Low-Fat and High-Quality Fermented Sausages
Abstract
1. Introduction
2. Materials and Methods
2.1. Lemon Albedo
2.2. Starter Culture
2.3. Protective Microbial Strain Selection
2.3.1. Lactiplantibacillus plantarum Producer Strains
2.3.2. Listeria Indicator Strains
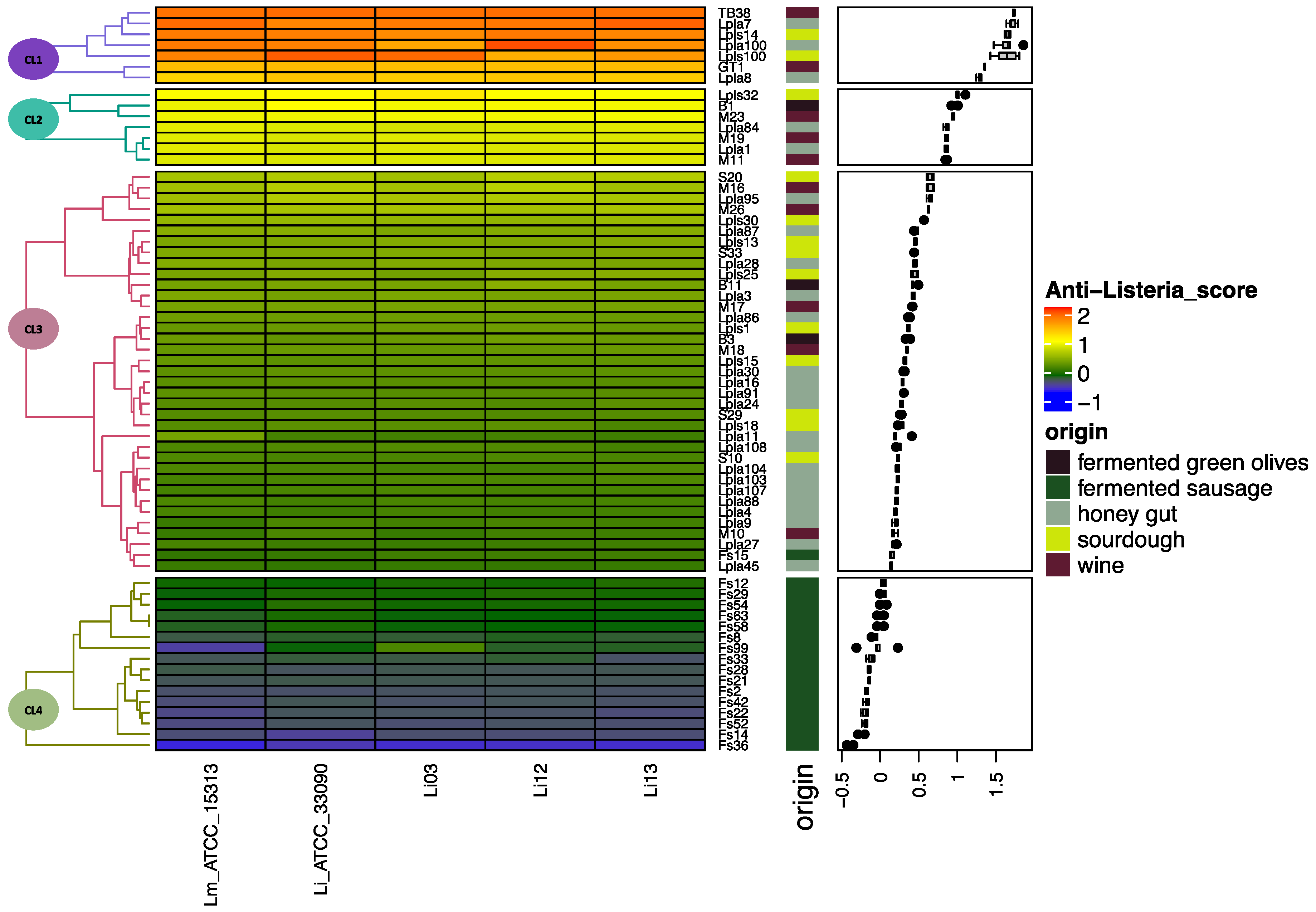
2.3.3. Screening of Anti-Listeria Activity of Lactiplantibacillus plantarum Strains
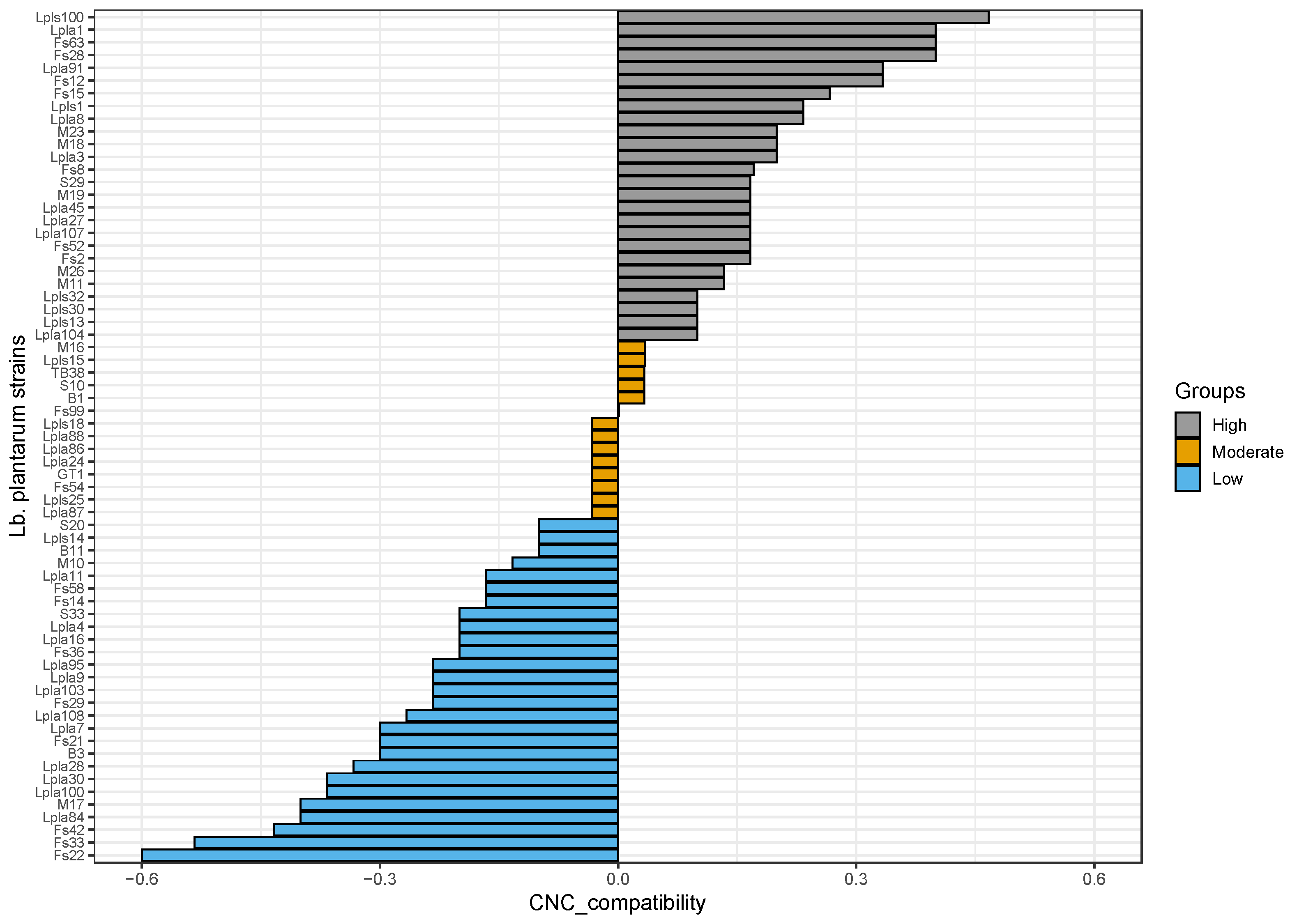
2.3.4. Detection of Interactions between Lactobacillus