Enterococci, from Harmless Bacteria to a Pathogen
Abstract
:1. Introduction
2. Emergence and Dissemination Trends of Vancomycin-Resistant Enterococci (VRE) Strains
3. The Public Health Impact of VRE
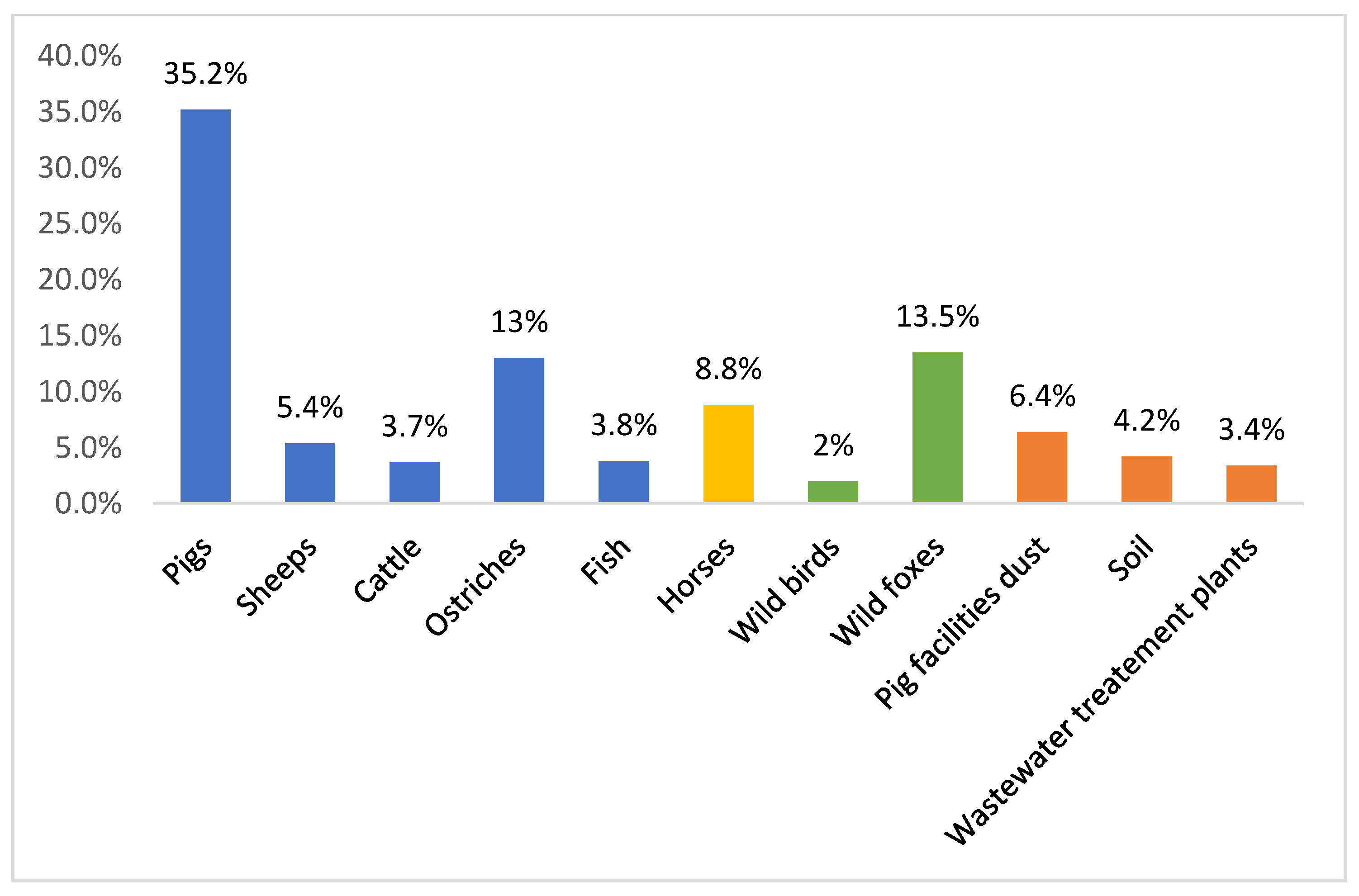
4. Persistence of VRE Strains in Portuguese Food Animals, an Example
Author Contributions
Funding
Conflicts of Interest
References
- Lengfelder, I.; Sava, I.G.; Hansen, J.J.; Kleigrewe, K.; Herzog, J.; Neuhaus, K.; Hoffman, T.; Sartor, R.B.; Haller, D. Complex bacterial consortia reprogram the colitogenic activity of Enterococcus faecalis in a gnotobiotic mouse model of chronic, immune-mediated colitis. Front. Immunol. 2019, 10, 1420. [Google Scholar] [CrossRef] [PubMed]
- Escobedo-Hinojosa, W.; Pardo-López, L. Analysis of bacterial metagenomes from the Southwestern Gulf of Mexico for pathogens detection. Pathog. Dis. 2017, 75, ftx058. [Google Scholar] [CrossRef] [PubMed]
- Comerlato, C.B.; Ritter, A.C.; Miyamoto, K.N.; Brandelli, A. Proteomic study of Enterococcus durans LAB18S growing on prebiotic oligosaccharides. Food Microbiol. 2020, 89, 103430. [Google Scholar] [CrossRef]
- Nilsson, O. Vancomycin resistant enterococci in farm animals—Occurrence and importance. Infect. Ecol Epidemiol. 2012, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Werner, G.; Coque, T.M.; Franz, C.M.A.P.; Grohmann, E.; Hegstad, K.; Jensen, L.; van Schaik, W.; Weaver, K. Antibiotic resistant enterococci—Tales of a drug resistance gene trafficker. Int. J. Med. Microbiol. 2013, 303, 360–379. [Google Scholar] [CrossRef]
- Dubin, K.; Pamer, E.G. Enterococci and their interactions with the intestinal microbiome. Microbiol. Spectr. 2016, 5. [Google Scholar] [CrossRef] [Green Version]
- Zaheer, R.; Cook, S.R.; Barbieri, R.; Goji, N.; Cameron, A.; Petkau, A.; Polo, R.O.; Tymensen, L.; Stamm, C.; Song, J.; et al. Surveillance of Enterococcus spp. reveals distinct species and antimicrobial resistance diversity across a One-Health continuum. Sci. Rep. 2020, 10, 3937. [Google Scholar] [CrossRef]
- Torres, C.; Alonso, C.A.; Ruiz-Ripa, L.; León-Sampedro, R.; del Campo, R.; Coque, T.M. Antimicrobial resistance in Enterococcus spp. of animal origin. Microbiol. Spectr. 2018, 6, 185–227. [Google Scholar] [CrossRef]
- Qiao, X.; Du, R.; Wang, Y.; Han, Y.; Zhou, Z. Isolation, characterisation and fermentation optimisation of bacteriocin-producing Enterococcus faecium. Waste Biomass Valorization 2019, 11, 3173–3181. [Google Scholar] [CrossRef]
- Fisher, K.; Phillips, C. The ecology, epidemiology and virulence of Enterococcus. Microbiology 2009, 155, 1749–1757. [Google Scholar] [CrossRef] [Green Version]
