Assessing the Immunochromatographic Test Strip for Serological Detection of Bovine Babesiosis in Uganda
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Sample Size Estimation
2.3. Preparation of Parasites and B. bigemina RAP-1/CT17 and B. bovis SBP-4 Recombinant Proteins
2.4. Enzyme-Linked Immunosorbent Assay (ELISA)
2.5. Preparation of the Immunochromatographic Test (ICT) Strips
2.6. Evaluation of Performance of the ICT Strips
2.7. Statistical Analysis
2.8. Ethical Statement
3. Results
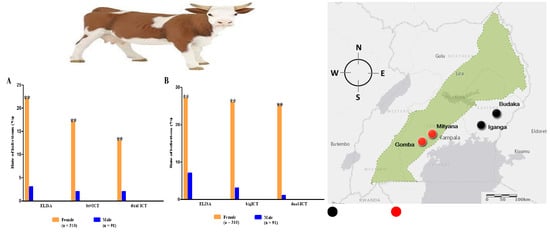
3.1. Seroprevalence of B. bigemina and B. bovis
3.2. Comparison between ELISA and ICT Depending on the Kappa Value
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ndambi, O.A.; Garcia, O.; Balikowa, D.; Kiconco, D.; Hemme, T.; Latacz-Lohmann, U. Milk production systems in Central Uganda: A farm economic analysis. Trop. Anim. Health Prod. 2008, 40, 269–279. [Google Scholar] [CrossRef]
- Balikowa, D. Dairy Development in Uganda: A Review of Uganda’s Dairy Industry; Dairy Development Authority: Kampala, Uganda, 2011. [Google Scholar]
- Ocaido, M.; Muwazi, R.; Opuda, J.A. Economic impact of ticks and tick-borne diseases on cattle production systems around Lake Mburo National Park in South Western Uganda. Trop. Anim. Health Prod. 2009, 41, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Tayebwa, D.S.; Vudriko, P.; Tuvshintulga, B.; Guswanto, A.; Nugraha, A.B.; Gantuya, S.; Batiha, G.E.-S.; Musinguzi, S.P.; Komugisha, M.; Bbira, J.S. Molecular epidemiology of Babesia species, Theileria parva, and Anaplasma marginale infecting cattle and the tick control malpractices in Central and Eastern Uganda. Ticks Tick Borne Dis. 2018, 9, 1475–1483. [Google Scholar] [CrossRef]
- Walker, A.R. Ticks of Domestic Animals in Africa: A Guide to Identification of Species; Bioscience Reports: Edinburgh, UK, 2003; Available online: http://www.biosciencereports.pwp.blueyonder.co.uk (accessed on 18 June 2020).
- Batiha, G.E.; Ali, H.; El-Mleeh, A.A.; Alsenosy, A.A.; Abdelsamei, E.K.; Abdel-Daim, M.M.; El-Sayed, Y.S.; Shaheen, H.M. In vitro study of ivermectin efficiency against the cattle tick, Rhipicephalus (Boophilus) annulatus, among cattle herds in El-Beheira, Egypt. Vet. World 2019, 12, 1319–1326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jongejan, F.; Uilenberg, G. The global importance of ticks. Parasitology 2004, 129, S3–S14. [Google Scholar] [CrossRef]
- Batiha, G.E.-S.; Beshbishy, A.M.; Tayebwa, D.S.; Shaheen, H.M.; Yokoyama, N.; Igarashi, I. Inhibitory effects of Syzygium aromaticum and Camellia sinensis methanolic extracts on the growth of Babesia and Theileria parasites. Ticks Tick Borne Dis. 2019, 10, 949–958. [Google Scholar] [CrossRef] [PubMed]