plantarum Strains and Conventional Starter Culture Strains
- -
- High compatibility: Indicator_compatibility ≥ 0.1
- -
- Moderate compatibility: −0.1 < Indicator_compatibility < 0.1
- -
- Low compatibility: Indicator_compatibility ≤ −0.1
2.4. Sausage Preparation
2.4.1. Conventional and Low-Fat Fermented Sausage
2.4.2. Inoculation of Salami with Listeria innocua
2.5. Physicochemical, Microbiological, and Sensory Analyses
2.5.1. Physicochemical and Microbiological Analyses
2.5.2. Listeria Detection
2.5.3. Fatty-Acid Determination of the Neutral Lipid Fraction
2.5.4. Sensory Analysis
2.6. Statistical Analysis
3. Results and Discussion
3.1. Anti-Listeria Lactiplantibacillus plantarum Strain Selection
3.1.1. Culture Starter Compatibility
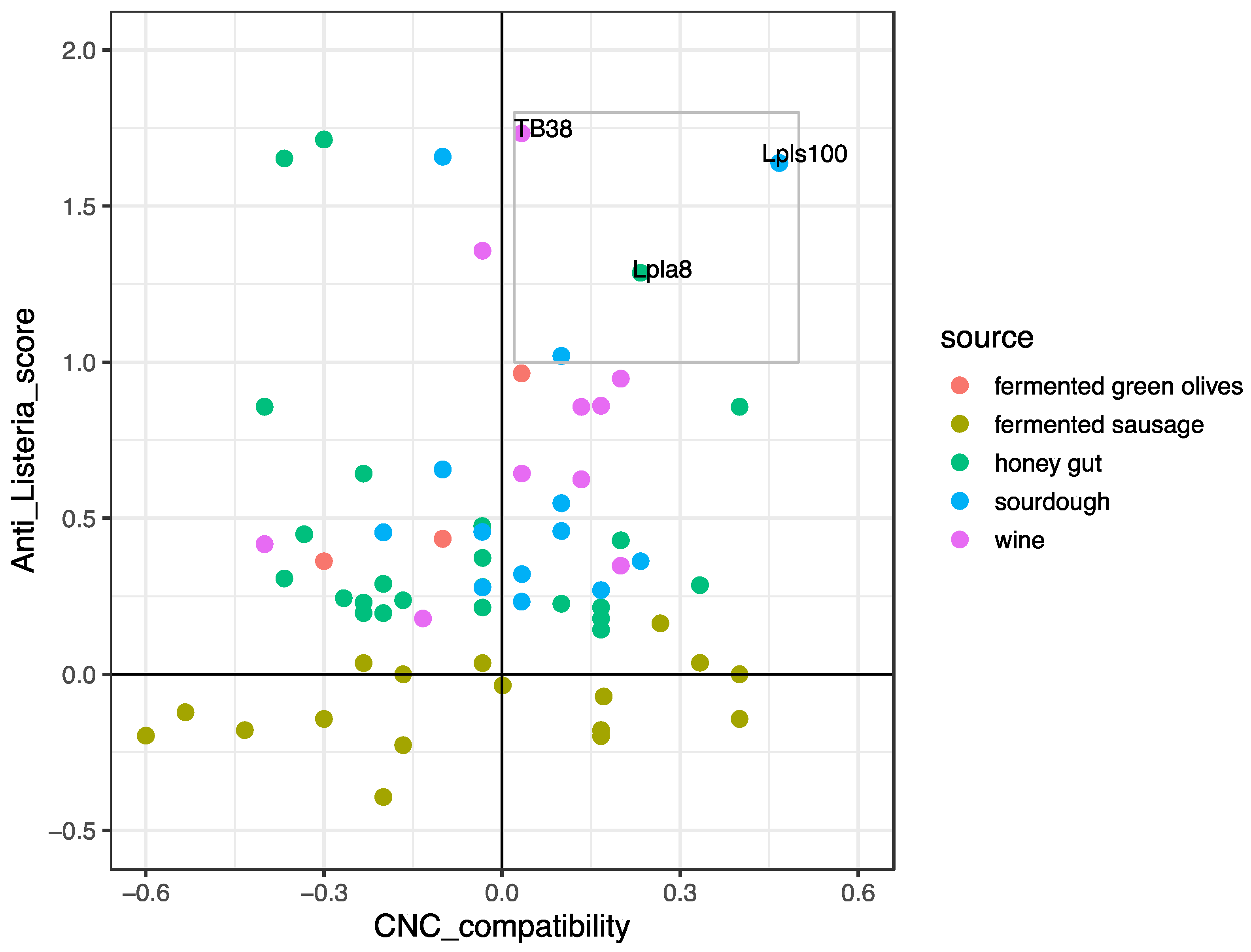
3.1.2. Protective Strain Choice
3.2. Quality Features of Low-Fat Fermented Sausages
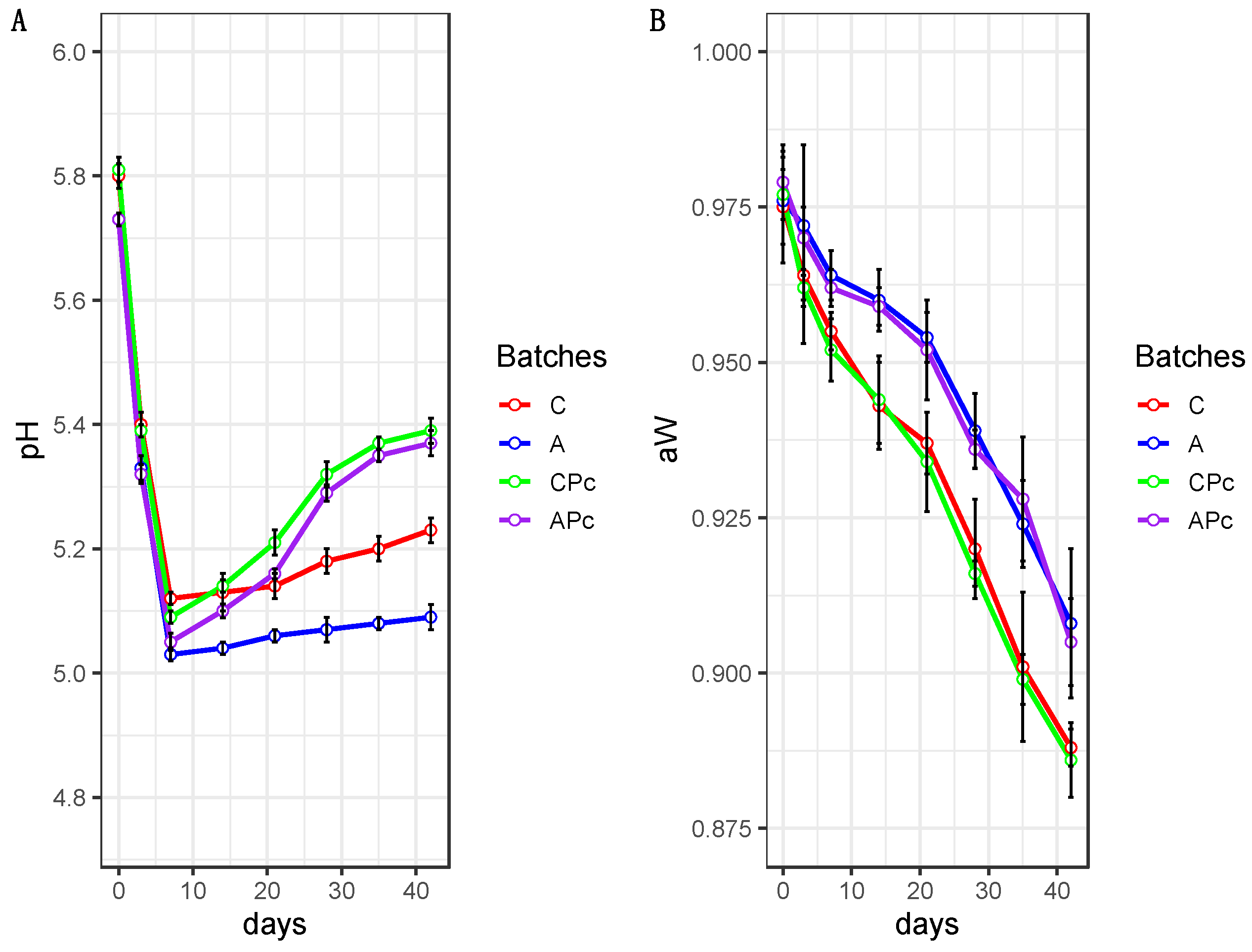
3.2.1. Physicochemical Features of Conventional and Low-Fat Sausages
