Structure and Antiparasitic Activity Relationship of Alkylphosphocholine Analogues against Leishmania donovani
Abstract
:1. Introduction
2. Methods and Reagents
2.1. Animals and Parasites
2.2. In Vitro Cytotoxicity against L. donovani and Uninfected Macrophages
2.3. In Vivo Cytotoxicity against L. donovani
2.4. Statistical Analysis
3. Result
3.1. The Effect of Varied Alkyl Carbon Chain Lengths of APCs against Leishmania donovani
3.2. The Effect of Charge on Tailed Molecules against Leishmania donovani
3.3. The Effect of Charge Separation on Tailed Molecules against L. donovani
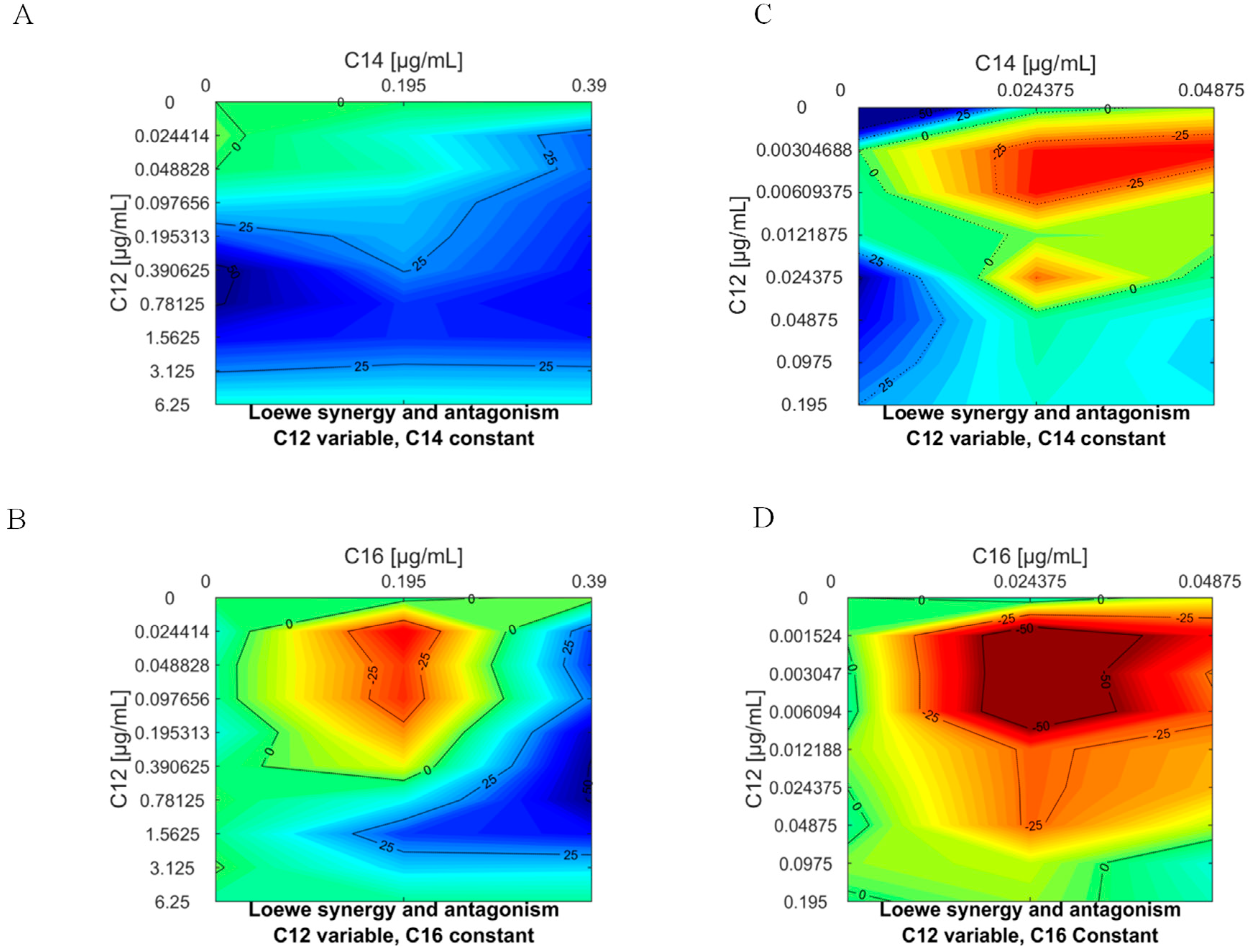
3.4. Studies to Determine if APC can Act Synergistically against L. donovani
3.5. In Vivo Efficacy of APC12 against L. donovani
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lindoso, J.A.L.; Moreira, C.H.V.; Cunha, M.A.; Queiroz, I.T. Visceral leishmaniasis and HIV co-infection current perspectives. HIV AIDS (Auckl) 2018, 10, 193–201. [Google Scholar] [PubMed] [Green Version]
- Chakravarty, J.; Sundar, S. Drug resistance in leishmaniasis. J. Glob. Infect. Dis. 2010, 2, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, V.; Singh, R.; Avishek, K.; Verma, A.; Deep, D.K.; Verma, S.; Salotra, P. Decline in clinical efficacy of oral miltefosine in treatment of post kala-azar dermal leishmaniasis (PKDL) in India. PLoS Negl. Trop. Dis. 2015, 9, e0004093. [Google Scholar]
- Sundar, S.; Singh, O.P.; Chakravarty, J. Visceral leishmaniasis elimination targets in India, strategies for preventing resurgence. Expert Rev. Anti-Infect. Ther. 2018, 16, 805–812. [Google Scholar] [CrossRef]
- Weng, H.B.; Chen, H.X.; Wang, M.W. Innovation in neglected tropical disease drug discovery and development. Infect. Dis. Poverty 2018, 7, 67. [Google Scholar] [CrossRef] [PubMed]
- Dorlo, T.P.; Balasegaram, M.; Beijnen, J.H.; de Vries, P.J. Miltefosine: A review of its pharmacology and therapeutic efficacy in the treatment of leishmaniasis. J. Antimicrob. Chemother. 2012, 67, 2576–2597. [Google Scholar] [CrossRef]
- Carnielli, J.B.T.; Crouch, K.; Forrester, S.; Silva, V.C.; Carvalho, S.F.G.; Damasceno, J.D.; Brown, E.; Dickens, N.J.; Costa, D.L.; Costa, C.H.N.; et al. Leishmania infantum genetic marker associated with miltefosine treatment failure for visceral leishmaniasis. EBioMedicine 2018, 36, 83–91. [Google Scholar] [CrossRef] [Green Version]
- Rijal, S.; Ostyn, B.; Uranw, S.; Rai, K.; Bhattarai, N.R.; Dorlo, T.P.; Beijnen, J.H.; Vanaerschot, M.; Decuypere, S.; Dhakal, S.S.; et al. Increasing failure of miltefosine in the treatment of Kala-azar in Nepal and the potential role of parasite drug resistance, reinfection, or noncompliance. Clin. Infect. Dis. 2013, 56, 1530–1538. [Google Scholar] [CrossRef] [Green Version]