- De Almeida, C.V.; Taddei, A.; Amedei, A. The controversial role of Enterococcus faecalis in colorectal cancer. Therap. Adv. Gastroenterol. 2018, 11, 1756284818783606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Kersh, T.A.; Marie, M.A.; Al-Sheikh, Y.A.; Al-Agamy, M.H.; Al Bloushy, A.A. Prevalence and risk factors of early fecal carriage of Enterococcus faecalis and Staphylococcus spp. and their antimicrobial resistant patterns among healthy neonates born in a hospital setting in central Saudi Arabia. Saudi Med. J. 2016, 37, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, Q.; Wu, X.; Qi, H.; Das, R.; Lin, H.; Shi, J.; Wang, S.; Yang, J.; Xue, Y.; et al. Vancomycin exposure caused opportunistic pathogens bloom in intestinal microbiome by simulator of the human intestinal microbial ecosystem (SHIME). Environ. Pollut. 2020, 265, 114399. [Google Scholar] [CrossRef]
- Arias, C.A.; Murray, B.E. The rise of the Enterococcus: Beyond vancomycin resistance. Nat. Rev. Microbiol. 2012, 10, 266–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hendrickx, A.P.A.; van Schaik, W.; Willems, R.J.L. The cell wall architecture of Enterococcus faecium: From resistance to pathogenesis. Future Microbiol. 2013, 8, 993–1010. [Google Scholar] [CrossRef] [PubMed]
- García-Solache, M.; Rice, L.B. The Enterococcus: A model of adaptability to its environment. Clin. Microbiol. Rev. 2019, 32, e00058-18. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, M.O.; Baptiste, K.E. Vancomycin-resistant enterococci: A review of antimicrobial resistance mechanisms and perspectives of human and animal health. Microb. Drug Resist. 2017, 24, 590–606. [Google Scholar] [CrossRef] [Green Version]
- Bonten, M.J.M.; Willems, R.; Weinstein, R.A. Vancomycin-resistant enterococci: Why are they here, and where do they come from? Lancet Infect. Dis. 2001, 1, 314–325. [Google Scholar] [CrossRef]
- Top, J.; Willems, R.; Bonten, M. Emergence of CC17 Enterococcus faecium: From commensal to hospital-adapted pathogen. FEMS Immunol. Med. Microbiol. 2008, 52, 297–308. [Google Scholar] [CrossRef] [Green Version]
- Rios, R.; Reyes, J.; Carvajal, L.P.; Rincon, S.; Panesso, D.; Echeverri, A.M.; Dihn, A.; Kolokotronis, S.-O.; Narechania, P.; Tran, T.T.; et al. Genomic epidemiology of vancomycin-resistant Enterococcus faecium (VREfm) in Latin America: Revisiting the global VRE population structure. Sci. Rep. 2020, 10, 5636. [Google Scholar] [CrossRef] [Green Version]
- Freitas, A.R.; Coque, T.M.; Novais, C.; Hammerum, A.M.; Lester, C.H.; Zervos, M.J.; Donabedian, S.; Jensen, L.B.; Francia, M.V.; Baquero, F.; et al. Human and swine hosts share vancomycin-resistant Enterococcus faecium CC17 and CC5 and Enterococcus faecalis CC2 clonal clusters harboring Tn1546 on indistinguishable plasmids. J. Clin. Microbiol. 2011, 49, 925–931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donabedian, S.M.; Perri, M.B.; Abdujamilova, N.; Gordoncillo, M.J.; Naqvi, A.; Reyes, K.C.; Zervus, M.J.; Bartlett, P. Characterization of vancomycin-resistant Enterococcus faecium isolated from swine in three Michigan counties. J. Clin. Microbiol. 2010, 48, 4156–4160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ECDC. Antimicrobial Consumption in the EU/EEA, Annual Epidemiological Report for 2018; European Centre for Disease Prevention and Control: Stockholm, Sweeden, 2019. [Google Scholar]