- Batiha, G.E.-S.; Magdy Beshbishy, A.; Adeyemi, O.S.; Nadwa, E.H.; Alkazmi, L.M.; Elkelish, A.A.; Igarashi, I. Phytochemical screening and antiprotozoal effects of the methanolic Berberis vulgaris and acetonic Rhus coriaria extracts. Molecules 2020, 25, 550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beshbishy, A.M.; Batiha, G.E.-S.; Alkazmi, L.; Nadwa, E.; Rashwan, E.; Abdeen, A.; Yokoyama, N.; Igarashi, I. Therapeutic effects of atranorin towards the proliferation of Babesia and Theileria parasites. Pathogens 2020, 9, 127. [Google Scholar] [CrossRef] [Green Version]
- Tanyel, E.; Guler, N.; Hokelek, M.; Ulger, F.; Sunbul, M. A case of severe babesiosis treated successfully with exchange transfusion. Int. J. Infect. Dis. 2015, 38, 83–85. [Google Scholar] [CrossRef] [Green Version]
- Batiha, G.E.-S.; Beshbishy, A.M.; Tayebwa, D.S.; Adeyemi, O.S.; Shaheen, H.; Yokoyama, N.; Igarashi, I. The effects of trans-chalcone and chalcone 4 hydrate on the growth of Babesia and Theileria. PLoS Negl. Trop. Dis. 2019, 13, e0007030. [Google Scholar] [CrossRef] [Green Version]
- Muhanguzi, D.; Matovu, E.; Waiswa, C. Prevalence and characterization of Theileria and Babesia species in cattle under different husbandry systems in western Uganda. Int. J. Anim. Vet. Adv. 2010, 2, 51–58. [Google Scholar]
- Beshbishy, A.M.; Batiha, G.E.-S.; Yokoyama, N.; Igarashi, I. Ellagic acid microspheres restrict the growth of Babesia and Theileria in vitro and Babesia microti in vivo. Parasites Vectors 2019, 12, 269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bock, R.; Jackson, L.; De Vos, A.; Jorgensen, W. Babesiosis of cattle. Parasitology 2004, 129, S247–S269. [Google Scholar] [CrossRef] [PubMed]
- El-Saber Batiha, G.; Magdy Beshbishy, A.; Stephen Adeyemi, O.; Nadwa, E.; Rashwan, E.; Yokoyama, N.; Igarashi, I. Safety and efficacy of hydroxyurea and eflornithine against most blood parasites Babesia and Theileria. PLoS ONE 2020, 15, e0228996. [Google Scholar] [CrossRef]
- Beshbishy, A.M.; Batiha, G.E.-S.; Adeyemi, O.S.; Yokoyama, N.; Igarashi, I. Inhibitory effects of methanolic Olea europaea and acetonic Acacia laeta on growth of Babesia and Theileria. Asian Pac. J. Trop. Med. 2019, 12, 425. [Google Scholar]
- Kivaria, F. The control of East Coast fever in Africa: A constant battle for impoverished dairy farmers. Vet. J. 2007, 174, 221–222. [Google Scholar] [CrossRef]
- Vudriko, P.; Okwee-Acai, J.; Tayebwa, D.S.; Byaruhanga, J.; Kakooza, S.; Wampande, E.; Omara, R.; Muhindo, J.B.; Tweyongyere, R.; Owiny, D.O. Emergence of multi-acaricide resistant Rhipicephalus ticks and its implication on chemical tick control in Uganda. Parasites Vectors 2016, 9, 4. [Google Scholar] [CrossRef]
- Nabukenya, I.; Rubaire-Akiiki, C.; Olila, D.; Ikwap, K.; Höglund, J. Ethnopharmacological practices by livestock farmers in Uganda: Survey experiences from Mpigi and Gulu districts. J. Ethnobiol. Ethnomed. 2014, 10, 9. [Google Scholar] [CrossRef] [Green Version]