- Goyal, V.; Burza, S.; Pandey, K.; Singh, S.N.; Singh, R.S.; Strub-Wourgaft, N.; Das, V.N.R.; Bern, C.; Hightower, A.; Rijal, S.; et al. Field effectiveness of new visceral leishmaniasis regimens after 1 year following treatment within public health facilities in Bihar, India. PLoS Negl. Trop. Dis. 2019, 26, 13–22. [Google Scholar] [CrossRef] [Green Version]
- Dorlo, T.P.; van Thiel, P.P.; Huitema, A.D.; Keizer, R.J.; de Vries, H.J.; Beijnen, J.H.; de Vries, P.J. Pharmacokinetics of miltefosine in Old World cutaneous leishmaniasis patients. Antimicrob. Antimicrobial Agents Chemother. 2008, 52, 2855–2860. [Google Scholar] [CrossRef] [Green Version]
- Croft, S.L.; Neal, R.A.; Pendergast, W.; Chan, J.H. The activity of alkyl phosphorylcholines and related derivatives against Leishmania donovani. Biochem. Pharmacol. 1987, 36, 2633–2636. [Google Scholar] [CrossRef]
- Croft, S.L.; Seifert, K.; Duchene, M. Anti-protozoal activities of phospholipid analogues. Mol. Biochem. Parasitol. 2003, 126, 165–172. [Google Scholar] [CrossRef]
- Zhang, R.R.; Grudzinski, J.J.; Mehta, T.I.; Burnette, R.R.; Hernandez, R.; Clark, P.A.; Lubin, J.A.; Pinchuk, A.N.; Jeffery, J.; Longino, M.; et al. In Silico Docking of Alkylphosphocholine Analogs to Human Serum AlbuminPredict Partitioning and Pharmacokinetics. Mol. Pharm. 2019, 16, 3350–3360. [Google Scholar] [CrossRef] [PubMed]
- Shaw, C.D.; Imamura, H.; Downing, T.; Blackburn, G.; Westrop, G.D.; Cotton, J.A.; Berriman, M.; Sanders, M.; Rijal, S.; Coombs, G.H.; et al. Genomic and Metabolomic Polymorphism among Experimentally Selected Paromomycin-Resistant Leishmania donovani Strains. Antimicrob. Agents Chemother. 2019, 64, e00904–e00919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alsaadi, M.; Italia, J.L.; Mullen, A.B.; Ravi Kumar, M.N.; Candlish, A.A.; Williams, R.A.; Shaw, C.D.; Al Gawhari, F.; Coombs, G.H.; Wiese, M.; et al. The efficacy of aerosol treatment with non-ionic surfactant vesicles containing amphotericin B in rodent models of leishmaniasis and pulmonary aspergillosis infection. J. Control. Release 2012, 28, 685–691. [Google Scholar] [CrossRef]
- Carter, K.C.; Mullen, A.B.; Sundar, S.; Kenney, R.T. Efficacies of vesicular and free sodium stibogluconate formulations against clinical isolates of Leishmania donovani. Antimicrob. Agents Chemother. 2001, 45, 3555–3559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakayama, G.R.; Caton, M.C.; Nova, M.P.; Parandoosh, Z. Assessment of the Alamar Blue assay for cellular growth and viability in vitro. J. Immunol. Methods 1997, 204, 205–208. [Google Scholar] [CrossRef]