- Terkuran, M.; Turhan, E.Ü.; Erginkaya, Z. The risk of vancomycin resistant enterococci infections from food industry. In Health and Safety Aspects of Food Processing Technologies; Malik, A., Erginkaya, Z., Erten, H., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 513–535. [Google Scholar] [CrossRef]
- Werner, G. Current trends of emergence and spread of vancomycin-resistant enterococci. In Antibiotic Resistant-Bacteria—A Continuous Challenge in the New Millenium; Pana, M., Ed.; IntechOpen: Rijeka, Croatia, 2012; pp. 303–354. [Google Scholar] [CrossRef] [Green Version]
- Ramos, S.; Igrejas, G.; Rodrigues, J.; Capelo-Martinez, J.-L.; Poeta, P. Genetic characterisation of antibiotic resistance and virulence factors in vanA-containing enterococci from cattle, sheep and pigs subsequent to the discontinuation of the use of avoparcin. Vet. J. 2012, 193, 301–303. [Google Scholar] [CrossRef] [PubMed]
- Wist, V.; Morach, M.; Schneeberger, M.; Cernela, N.; Stevens, M.J.A.; Zurfluh, K.; Stephan, R.; Nüesch-Inderbinen, M. Phenotypic and genotypic traits of vancomycin-resistant enterococci from healthy food-producing animals. Microorganisms 2020, 8, 261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orsi, G.B.; Ciorba, V. Vancomycin resistant enterococci healthcare associated infections. Ann Ig 2013, 25, 485–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Markwart, R.; Willrich, N.; Haller, S.; Noll, I.; Koppe, U.; Werner, G.; Eckmanns, T.; Reuss, A. The rise in vancomycin-resistant Enterococcus faecium in Germany: Data from the German Antimicrobial Resistance Surveillance (ARS). Antimicrob. Resist. Infect. Control 2019, 8, 147. [Google Scholar] [CrossRef]
- Reinseth, I.S.; Ovchinnikov, K.V.; Tønnesen, H.H.; Carlsen, H.; Diep, D.B. The increasing issue of vancomycin-resistant enterococci and the bacteriocin solution. Probiotics Antimicrob. Proteins 2019. [Google Scholar] [CrossRef]
- Ford, C.D.; Lopansri, B.K.; Gazdik, M.A.; Webb, B.; Snow, G.L.; Hoda, D.; Adams, B.; Petersen, F.B. Room contamination, patient colonization pressure, and the risk of vancomycin-resistant Enterococcus colonization on a unit dedicated to the treatment of hematologic malignancies and hematopoietic stem cell transplantation. Am. J. Infect. Control 2016, 44, 1110–1115. [Google Scholar] [CrossRef]
- Suleyman, G.; Alangaden, G.; Bardossy, A.C. The role of environmental contamination in the transmission of nosocomial pathogens and healthcare-associated infections. Curr. Infect. Dis. Rep. 2018, 20, 12. [Google Scholar] [CrossRef]
- Drees, M.; Snydman, D.R.; Schmid, C.H.; Barefoot, L.; Hansjosten, K.; Vue, P.M.; Cronin, M.; Nasraway, S.A.; Golan, Y. Prior environmental contamination increases the risk of acquisition of vancomycin-resistant enterococci. Clin. Infect. Dis. 2008, 46, 678–685. [Google Scholar] [CrossRef]