- Batiha, G.E.; Beshbishy, A.M.; Tayebwa, D.S.; Adeyemi, O.S.; Yokoyama, N.; Igarashi, I. Anti-piroplasmic potential of the methanolic Peganum harmala seeds and ethanolic Artemisia absinthium leaf extracts. J. Protozool. Res. 2019, 29, 8–25. [Google Scholar]
- Al-Yousif, Y.; Anderson, J.; Chard-Bergstrom, C.; Kapil, S. Development, evaluation, and application of lateral-flow immunoassay (immunochromatography) for detection of rotavirus in bovine fecal samples. Clin. Diagn. Lab. Immunol. 2002, 9, 723–725. [Google Scholar] [CrossRef] [Green Version]
- Berens, S.J.; Brayton, K.A.; Molloy, J.B.; Bock, R.E.; Lew, A.E.; McElwain, T.F. Merozoite surface antigen 2 proteins of Babesia bovis vaccine breakthrough isolates contain a unique hypervariable region composed of degenerate repeats. Infect. Immun. 2005, 73, 7180–7189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, H.; Reckmann, I.; Hollingdale, M.R.; Bujard, H.; Robson, K.; Crisanti, A. Thrombospondin related anonymous protein (TRAP) of Plasmodium falciparum binds specifically to sulfated glycoconjugates and to HepG2 hepatoma cells suggesting a role for this molecule in sporozoite invasion of hepatocytes. EMBO J. 1993, 12, 2881–2889. [Google Scholar] [CrossRef] [PubMed]
- Norimine, J.; Suarez, C.E.; McElwain, T.F.; Florin-Christensen, M.; Brown, W.C. Immunodominant epitopes in Babesia bovis rhoptry-associated protein 1 that elicit memory CD4+-T-lymphocyte responses in B. bovis-immune individuals are located in the amino-terminal domain. Infect. Immun. 2002, 70, 2039–2048. [Google Scholar] [CrossRef] [Green Version]
- Ruef, B.J.; Dowling, S.C.; Conley, P.G.; Perryman, L.E.; Brown, W.C.; Jasmer, D.P.; Rice-Ficht, A.C. A unique Babesia bovis spherical body protein is conserved among geographic isolates and localizes to the infected erythrocyte membrane. Mol. Biochem. Parasitol. 2000, 105, 1–12. [Google Scholar] [CrossRef]
- Kim, C.-M.; Blanco, L.B.C.; Alhassan, A.; Iseki, H.; Yokoyama, N.; Xuan, X.; Igarashi, I. Development of a rapid immunochromatographic test for simultaneous serodiagnosis of bovine babesioses caused by Babesia bovis and Babesia bigemina. Am. J. Trop. Med. Hyg. 2008, 78, 117–121. [Google Scholar] [CrossRef]
- Ibrahim, H.M.; Moumouni, P.F.A.; Mohammed-Geba, K.; Sheir, S.K.; Hashem, I.S.; Cao, S.; Terkawi, M.A.; Kamyingkird, K.; Nishikawa, Y.; Suzuki, H. Molecular and serological prevalence of Babesia bigemina and Babesia bovis in cattle and water buffalos under small-scale dairy farming in Beheira and Faiyum Provinces, Egypt. Vet. Parasitol. 2013, 198, 187–192. [Google Scholar] [CrossRef]
- Li, Y.; Luo, Y.; Cao, S.; Terkawi, M.A.; Lan, D.; Long, P.T.; Yu, L.; Zhou, M.; Gong, H.; Zhang, H. Molecular and seroepidemiological survey of Babesia bovis and Babesia bigemina infections in cattle and water buffaloes in the central region of Vietnam. Trop. Biomed. 2014, 31, 406–413. [Google Scholar]