- Ahmed, S.A.; Gogal, R.M., Jr.; Walsh, J.E. A new rapid and simple non-radioactive assay to monitor and determine the proliferation of lymphocytes: An alternative to [3H]thymidine incorporation assay. J. Immunol. Methods 1994, 170, 211–224. [Google Scholar] [CrossRef]
- Di Veroli, G.Y.; Fornari, C.; Wang, D.; Mollard, S.; Bramhall, J.L.; Richards, F.M.; Jodrell, D.I. Combenefit: An interactive platform for the analysis and visualization of drug combinations. Bioinformatics 2016, 32, 2866–2868. [Google Scholar] [CrossRef]
- Van Blitterswijk, W.J.; Verheij, M. Anticancer mechanisms and clinical application of alkylphospholipids. Biochim. Biophys. Acta 2013, 1831, 663–674. [Google Scholar] [CrossRef]
- Mooney, R.; Masala, M.; Martial, T.; McGinness, C.; Henriquez, F.; Williams, R. Alkyl-carbon chain length of cationic compounds and derivatives are key determinants of their anti- Acanthamoeba activities. Scietific Rep. 2020, 10, 6420–6430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walochnik, J.; Duchene, M.; Seifert, K. Cytotoxic activity of alkylphosphocholines against clinical isolates of Acanthamoeba spp. Antimicrob. Agents Chemother. 2001, 46, 695–701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weichert, J.P.; Clark, P.A.; Kandela, I.K.; Vaccaro, A.M.; Clarke, W.; Longino, M.A.; Pinchuk, A.N.; Farhoud, M.; Swanson, K.I.; Floberg, J.M.; et al. Alkylphosphocholine analogs for broad-spectrum cancer imaging and therapy. Sci. Transl. Med. 2014, 6, 240–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinchuk, A.N.; Rampy, M.A.; Longino, M.A.; Skinner, R.W.; Gross, M.D.; Weichert, J.P.; Counsell, R.E. Synthesis and structure-activity relationship effects on the tumor avidity of radioiodinated phospholipid ether analogues. J. Med. Chem. 2006, 49, 2155–2165. [Google Scholar] [CrossRef]
- Obando, D.; Widmer, F.; Wright, L.C.; Sorrell, T.C.; Jolliffe, K.A. Synthesis, antifungal and antimicrobial activity of alkylphospholipids. Bioorg. Med. Chem. 2007, 15, 5158–5165. [Google Scholar] [CrossRef]
- Palusinska-Szysz, M.; Kania, M.; Turska-Szewczuk, A.; Danikiewicz, W.; Russa, R.; Fuchs, B. Identification of unusual phospholipid fatty acyl compositions of Acanthamoeba castellanii. PLoS ONE 2014, 9, e101243. [Google Scholar] [CrossRef] [Green Version]
- Williams, R.A.; Smith, T.K.; Cull, B.; Mottram, J.C.; Coombs, G.H. ATG5 is essential for ATG8-dependent autophagy and mitochondrial homeostasis in Leishmania major. PLoS Pathog. 2012, 8, e1002695. [Google Scholar] [CrossRef] [Green Version]