- Reyes, K.; Bardossy, A.C.; Zervos, M. Vancomycin-resistant enterococci. Epidemiology, infection, prevention and control. Infect. Dis Clin. N. Am. 2016, 30, 953–965. [Google Scholar] [CrossRef] [PubMed]
- Correa-Martinez, C.L.; Tönnies, H.; Froböse, N.J.; Mellmann, A.; Kampmeier, S. Transmission of vancomycin-resistant enterococci in the hospital setting: Uncovering the patient-environment interplay. Microorganisms 2020, 8, 203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbo, L.; Shukla, B.S.; Giles, A.; Aragon, L.; Jimenez, A.; Camargo, J.F.; Simkins, J.; Sposato, K.; Tran, T.C.; Diaz, L.; et al. Linezolid- and vancomycin-resistant Enterococcus faecium in solid organ transplant recipients: Infection control and antimicrobial stewardship using whole genome sequencing. Clin. Infect. Dis. 2019, 69, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Liese, J.; Schüle, L.; Oberhettinger, P.; Tschörner, L.; Nguyen, T.; Dörfel, D.; Vogel, W.; Marschal, M.; Autenrieth, I.; Willmann, M.; et al. Expansion of vancomycin-resistant Enterococcus faecium in an academic tertiary hospital in Southwest Germany: A large scale whole-genome-based outbreak investigation. Antimicrob. Agents Chemother. 2019, 63, e01978-18. [Google Scholar] [CrossRef] [Green Version]
- Raven, K.E.; Gouliouris, T.; Brodrick, H.; Coll, F.; Brown, N.M.; Reynolds, R.; Reuter, S.; Török, M.E.; Parkhill, J.; Peacock, S.J. Complex routes of nosocomial vancomycin-resistant Enterococcus faecium transmission revealed by genome sequencing. Clin. Infect. Dis. 2017, 64, 886–893. [Google Scholar] [CrossRef] [PubMed]
- Dalton, K.R.; Rock, C.; Carroll, K.C.; Davis, M.F. One Health in hospitals: How understanding the dynamics of people, animals, and the hospital built-environment can be used to better inform interventions for antimicrobial-resistant gram-positive infections. Antimicrob. Resist. Infect. Control 2020, 9, 78. [Google Scholar] [CrossRef]
- Contreras, G.; Munita, J.M.; Arias, C.A. Novel strategies for the management of vancomycin-resistant enterococcal infections. Curr. Infect. Dis. Rep. 2019, 21, 22. [Google Scholar] [CrossRef]
- Zhen, X.; Lundborg, C.S.; Sun, X.; Hu, X.; Dong, H. Economic burden of antibiotic resistance in ESKAPE organisms: A systematic review. Antimicrob. Resist. Infect. Control 2019, 8, 137. [Google Scholar] [CrossRef] [Green Version]
- Bradley, J.S. Which antibiotic for resistant Gram-positives, and why? J. Infect. 2014, 68, S63–S75. [Google Scholar] [CrossRef]
- Irfan, S.; Qamar, S. A case report on isolation of linezolid- and vancomycin-resistant Enterococcus faecium species from cerebrospinal fluid of a patient suffering from ventriculoperitoneal shunt-associated meningitis. Cureus 2019, 11, e5631. [Google Scholar] [CrossRef] [Green Version]