- Terkawi, M.A.; Huyen, N.X.; Shinuo, C.; Inpankaew, T.; Maklon, K.; Aboulaila, M.; Ueno, A.; Goo, Y.-K.; Yokoyama, N.; Jittapalapong, S. Molecular and serological prevalence of Babesia bovis and Babesia bigemina in water buffaloes in the northeast region of Thailand. Vet. Parasitol. 2011, 178, 201–207. [Google Scholar] [CrossRef]
- Guswanto, A.; Allamanda, P.; Mariamah, E.S.; Sodirun, S.; Wibowo, P.E.; Indrayani, L.; Nugroho, R.H.; Wirata, I.K.; Jannah, N.; Dias, L.P. Molecular and serological detection of bovine babesiosis in Indonesia. Parasites Vectors 2017, 10, 550. [Google Scholar] [CrossRef] [Green Version]
- Dohoo, I.R.; Martin, W.; Stryhn, H.E. Veterinary Epidemiologic Research; VER Inc.: Charlottetown, PE, Canada, 2003. [Google Scholar]
- Batiha, G.E.-S.; Beshbishy, A.M.; Alkazmi, L.; Adeyemi, O.S.; Nadwa, E.; Rashwan, E.; El-Mleeh, A.; Igarashi, I. Gas chromatography-mass spectrometry analysis, phytochemical screening and antiprotozoal effects of the methanolic Viola tricolor and acetonic Laurus nobilis extracts. BMC Complment. Med. Ther. 2020, 20, 1–14. [Google Scholar] [CrossRef]
- Fleiss, J.L.; Levin, B.; Paik, M.C. Statistical Methods for Rates and Proportions, 3rd ed.; Wiley-InterScience: Hoboken, NJ, USA, 2013. [Google Scholar]
- Schischke, A. Cross-Sectional Study of the Prevalence of Babesia Bigemina in Uganda. Bachelor’s Thesis, Swedish University of Agricultural Sciences, Uppsala, Sweden, 2015; p. 29. [Google Scholar]
- Kabi, F.; Magona, J.; Nasinyama, G.; Walubengo, J. Sero-prevalences of Tick-borne infections among the Nkedi Zebu and Ankole cattle in Soroti district, Uganda. J. Protozool. Res. 2008, 18, 61–70. [Google Scholar]
- Rubaire-Akiiki, C.; Okello-Onen, J.; Nasinyama, G.; Vaarst, M.; Kabagambe, E.; Mwayi, W.; Musunga, D.; Wandukwa, W. The prevalence of serum antibodies to tick-borne infections in Mbale District, Uganda: The effect of agro-ecological zone, grazing management and age of cattle. J. Insect Sci. 2004, 4, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barros, S.L.; Madruga, C.R.; Araújo, F.R.; Menk, C.F.; de Almeida, M.A.O.; Melo, E.P.; Kessler, R.H. Serological survey of Babesia bovis, Babesia bigemina, and Anaplasma marginale antibodies in cattle from the semi-arid region of the state of Bahia, Brazil, by enzyme-linked immunosorbent assays. Memórias do Instituto Oswaldo Cruz 2005, 100, 513–517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guswanto, A.; Allamanda, P.; Mariamah, E.S.; Munkjargal, T.; Tuvshintulga, B.; Takemae, H.; Sivakumar, T.; AbouLaila, M.; Terkawi, M.A.; Ichikawa-Seki, M. Evaluation of immunochromatographic test (ICT) strips for the serological detection of Babesia bovis and Babesia bigemina infection in cattle from Western Java, Indonesia. Vet. Parasitol. 2017, 239, 76–79. [Google Scholar] [CrossRef]
- Huang, X.; Xuan, X.; Hirata, H.; Yokoyama, N.; Xu, L.; Suzuki, N.; Igarashi, I. Rapid immunochromatographic test using recombinant SAG2 for detection of antibodies against Toxoplasma gondii in cats. J. Clin. Microbiol. 2004, 42, 351–353. [Google Scholar] [CrossRef] [Green Version]