- Rakotomanga, M.; Saint-Pierre-Chazalet, M.; Loiseau, P.M. Alteration of fatty acid and sterol metabolism in miltefosine-resistant Leishmania donovani promastigotes and consequences for drug-membrane interactions. Antimicrob. Agents Chemother. 2005, 49, 2677–2689. [Google Scholar] [CrossRef] [Green Version]
- Barioni, M.B.; Ramos, A.P.; Zaniquelli, M.E.D.; Acuña, A.U.; Ito, A.S. Miltefosine and BODIPY-labeled alkylphosphocholine with leishmanicidal activity: Aggregation properties and interaction with model membranes. Biophys. Chem. 2015, 196, 92–99. [Google Scholar] [CrossRef]
- Kuiper, J.M.; Buwalda, R.T.; Hulst, R.; Engberts, J.B.F.N. Novel Pyridinium Surfactants with Unsaturated Alkyl Chains. Aggregation Behaviour and Interactions with Methyl Orange in Aqueous Solution. Langmuir 2001, 17, 5216–5224. [Google Scholar] [CrossRef] [Green Version]
- Yaseen, M.; Lu, J.R.; Webster, J.R.P.; Penfold, J. Adsorption of single chain Zwitterionic phosphocholine surfactants: Effects of length of alkyl chain and head group linker. Biophys. Chem. 2005, 117, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Varela-M, R.E.; Villa-Pulgarin, J.A.; Yepes, E.; Müller, I.; Modolell, M.; Muñoz, D.L.; Robledo, S.M.; Muskus, C.E.; López-Abán, J.; Muro, A.; et al. In vitro and in vivo efficacy of ether lipid edelfosine against Leishmania spp. and SbV-resistant parasites. PLoS Negl. Trop. Dis. 2012, 6, e1612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coghi, P.; Vaiana, N.; Pezzano, M.G.; Rizzi, L.; Kaiser, M.; Brun, R.; Romeo, S. Parallel synthesis and antileishmanial activity of ether-linked phospholipids. Bioorg. Med. Chem. Lett. 2008, 18, 4658–4660. [Google Scholar] [CrossRef]
- Azzouz, S.; Maache, M.; Garcia, R.G.; Osuna, A. Leishmanicidal activity of edelfosine, miltefosine and ilmofosine. Basic Clin. Pharmacol. Toxicol. 2005, 96, 60–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabrera-Serra, M.G.; Lorenzo-Morales, J.; Valladares, B.; Pinero, J.E. In vitro activity of perifosine: A novel alkylphospholipid against the promastigote stage of Leishmania species. Parasitol. Res. 2007, 100, 1155–1157. [Google Scholar] [CrossRef] [PubMed]
- Cabrera-Serra, M.G.; Valladares, B.; Pinero, J.E. In vivo activity of perifosine against Leishmania amazonensis. Acta Trop. 2008, 108, 20–25. [Google Scholar] [CrossRef]
- Oliver, R.C.; Lipfert, J.; Fox, D.A.; Lo, R.H.; Doniach, S.; Columbus, L. Dependence of micelle size and shape on detergent alkyl chain length and head group. PLoS ONE 2013, 8, e62488. [Google Scholar] [CrossRef]
- Vieira, D.B.; Carmona-Ribeiro, A.M. Cationic lipids and surfactants as antifungal agents: Mode of action. J. Antimicrob. Chemother. 2006, 58, 760–767. [Google Scholar] [CrossRef]
