Food Sci. Technol. 2015, 63, 807–813. [Google Scholar] [CrossRef]
- Aljewicz, M.; Cichosz, G. Protective effects of Lactobacillus cultures in Dutch-type cheese-like products. LWT Food Sci. Technol. 2015, 63. [Google Scholar] [CrossRef]
- Beristain-Bauza, S.C.; Mani-López, E.; Palou, E.; López-Malo, A. Antimicrobial activity and physical properties of protein films added with cell-free supernatant of Lactobacillus rhamnosus. Food Control. 2016, 62, 44–51. [Google Scholar] [CrossRef]
- Zagorec, M.; Champomier-Vergès, M.-C. Lactobacillus sakei: A Starter for Sausage Fermentation, a Protective Culture for Meat Products. Microorganisms 2017, 5, 56. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhu, L.; Dong, P.; Liang, R.; Mao, Y.; Qiu, S.; Luo, X. Bio-protective potential of lactic acid bacteria: Effect of Lactobacillus sakei and Lactobacillus curvatus on changes of the microbial community in vacuum-packaged chilled beef. Asian-Australas. J. Anim. Sci. 2017, 31, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Lawton, M.R.; Jencarelli, K.G.; Kozak, S.M.; Alcaine, S.D. Short communication: Evaluation of commercial meat cultures to inhibit Listeria monocytogenes in a fresh cheese laboratory model. J. Dairy Sci. 2020, 103, 1269–1275. [Google Scholar] [CrossRef] [PubMed]
- Gensler, C.A.; Brown, S.R.B.; Aljasir, S.F.; D’Amico, D. Compatibility of commercially produced protective cultures with common cheesemaking cultures and their antagonistic effect on foodborne pathogens. J. Food Prot. 2020, 83, 1010–1019. [Google Scholar] [CrossRef] [PubMed]
- Fanning, S.; Proos, S.; Jordan, K.; Srikumar, S. A review on the applications of next generation sequencing technologies as applied to food-related microbiome studies. Front. Microbiol. 2017, 8, 1–16. [Google Scholar] [CrossRef]
| Strain | Use |
|---|---|
| Streptococcus thermophilus ST022 | Starter culture |
| Lacticaseibacillus rhamnosus UC8490 | Adjunct culture |
| Lactiplantibacillus plantarum UC8491 | Adjunct culture |
| Latilactobacillus curvatus UC8266 | Adjunct culture |
| Latilactobacillus curvatus UC8265 | Adjunct culture |
| Lacticaseibacillus casei UC8561 | Adjunct culture |
| Ligilactobacillus acidipiscis UC8115 | Adjunct culture |
| Lactiplantibacillus plantarum LP48 | Adjunct culture |
| Lactiplantibacillus plantarum LP52 | Adjunct culture |
| Latilactobacillus sakei LSK04 | Adjunct culture |
| Lacticaseibacillus casei LC10 | Adjunct culture |
| Lacticaseibacillus rhamnosus LRH05 | Adjunct culture |
| Carnobacterium maltaromaticum CNB04 | Adjunct culture |
| Carnobacterium divergens CNB05 | Adjunct culture |
| Carnobacterium maltaromaticum CNB06 | Adjunct culture |
| Obesumbacterium proteus UC7452, | Indicator/Spiking |
| Obesumbacterium proteus UC7453 | Indicator/Spiking |
| Obesumbacterium proteus UC7454 | Indicator/Spiking |
| Obesumbacterium proteus UC7456 | Indicator/Spiking |
| Obesumbacterium proteus UC7457 | Indicator/Spiking |
| Obesumbacterium proteus UC7459 | Indicator/Spiking |
| Pseudomonas libanensis UC7310 | Indicator/Spiking |
| Pseudomonas fragi UC7455 | Indicator/Spiking |
| Pseudomonas gessardii UC7458 | Indicator/Spiking |
| Aerococcus urinaequi UC7460 | Indicator/Spiking |
| Species | Frequency of Isolation (%) | Production Period |
|---|---|---|
| Obesumbacterium proteus | 42.6 | summer and autumn |
| Aerococcus urinaeequi | 8.8 | summer |
| Enterococcus faecalis | 6.4 | summer |
| Latilactobacillus graminis | 5.8 | summer |
| Pseudomonas brennerii | 5.3 | autumn |
| Carnobacterium gallinarum | 4.7 | summer |
| Pseudomonas libanensis | 4.7 | summer |
| Leuconostoc pseudomesenteroides | 4.0 | summer |
| Hafnia alvei | 2.9 | autumn |
| Pseudomonas fragi | 2.3 | summer |
| Pseudomonas psychrophila | 1.8 | summer |
| Pseudomonas gessardii | 1.7 | autumn |
| Exiguobacterium acetylicum | 1.2 | summer |
| Macrococcus caseoliticus | 1.2 | summer |
| Staphylococcus vitulinus | 1.2 | summer |
| Lacticaseibacillus paracasei | 1.2 | summer |
| Pseudomonas proteolytica | 1.2 | autumn |
| Serratia liquefaciens | 1.2 | summer |
| E. pseudoavium/devriesei | 0.6 | summer |
| Lactiplantibacillus paraplantarum | 0.6 | summer |
| Leuconostoc mesenteroides | 0.6 | summer |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bassi, D.; Gazzola, S.; Sattin, E.; Dal Bello, F.; Simionati, B.; Cocconcelli, P.S. Lactic Acid Bacteria Adjunct Cultures Exert a Mitigation Effect against Spoilage Microbiota in Fresh Cheese. Microorganisms 2020, 8, 1199. https://doi.org/10.3390/microorganisms8081199
Bassi D, Gazzola S, Sattin E, Dal Bello F, Simionati B, Cocconcelli PS. Lactic Acid Bacteria Adjunct Cultures Exert a Mitigation Effect against Spoilage Microbiota in Fresh Cheese. Microorganisms. 2020; 8(8):1199. https://doi.org/10.3390/microorganisms8081199
Chicago/Turabian StyleBassi, Daniela, Simona Gazzola, Eleonora Sattin, Fabio Dal Bello, Barbara Simionati, and Pier Sandro Cocconcelli. 2020. "Lactic Acid Bacteria Adjunct Cultures Exert a Mitigation Effect against Spoilage Microbiota in Fresh Cheese" Microorganisms 8, no. 8: 1199. https://doi.org/10.3390/microorganisms8081199
APA StyleBassi, D., Gazzola, S., Sattin, E., Dal Bello, F., Simionati, B., & Cocconcelli, P. S. (2020). Lactic Acid Bacteria Adjunct Cultures Exert a Mitigation Effect against Spoilage Microbiota in Fresh Cheese. Microorganisms, 8(8), 1199. https://doi.org/10.3390/microorganisms8081199