- Gnoth, C.; Johnson, S. Strips of hope: Accuracy of home pregnancy tests and new developments. Geburtshilfe Frauenheilkd. 2014, 74, 661–669. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, K.; Yu, W.; Kelly, L.; Bermudez, R.; Renteria, T.; Dajer, A.; Gutierrez, E.; Williams, J.; Algire, J.; Torioni de Eschaide, S. Development of a lateral flow assay for rapid detection of bovine antibody to Anaplasma marg. J. Immunoass. Immunochem. 2007, 29, 10–18. [Google Scholar] [CrossRef]
- Muhanguzi, D.; Byaruhanga, J.; Amanyire, W.; Ndekezi, C.; Ochwo, S.; Nkamwesiga, J.; Mwiine, F.N.; Tweyongyere, R.; Fourie, J.; Madder, M. Invasive cattle ticks in East Africa: Morphological and molecular confirmation of the presence of Rhipicephalus microplus in South-Eastern Uganda. Parasites Vectors 2020, 13, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Ringo, A.E.; Moumouni, P.F.A.; Lee, S.-H.; Liu, M.; Khamis, Y.H.; Gao, Y.; Guo, H.; Zheng, W.; Efstratiou, A.; Galon, E.M. Molecular detection and characterization of tick-borne protozoan and rickettsial pathogens isolated from cattle on Pemba Island, Tanzania. Ticks Tick Borne Dis. 2018, 9, 1437–1445. [Google Scholar] [CrossRef]
- Woodford, J.; Jones, T.; Rae, P.; Boid, R.; Bell-Sakyi, L. Seroepidemiological studies of bovine babesiosis on Pemba Island, Tanzania. Vet. Parasitol. 1990, 37, 175–184. [Google Scholar] [CrossRef]
- Hinz, R.; Schwarz, N.G.; Hahn, A.; Frickmann, H. Serological approaches for the diagnosis of schistosomiasis—A review. Mol. Cell. Probes 2017, 31, 2–21. [Google Scholar] [CrossRef] [PubMed]
- Terkawi, M.A.; Kameyama, K.; Rasul, N.H.; Xuan, X.; Nishikawa, Y. Development of an immunochromatographic assay based on dense granule protein 7 for serological detection of Toxoplasma gondii infection. Clin. Vaccine Immunol. 2013, 20, 596–601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sivakumar, T.; Tuvshintulga, B.; Kothalawala, H.; Silva, S.S.P.; Lan, D.T.B.; Long, P.T.; Ybañez, A.P.; Ybañez, R.H.D.; Francisco Benitez, D.; Tayebwa, D.S. Host range and geographical distribution of Babesia sp. Mymensingh. Transbound. Emerg. Dis. 2020. [Google Scholar] [CrossRef] [PubMed]
| Parasite Species | bovICT/bigICT a | ELISA b | Dual-ICT b | ICT/ELISA c | PCR d | |||
|---|---|---|---|---|---|---|---|---|
| (+) | (-) | (+) | (-) | |||||
| B. bovis | ||||||||
| (+) | 17 (4.2%) | 3 (0.75%) | 14 (3.5%) | 13 (3.2%) | 4 (1%) | 39 (9.7%) | 0% | |
| (−) | 384 (95.8%) | 22 (5.5%) | 362 (90.3%) | 1 (0.2%) | 383 (95.5%) | 362 (90.3%) | ||
| Total | 401 | 25 (6.2%) | 376 (93.8%) | 14 (3.5%) | 387 (96.5%) | 401 | ||
| B. bigemina | ||||||||
| (+) | 27 (6.7%) | 8 (2%) | 19 (4.7%) | 23 (5.7%) | 4 (1%) | 53 (13.2%) | 13.6% | |
| (−) | 374 (93.3%) | 26 (6.5%) | 348 (86.8%) | 2 (0.5%) | 372 (92.8%) | 348 (86.6%) | ||