- Carmona-Ribeiro, A.M.; de Melo Carrasco, L.D. Cationic antimicrobial polymers and their assemblies. Int. J. Mol. Sci. 2013, 14, 9906–9946. [Google Scholar] [CrossRef] [Green Version]
- Pimenta, P.F.P.; De Souza, W. Leishmania mexicana amazonensis: Surface charge of amastigote and promastigote forms. Exp. Parasitol. 1983, 56, 194–206. [Google Scholar] [CrossRef]
- Saraiva, E.M.; Vannier-Santos, M.A.; Silva-Filho, F.C.; De Souza, W. Anionic site behaviour in Leishmania and its role in the parasite-macrophage interaction. J. Cell Sci. 1989, 93, 481–489. [Google Scholar]
- Inácio, Â.S.; Domingues, N.S.; Nunes, A.; Martins, P.T.; Moreno, M.J.; Estronca, L.M.; Fernandes, R.; Moreno, A.J.; Borrego, M.J.; Gomes, J.P.; et al. Quaternary ammonium surfactant structure determines selective toxicity towards bacteria: Mechanisms of action and clinical implications in antibacterial prophylaxis. J. Antimicrob. Chemother. 2016, 71, 641–654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suárez, M.J.; Mosquera, V. Mixed micelles of n-alkyltrimethylammonium bromides: Influence of alkyl chain length. Phys. Chem. Chem. Phys. 1999, 1, 3583–3587. [Google Scholar]
- Khan, A.; Marques, E.F. Synergism and polymorphism in mixed surfactant systems. Curr. Opin. Colloid Interface Sci. 1999, 4, 402–410. [Google Scholar] [CrossRef] [Green Version]
- Feng, S.; Catron, N.D.; Zhu, A.; Lipari, J.M.; Wu, J.; Gao, Y.; Zhang, G.G.Z. Predictive Modeling of Micellar Solubilization by Single and Mixed Nonionic Surfactants. J. Pharm. Sci. 2018, 107, 2079–2090. [Google Scholar] [CrossRef] [PubMed]
- van Griensven, J.; Balasegaram, M.; Meheus, F.; Alvar, J.; Lynen, L.; Boelaert, M. Combination therapy for visceral leishmaniasis. Lancet Infect. Dis. 2010, 10, 184–194. [Google Scholar] [CrossRef]
- Sundar, S.; Rai, M.; Chakravarty, J.; Agarwal, D.; Agrawal, N.; Vaillant, M.; Olliaro, P.; Murray, H.W. New treatment approach in Indian visceral leishmaniasis: Single-dose liposomal amphotericin B followed by short-course oral miltefosine. Clin. Infect. Dis. 2008, 47, 1000–1006. [Google Scholar] [CrossRef] [Green Version]
- Sundar, S.; Sinha, P.K.; Rai, M.; Verma, D.K.; Nawin, K.; Alam, S.; Chakravarty, J.; Vaillant, M.; Verma, N.; Pandey, K.; et al. Comparison of short-course multidrug treatment with standard therapy for visceral leishmaniasis in India: An open-label, non-inferiority, randomised controlled trial. Lancet 2011, 377, 477–486. [Google Scholar] [CrossRef]