- Marshall, S.H.; Donskey, C.J.; Hutton-Thomas, R.; Salata, R.A.; Rice, L.B. Gene dosage and linezolid resistance in Enterococcus faecium and Enterococcus faecalis. Antimicrob. Agents Chemother. 2002, 46, 3334–3336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bourgeois-Nicolaos, N.; Massias, L.; Couson, B.; Butel, M.-J.; Andremont, A.; Doucet-Populaire, F. Dose dependence of emergence of resistance to linezolid in Enterococcus faecalis in vivo. J. Infect. Dis. 2007, 195, 1480–1488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freitas, A.R.; Tedim, A.P.; Duarte, B.; Elghaieb, H.; Abbassi, M.S.; Hassen, A.; Read, A.; Alves, V.; Novais, C.; Peixe, L. Linezolid-resistant (Tn6246::fexB-poxtA) Enterococcus faecium strains colonizing humans and bovines on different continents: Similarity without epidemiological link. J. Antimicrob. Chemother. 2020, dkaa227. [Google Scholar] [CrossRef] [PubMed]
- Egan, S.A.; Corcoran, S.; McDermott, H.; Fitzpatrick, M.; Hoyne, A.; McCormack, O.; Cullen, A.; Brennan, G.I.; O’Connell, B.; Coleman, D.C. A hospital outbreak of linezolid-resistant and vancomycin-resistant ST80 Enterococcus faecium harbouring an optrA-encoding conjugative plasmid investigated by whole-genome sequencing. J. Hosp. Infect. 2020, S0195-6701, 30244–30249. [Google Scholar] [CrossRef] [PubMed]
- Kerschner, H.; Cabal, A.; Hartl, R.; Machherndl-Spandl, S.; Allerberger, F.; Ruppitsch, W.; Apfalter, P. Hospital outbreak caused by linezolid resistant Enterococcus faecium in Upper Austria. Antimicrob. Resist. Infect. Control 2019, 8, 150. [Google Scholar] [CrossRef] [Green Version]
- Hollenbeck, B.L.; Rice, L.B. Intrinsic and acquired resistance mechanisms in enterococcus. Virulence 2012, 3, 421–433. [Google Scholar] [CrossRef] [Green Version]
- Monteiro da Silva, B.N.; Faria, A.R.; Souza, S.d.S.R.; Colodette, S.S.; Morais, J.M.; Teixeira, L.M.; Merquior, V.L.C. Expression of VanA-type vancomycin resistance in a clinical isolate of Enterococcus faecium showing insertion of IS19 in the vanS gene. Int. J. Antimicrob. Agents 2020, 55, 105897. [Google Scholar] [CrossRef]
- Kohler, V.; Vaishampayan, A.; Grohmann, E. Broad-host-range Inc18 plasmids: Occurrence, spread and transfer mechanisms. Plasmid 2018, 99, 11–21. [Google Scholar] [CrossRef]
- Rice, L.B. Antimicrobial stewardship and antimicrobial resistance. Med. Clin. N. Am. 2018, 102, 805–818. [Google Scholar] [CrossRef]
- Lemen, S.W.; Lewalter, K. Antibiotic stewardship and horizontal infection control are more effective than screening, isolation and eradication. Infection 2018, 46, 581–590. [Google Scholar] [CrossRef] [Green Version]
- Akpan, M.R.; Isemin, N.U.; Udoh, A.E.; Ashiru-Oredope, D. Implementation of antimicrobial stewardship programmes in African countries: A systematic literature review. J. Glob. Antimicrob. Resist. 2020, 22, 317–324. [Google Scholar] [CrossRef] [PubMed]
- EU. EU Guidelines for the Prudent Use of Antimicrobials Inhuman Health (2017/C 212/01). Available online: https://ec.europa.eu/health/amr/sites/amr/files/amr_guidelines_prudent_use_en.pdf (accessed on 14 July 2020).