| Total | 401 | 34 (8.5%) | 367 (91.5%) | 25 (6.2%) | 376 (93.8%) | 401 | ||
| Sampling Location (District) | No. of Samples | No. of Positive (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| B. bovis | B. bigemina | Mixed Infection | ||||||||
| bovELISA | bovICT | Dual-ICT | bigELISA | bigICT | Dual-ICT | bov/big-ELISA | bov/big-ICT | Dual-ICT | ||
| Gomba | 105 | 19 (17.7%) | 7 (6.7%) | 6 (5.7%) | 11 (10.2%) | 13 (12.4%) | 13 (12.4%) | 3 (2.8%) | 1 (0.9%) | 2 (1.9%) |
| Mityana | 95 | 5 (5.2%) | 3 (3.1%) | 2 (2.1%) | 6 (6.3%) | 5 (5.3%) | 6 (6.3%) | 1 (1.1%) | 0 (0%) | 0 (0%) |
| Iganga | 100 | 1 (1%) | 2 (2%) | 2 (2%) | 6 (6%) | 2 (2%) | 3 (3%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Buddaka | 101 | 0 (0%) | 5 (5%) | 4 (3.9%) | 11 (10.6%) | 7 (6.7%) | 3 (2.9%) | 0 (0%) | 3 (2.9%) | 3 (2.9%) |
| Total | 401 | 25 (6.2%) | 17 (4.3%) | 14 (3.7%) | 34 (8.4%) | 27 (6.7%) | 25 (6.2%) | 4 (1%) | 4 (1%) | 5 (1.2%) |
| Diagnostic Methods | Kappa Value | 95% CI a | Agreement b |
|---|---|---|---|
| bovICT and bovELISA | 0.088 | 0.055 to 0.231 | Slight |
| dual-ICT and bovELISA | 0.115 | 0.042 to 0.272 | Slight |
| bovICT and dual-ICT | 0.816 | 0.672 to 0.960 | Very good |
| bigICT and bigELISA | 0.191 | 0.042 to 0.341 | Slight |
| dual-ICT and bigELISA | 0.173 | 0.024 to 0.322 | Slight |
| bigICT and dual-ICT | 0.863 | 0.764 to 0.963 | Very good |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stuart Tayebwa, D.; Magdy Beshbishy, A.; Batiha, G.E.-S.; Komugisha, M.; Joseph, B.; Vudriko, P.; Yahia, R.; Alkazmi, L.; Hetta, H.F.; Yokoyama, N.; et al. Assessing the Immunochromatographic Test Strip for Serological Detection of Bovine Babesiosis in Uganda. Microorganisms 2020, 8, 1110. https://doi.org/10.3390/microorganisms8081110
Stuart Tayebwa D, Magdy Beshbishy A, Batiha GE-S, Komugisha M, Joseph B, Vudriko P, Yahia R, Alkazmi L, Hetta HF, Yokoyama N, et al. Assessing the Immunochromatographic Test Strip for Serological Detection of Bovine Babesiosis in Uganda. Microorganisms. 2020; 8(8):1110. https://doi.org/10.3390/microorganisms8081110
Chicago/Turabian StyleStuart Tayebwa, Dickson, Amany Magdy Beshbishy, Gaber El-Saber Batiha, Mariam Komugisha, Byaruhanga Joseph, Patrick Vudriko, Ramadan Yahia, Luay Alkazmi, Helal F. Hetta, Naoaki Yokoyama, and et al. 2020. "Assessing the Immunochromatographic Test Strip for Serological Detection of Bovine Babesiosis in Uganda" Microorganisms 8, no. 8: 1110. https://doi.org/10.3390/microorganisms8081110
APA StyleStuart Tayebwa, D., Magdy Beshbishy, A., Batiha, G. E.-S., Komugisha, M., Joseph, B., Vudriko, P., Yahia, R., Alkazmi, L., Hetta, H. F., Yokoyama, N., & Igarashi, I. (2020). Assessing the Immunochromatographic Test Strip for Serological Detection of Bovine Babesiosis in Uganda. Microorganisms, 8(8), 1110. https://doi.org/10.3390/microorganisms8081110