- Rahman, R.; Goyal, V.; Haque, R.; Jamil, K.; Faiz, A.; Samad, R.; Ellis, S.; Balasegaram, M.; den Boer, M.; Rijal, S.; et al. Safety and efficacy of short course combination regimens with AmBisome, miltefosine and paromomycin for the treatment of visceral leishmaniasis (VL) in Bangladesh. PLoS Negl. Trop. Dis. 2017, 11, e0005635. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.Z.; LeCluyse, E.L.; Thakker, D.R. Dodecylphosphocholine-mediated enhancement of paracellular permeability and cytotoxicity in Caco-2 cell monolayers. J. Pharm. Sci. 1999, 88, 1161–1168. [Google Scholar] [CrossRef]
- Menez, C.; Buyse, M.; Chacun, H.; Farinotti, R.; Barratt, G. Modulation of intestinal barrier properties by miltefosine. Biochem. Pharmacol. 2006, 71, 486–496. [Google Scholar] [CrossRef] [PubMed]
- Ménez, C.; Buyse, M.; Dugave, C.; Farinotti, R.; Barratt, G. Intestinal Absorption of Miltefosine: Contribution of Passive Paracellular Transport. Pharm. Res. 2007, 24, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Ménez, C.; Buyse, M.; Farinotti, R.; Barratt, G. Inward translocation of the phospholipid analogue miltefosine across Caco-2 cell membranes exhibits characteristics of a carrier-mediated process. Lipids 2007, 42, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Falavigna, M.; Klitgaard, M.; Steene, E.; Flaten, G.E. Mimicking regional and fasted/fed state conditions in the intestine with the mucus-PVPA in vitro model: The impact of pH and simulated intestinal fluids on drug permeability. Eur. J. Pharm. Sci. 2019, 132, 44–54. [Google Scholar] [CrossRef]
- Ouyang, H.; Morris-Natschke, S.L.; Ishaq, K.S.; Ward, P.; Liu, D.; Leonard, S.; Thakker, D.R. Structure-activity relationship for enhancement of paracellular permeability across Caco-2 cell monolayers by 3-alkylamido-2-alkoxypropylphosphocholines. J. Med. Chem. 2002, 45, 2857–2866. [Google Scholar] [CrossRef]
- Carter, K.C.; Sundar, S.; Spickett, C.; Pereira, O.C.; Mullen, A.B. The in vivo susceptibility of Leishmania donovani to sodium stibogluconate is drug specific and can be reversed by inhibiting glutathione biosynthesis. Antimicrob. Agents Chemother. 2003, 47, 1529–1535. [Google Scholar] [CrossRef] [Green Version]
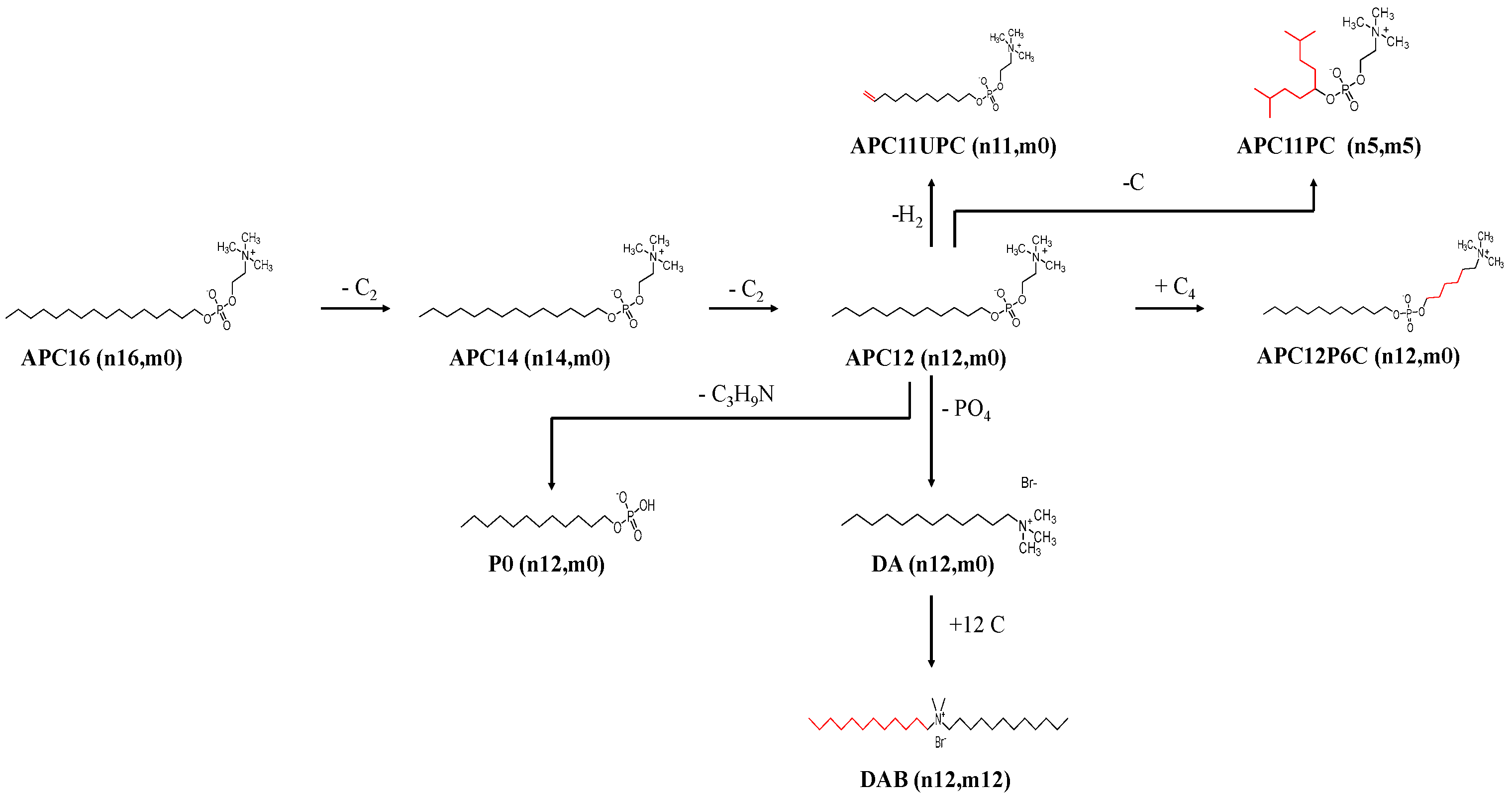
| Compound | Chain Length and Number (n,m) | Charge | Promastigotes µg/mL (µM) | Amastigote µg/mL (µM) | Macrophage µg/mL (µM) | Selectivity Index (SI50) | Promast: Amast Ratio | Molecular Mass | CMC µg/mL (µM) |
|---|---|---|---|---|---|---|---|---|---|
| Modification of tail | |||||||||
| APC12 | n-12; m-0 | Zwitterionic | ** 0.16 ± 0.00 (0.46 ± 0.00) | ** 0.01 ± 0.00 (0.026 ± 0.00) | 35.38 ± 1.74 (100.64 ± 4.95) | 3870.77 | 16.46 | 351.5 | 351.50 (1.00) |
| APC14 | n-14; m-0 | Zwitterionic | ** 0.20 ± 0.00 (0.53 ± 0.00) | ** 0.03 ± 0.02 (0.066 ± 0.05) | 40.89 ± 4.42 (107.75 ± 11.65) | 1632.58 | 7.97 | 379.5 | 45.54 (1.2 × 10-5) |