- CDC. Core Elements of Hospital Antibiotic Stewardship Programs; US Department of Health and Human Services: Washington, DC, USA; CDC: Atlanta, GA, USA, 2019. Available online: https://www.cdc.gov/antibiotic-use/core-elements/hospital.html (accessed on 14 July 2020).
- Gao, L.; Hu, J.; Zhang, X.; Wei, L.; Li, S.; Miao, Z.; Chai, T. Application of swine manure on agricultural fields contributes to extended- spectrum ß-lactamase producing Escherichia coli spread in Tai’an, China. Front. Microbiol. 2015, 6, 313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manson, J.M.; Hancock, L.E.; Gilmore, M.S. Mechanism of chromosomal transfer of Enterococcus faecalis pathogenicity island, capsule, antimicrobial resistance, and other traits. Proc. Natl. Acad. Sci. USA 2010, 107, 12269–12274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breuer, R.J.; Hirt, H.; Dunny, G.M. Mechanistic features of the enterococcal pCF10 sex pheromone response and the biology of Enterococcus faecalis in its natural habitat. J. Bacteriol. 2018, 200, e00733-17. [Google Scholar] [CrossRef] [Green Version]
- Ch’ng, J.-H.; Chong, K.K.L.; Lam, L.N.; Wong, J.J.; Kline, K.A. Biofilm-associated infection by enterococci. Nat. Rev. Microbiol. 2019, 17, 82–94. [Google Scholar] [CrossRef]
- Fiore, E.; Van Tyne, D.; Gilmore, M.S. Pathogenicity of enterococci. Microbiol. Spectr. 2019, 7. [Google Scholar] [CrossRef]
- Guzman Prieto, A.M.; van Schaik, W.; Rogers, M.R.C.; Coque, T.M.; Baquero, F.; Corander, J.; Willems, R.J.L. Global emergence and dissemination of enterococci as nosocomial pathogens: Attack of the clones? Front. Microbiol. 2016, 7, 788. [Google Scholar] [CrossRef] [Green Version]
- Lee, T.; Pang, S.; Abraham, S.; Coombs, G.W. Antimicrobial-resistant CC17 Enterococcus faecium: The past, the present and the future. J. Glob. Antimicrob. Resist. 2019, 16, 36–47. [Google Scholar] [CrossRef] [Green Version]
- Ekwanzala, M.D.; Dewar, J.B.; Kamika, I.; Momba, M.N.B. Comparative genomics of vancomycin-resistant Enterococcus spp. revealed common resistome determinants from hospital wastewater to aquatic environments. Sci. Total Environ. 2020, 719, 137275. [Google Scholar] [CrossRef]
- Araújo, C.; Torres, C.; Gonçalves, A.; Carneiro, C.; López, M.; Radhouani, H.; Pardal, M.; Igrejas, G.; Poeta, P. Genetic detection and multilocus sequence typing of vanA-containing Enterococcus strains from mullets fish (Liza ramada). Microb. Drug Resist. 2011, 17, 357–361. [Google Scholar] [CrossRef]
- Araújo, C.; Torres, C.; Silva, N.; Carneiro, C.; Gonçalves, A.; Radhouani, H.; Correia, S.; Costa, P.M.; Pacheco, R.; Zarazaga, M.; et al. Vancomycin-resistant enterococci from Portuguese wastewater treatment plants. J. Basic Microbiol. 2010, 50, 605–609. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.; Poeta, P.; Silva, N.; Araújo, C.; López, M.; Ruiz, E.; Ulyiakina, I.; Direitinho, J.; Igrejas, G.; Torres, C. Characterization of vancomycin-resistant enterococci isolated from fecal samples of ostriches by molecular methods. Foodborne Pathog. Dis. 2010, 7, 1133–1136. [Google Scholar] [CrossRef] [PubMed]