| APC16 | n-12; m-0 | Zwitterionic | 0.70 ± 0.00 (1.72 ± 0.00) | 0.10 ± 0.01 (0.25 ± 0.02) | 5.08 ± 8.29 (12.47 ± 20.34) | 49.88 | 7.00 | 407.5 | 0.41 (1.3 × 10-5) |
| APC11PC | n-6; m-5 | Zwitterionic | b >6.25 ± 0.06 (> 18.63 ± 0.18) | b 0.19 ± 0.08 (0.55 ± 0.24) | 61.90 ± 0.01 (184.56 ± 0.03) | 335.56 | >33.60 | 335.4 | NA |
| APC11UPC | n-12; m-0 | Zwitterionic | b 1.80 ± 0.02 (5.37 ± 0.06) | 0.01 ± 0.06 (0.04 ± 0.18) | 54.00 ± 0.13 (161.00 ± 0.39) | 4025.00 | 135.34 | 335.4 | NA |
| Modification of the linker | |||||||||
| APC12P6C | n-12; n-0 | Zwitterionic | b 33.50 ± 0.06 (82.21 ± 0.15) | b 1.01 ± 0.07 (2.47 ± 0.17) | 14.10 ± 1.08 (34.60 ± 2.65) | 14.01 | 33.33 | 407.5 | NA |
| Modification of the head | |||||||||
| DA | n-12; m-0 | Cationic | b 0.09 ± 0.00 (0.05 ± 0.00) | 0.47 ± 0.00 (2.54 ± 0.00) | 37.60 ± 0.01 (202.91 ± 0.05) | 79.88 | 0.18 | 185.3 | NA |
| DAB | n-12; m-12 | Cationic | b 0.04 ± 0.05 (0.091 ± 0.11) | b 0.06 ± 0.00 (0.119 ± 0.00) | 82.76 ± 0.01 (178.91 ± 0.02) | 1503.45 | 0.79 | 462.6 | NA |
| PO | n-12; m-0 | Anionic | b >6.25 ± 0.01 (> 25.07 ± 0.04) | b >2.00 ± 0.01 (> 8.02 ± 0.04) | 100.00 ± 0.01 (401.11 ± 0.04) | >50.00 | >3.12 | 249.31 | NA |
| Strain | Antimony Resistance | Drug | Mean IC50 µg/mL (µM) | Promast: Amast Ratio | Cross-Resistance Index (CRI50) (Promast/Amast) | Selectivity Index (SI50) | |
|---|---|---|---|---|---|---|---|
| Promastigotes | Amastigotes | ||||||
| 282/4 | Sb-sensitive | APC12 | ** 0.55 ± 0.02 (1.56 ± 0.06) | ** 0.26 ± 0.01 (0.74 ± 0.03) | 2.09 | NA/NA | 136.08 |
| APC14 | ** 0.44 ± 0.07 (1.16 ± 0.18) | ** 0.12 ± 0.02 (0.32 ± 0.05) | 3.64 | NA/NA | 340.75 | ||
| APC16 | 0.84 ± 0.05 (2.06 ± 0.12) | 0.31 ± 0.02 (0.76 ± 0.05) | 2.68 | NA/NA | 16.39 | ||
| 087/11 | Sb-intermediate | APC12 | ** 0.42 ± 0.02 (1.19 ± 0.06) | ** 0.20 ± 0.07 (0.57 ± 0.20) | 2.11 | 0.76/0.77 | 176.90 |
| APC14 | ** 0.21 ± 0.00 (0.55 ± 0.00) | ** 0.19 ± 0.01 (0.50 ± 0.03) | 1.05 | 0.48/1.58 | 215.21 | ||
| APC16 | 1.49 ± 0.6 (3.66 ± 1.47) | 0.29 ± 0.01 (0.71 ± 0.02) | 5.15 | 1.77/1.76 | 17.52 | ||
| 275/18 | Sb-resistant | APC12 | ** 0.49 ± 0.03 (1.39 ± 0.09) | ** 0.23 ± 0.01 (0.65 ± 0.03) | 2.10 | 0.89/0.88 | 153.83 |
| APC14 | ** 0.37 ± 0.05 (0.97 ± 0.13) | ** 0.28 ± 0.00 (0.74 ± 0.00) | 1.31 | 0.84/2.33 | 146.04 | ||
| APC16 | 1.26 ± 0.06 (3.09 ± 0.15) | 0.51 ± 0.00 (1.25 ± 0.00) | 2.49 | 1.50/1.65 | 9.96 | ||
| Strain | Antimony Resistance | Drug | Mean IC50 Value µg/mL (µM) | Promast: Amast Ratio | Cross-Resistance Index (CRI50) (Promast/amast) | Selectivity Index (SI50) | |
|---|---|---|---|---|---|---|---|
| Promastigotes | Amastigotes | ||||||
| 282/4 | Milt-Sb-sensitive | APC12 | >125.00 ± 0.00 (>355.60 ± 0.00) | **0.08 ± 0.17 (0.23 ± 0.48) | >1562.5 | NA/NA | 442.25 |
| APC14 | >125.00 ± 0.00 (329.40 ± 0.00) | >1.73 ± 00 (>4.56 ± 00) | >72.25 | NA/NA | >23.64 | ||
| APC16 | >125.00 ± 0.00 (306.70 ± 0.00) | >1.61 ± 00 (>3.95 ± 00) | >77.64 | NA/NA | >3.16 | ||
| 087/11 | Milt-Sb-intermediate | APC12 | ** 184.00 ± 0.10 (0.52 ± 0.22) | ** 6.17 ± 0.01 (17.55 ± 0.03) | 29.82 | >1.47/77.13 | 5.73 |
| APC14 | 232.00 ± 0.30 (0.63 ± 0.11) | 10.03 ± 0.00 (26.43 ± 0.00) | 23.13 | >1.86/> 5.95 | 4.08 | ||
| APC16 | 242.00 ± 0.24 (0.56 ± 0.12) | 12.65 ± 0.00 (31.04 ± 0.00) | 19.13 | >1.94/> 7.86 | 0.40 | ||
| 275/18 | Milt-Sb-resistant | APC12 | >125.00 ± 0.00 (>355.60 ± 0.00) | ** 4.45 ± 0.01 (12.66 ± 0.03) | >28.09 | 1.00/5.62 | 7.95 |
| APC14 | >125.00 ± 0.00 (329.40 ± 0.00) | ** 34.85 ± 0.02 (91.83 ± 0.05) | 3.59 | 1.00/> 20.144 | 1.17 | ||
| APC16 | >125.00 ± 0.00 (306.70 ± 0.00) | >61.36 ± 0.00 (>150.58 ± 0.0) | 2.04 | 1.00/> 38.11 | >0.08 | ||
| Treatment | Mean Parasite Burden ± SD | ||
|---|---|---|---|
| Spleen | Liver | Bone Marrow | |
| Experiment 1: Oral administration | |||
| Control | 198 ± 62 | 1000 ± 286 | 225 ± 100 |
| MIL 40 mg/kg oral | 73 ± 68 * (53 ± 31) | 433 ± 46 ** (40 ± 25) | 197 ± 157 (30 ± 35) |
| APC12 40 mg/kg oral | 275 ± 96 b (8 ± 18) | 880 ± 276 a (36 ± 34) | 210 ± 90 (54 ± 34) |
| Experiment 2: Intravenous administration | |||
| Control | 305 ± 97 | 1299 ± 158 | 458 ± 129 |
| APC12 40 mg/kg IV | 112 ± 30 * (60 ± 11) | 434 ± 197 ** (60 ± 19) | 555 ± 254 (8 ± 14) |
| APC12 80 mg/kg oral | 257 ± 73 c (13 ± 27) | 939 ± 170 c (27 ± 16) | 607 ± 115 (2 ± 4) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, H.; Carter, K.C.; Williams, R.A.M. Structure and Antiparasitic Activity Relationship of Alkylphosphocholine Analogues against Leishmania donovani. Microorganisms 2020, 8, 1117. https://doi.org/10.3390/microorganisms8081117
Ahmed H, Carter KC, Williams RAM. Structure and Antiparasitic Activity Relationship of Alkylphosphocholine Analogues against Leishmania donovani. Microorganisms. 2020; 8(8):1117. https://doi.org/10.3390/microorganisms8081117
Chicago/Turabian StyleAhmed, Humera, Katharine C. Carter, and Roderick A.M. Williams. 2020. "Structure and Antiparasitic Activity Relationship of Alkylphosphocholine Analogues against Leishmania donovani" Microorganisms 8, no. 8: 1117. https://doi.org/10.3390/microorganisms8081117
APA StyleAhmed, H., Carter, K. C., & Williams, R. A. M. (2020). Structure and Antiparasitic Activity Relationship of Alkylphosphocholine Analogues against Leishmania donovani. Microorganisms, 8(8), 1117. https://doi.org/10.3390/microorganisms8081117