- Moura, I.; Radhouani, H.; Torres, C.; Poeta, P.; Igrejas, G. Detection and genetic characterisation of vanA-containing Enterococcus strains in healthy Lusitano horses. Equine Vet. J. 2010, 42, 181–183. [Google Scholar] [CrossRef]
- Radhouani, H.; Igrejas, G.; Carvalho, C.; Pinto, L.; Gonçalves, A.; Lopez, M.; Sargo, R.; Cardoso, L.; Martinho, A.; Rego, V.; et al. Clonal lineages, antibiotic resistance and virulence factors in vancomycin-resistant enterococci isolated from fecal samples of red foxes (Vulpes vulpes). J. Wildl. Dis. 2011, 47, 769–773. [Google Scholar] [CrossRef] [PubMed]
- Braga, T.M.; Pomba, C.; Lopes, M.F.S. High-level vancomycin resistant Enterococcus faecium related to humans and pigs found in dust from pig breeding facilities. Vet. Microbiol. 2013, 161, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.; Igrejas, G.; Carvalho, I.; Peixoto, F.; Cardoso, L.; Pereira, J.E.; del Campo, R.; Poeta, P. Genetic characterization of vanA-Enterococcus faecium isolates from wild red-legged partridges in Portugal. Microb. Drug Resist. 2017, 24, 89–94. [Google Scholar] [CrossRef]
- Silva, V.; Peixoto, F.; Igrejas, G.; Parelho, C.; Garcia, P.; Carvalho, I.; Sousa, M.; Pereira, J.E.; Rodrigues, A.; Poeta, P. First report on vanA-Enterococcus faecalis recovered from soils subjected to long-term livestock agricultural practices in Azores Archipelago. Int. J. Environ. Res. 2018, 12, 39–44. [Google Scholar] [CrossRef]
- Aarestrup, F.M. Characterization of glycopeptide-resistant Enterococcus faecium (GRE) from broilers and pigs in Denmark: Genetic evidence that persistence of GRE in pig herds is associated with coselection by resistance to macrolides. J. Clin. Microbiol. 2000, 38, 2774–2777. [Google Scholar] [CrossRef] [Green Version]
- Sabença, C.; de Sousa, T.; Oliveira, S.; Viala, D.; Théron, L.; Chambon, C.; Hébraud, M.; Beyrouthy, R.; Bonnet, R.; Caniça, M. Next-generation sequencing and MALDI mass spectrometry in the study of multiresistant processed meat vancomycin-resistant enterococci (VRE). Biology (Basel) 2020, 9, 89. [Google Scholar] [CrossRef]
- Mazaheri Nezhad Fard, R.; Soltan Dallal, M.M.; Abbaspour, M.; Rajabi, Z. Study of VanA, B, C, D, E genes in vancomycin resistant enterococci isolated from retailed dried vegetables in Tehran, Iran. Int. J. Enteric. Pathog. 2019, 7, 9–14. [Google Scholar] [CrossRef]
- Jahansepas, A.; Sharifi, Y.; Aghazadeh, M.; Rezaee, M.A. Comparative analysis of Enterococcus faecalis and Enterococcus faecium strains isolated from clinical samples and traditional cheese types in the Northwest of Iran: Antimicrobial susceptibility and virulence traits. Arch. Microbiol. 2019, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Al-Shammary, A.H.A. Run-off patterns of vancomycin resistant enterococci (VRE clones) in cows raw milk and imported milk powders at Baghdad markets. Iraqi J. Vet. Med. (ISSN-P 1609-5693 ISSN-E 2410-7409) 2019, 43, 61–66. [Google Scholar] [CrossRef]
- Alonso, V.P.P.; Queiroz, M.M.; Gualberto, M.L.; Nascimento, M.S. Klebsiella pneumonia carbapenemase (KPC), methicillin-resistant Staphylococcus aureus (MRSA), and vancomycin-resistant Enterococcus spp. (VRE) in the food production chain and biofilm formation on abiotic surfaces. Curr. Opin. Food Sci. 2019, 26, 79–86. [Google Scholar] [CrossRef]
- Novais, C.; Coque, T.M.; Costa, M.J.; Sousa, J.C.; Baquero, F.; Peixe, L.V. High occurrence and persistence of antibiotic-resistant enterococci in poultry food samples in Portugal. J. Antimicrob. Chemother. 2005, 56, 1139–1143. [Google Scholar] [CrossRef] [PubMed]
- Freitas, A.R.; Novais, C.; Correia, R.; Monteiro, M.; Coque, T.M.; Peixe, L. Non-susceptibility to tigecycline in enterococci from hospitalized patients, food products and community sources. Int. J. Antimicrob. Agents 2011, 38, 174–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbosa, J.; Ferreira, V.; Teixeira, P. Antibiotic susceptibility of enterococci isolated from traditional fermented meat products. Food Microbiol. 2009, 26, 527–532. [Google Scholar] [CrossRef]
- Ribeiro, T.; Oliveira, M.; Fraqueza, M.J.; Lauková, A.; Elias, M.; Tenreiro, R.; Barreto, A.S.; Semedo-Lemsaddek, R. Antibiotic resistance and virulence factors among enterococci isolated from chouriço, a raditional Portuguese dry fermented sausage. J. Food Prot. 2011, 74, 465–469. [Google Scholar] [CrossRef] [Green Version]
- Domingos-Lopes, M.; Stanton, C.; Ross, P.R.; Dapkevicius, M.L.N.E.; Silva, C.C.G. Genetic diversity, safety and technological characterization of lactic acid bacteria isolated from artisanal Pico cheese. Food Microbiol. 2017, 63, 178–190. [Google Scholar] [CrossRef]
- Câmara, S.P.A.; Dapkevicius, A.; Silva, C.C.G.; Malcata, F.X.; Dapkevicius, M.L.N.E. Artisanal Pico cheese as reservoir of Enterococcus species possessing virulence and antibiotic resistance properties: Implications for food safety. Food Biotechnol. 2020, 34, 25–41. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos, S.; Silva, V.; Dapkevicius, M.d.L.E.; Igrejas, G.; Poeta, P. Enterococci, from Harmless Bacteria to a Pathogen. Microorganisms 2020, 8, 1118. https://doi.org/10.3390/microorganisms8081118
Ramos S, Silva V, Dapkevicius MdLE, Igrejas G, Poeta P. Enterococci, from Harmless Bacteria to a Pathogen. Microorganisms. 2020; 8(8):1118. https://doi.org/10.3390/microorganisms8081118
Chicago/Turabian StyleRamos, Sónia, Vanessa Silva, Maria de Lurdes Enes Dapkevicius, Gilberto Igrejas, and Patrícia Poeta. 2020. "Enterococci, from Harmless Bacteria to a Pathogen" Microorganisms 8, no. 8: 1118. https://doi.org/10.3390/microorganisms8081118
APA StyleRamos, S., Silva, V., Dapkevicius, M. d. L. E., Igrejas, G., & Poeta, P. (2020). Enterococci, from Harmless Bacteria to a Pathogen. Microorganisms, 8(8), 1118. https://doi.org/10.3390/microorganisms8081118