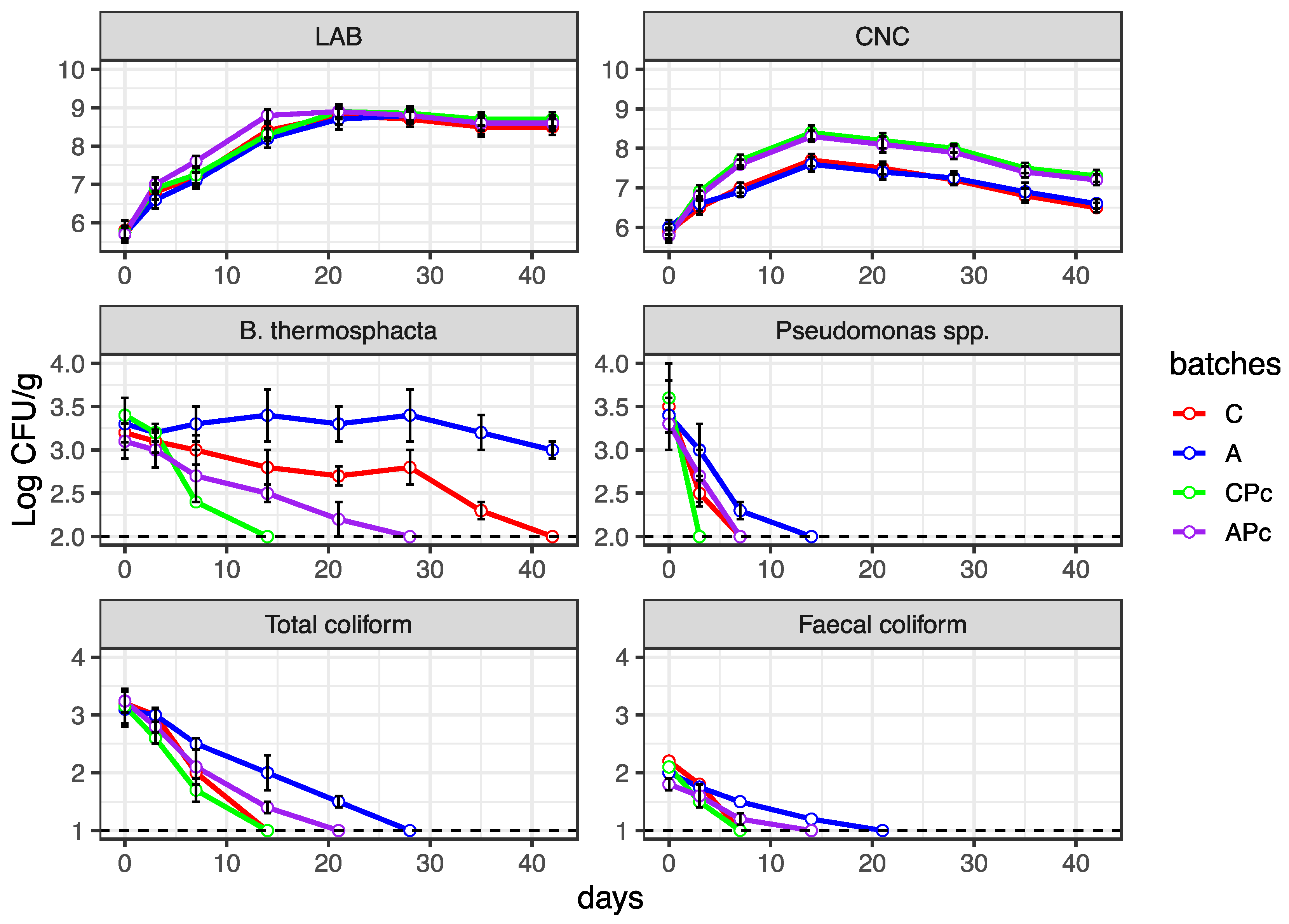
3.2.2. Microbiological Features of Conventional and Low-Fat sausages
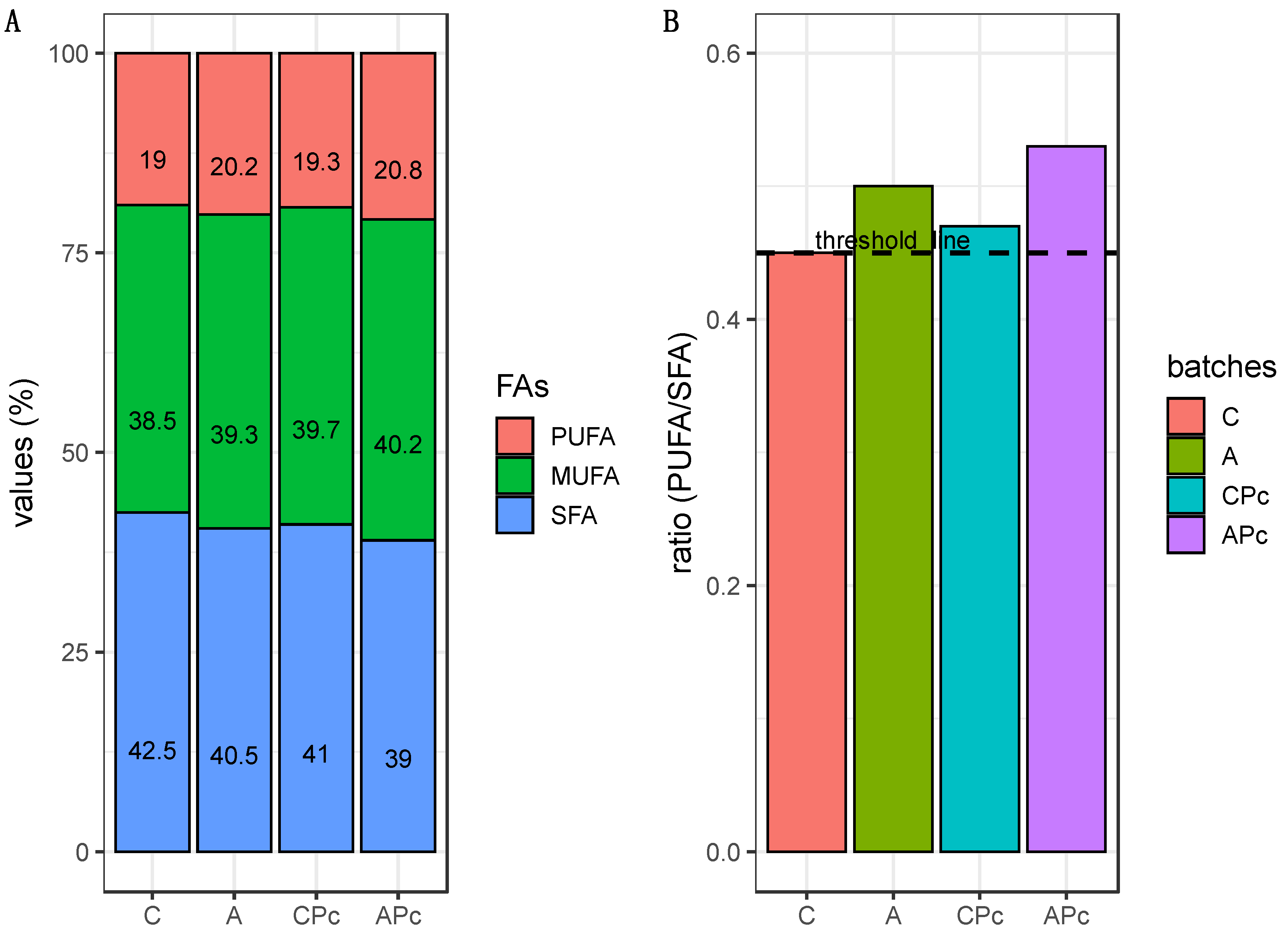
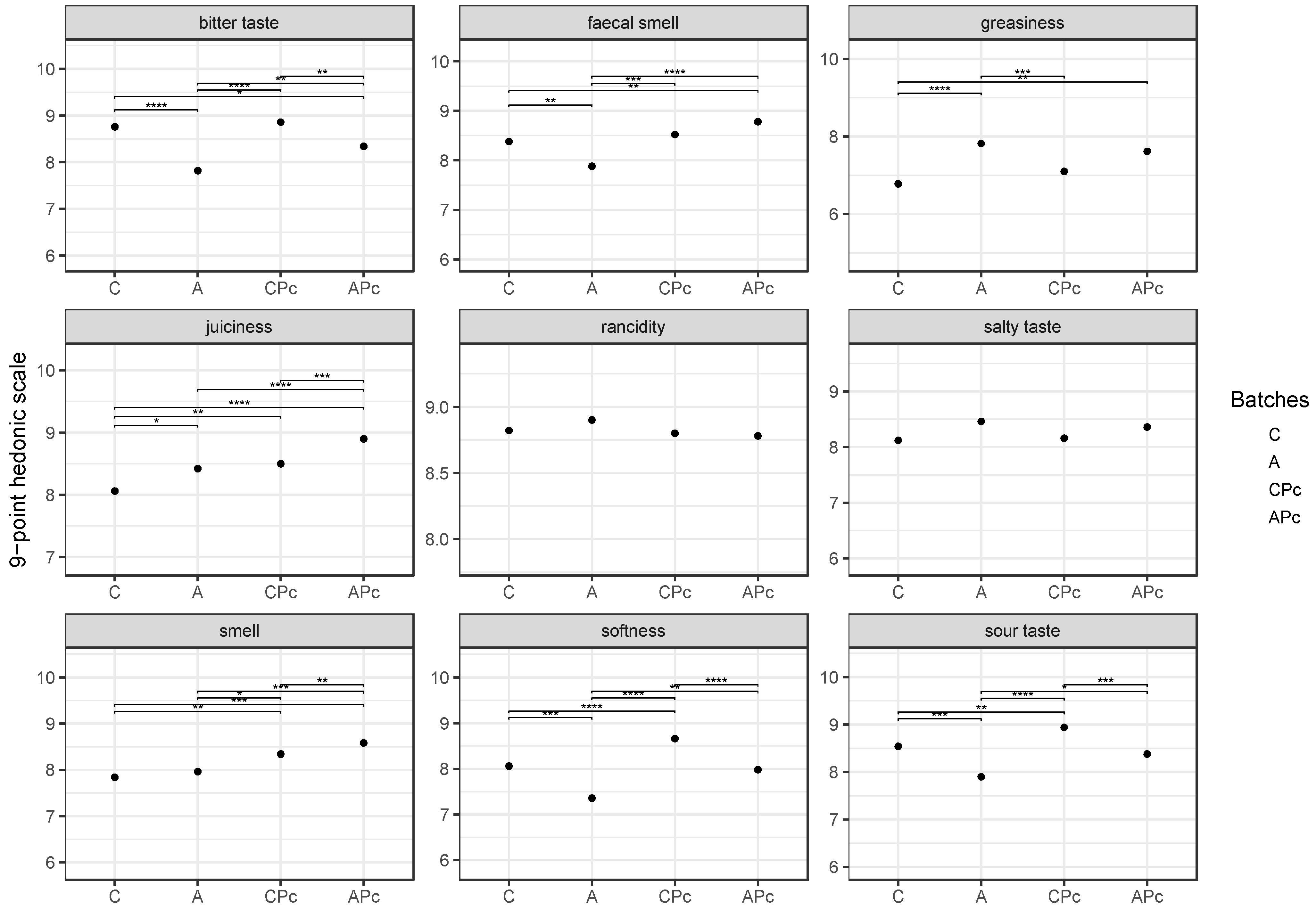
3.2.3. Final Fatty Acid Composition and Sensory Features
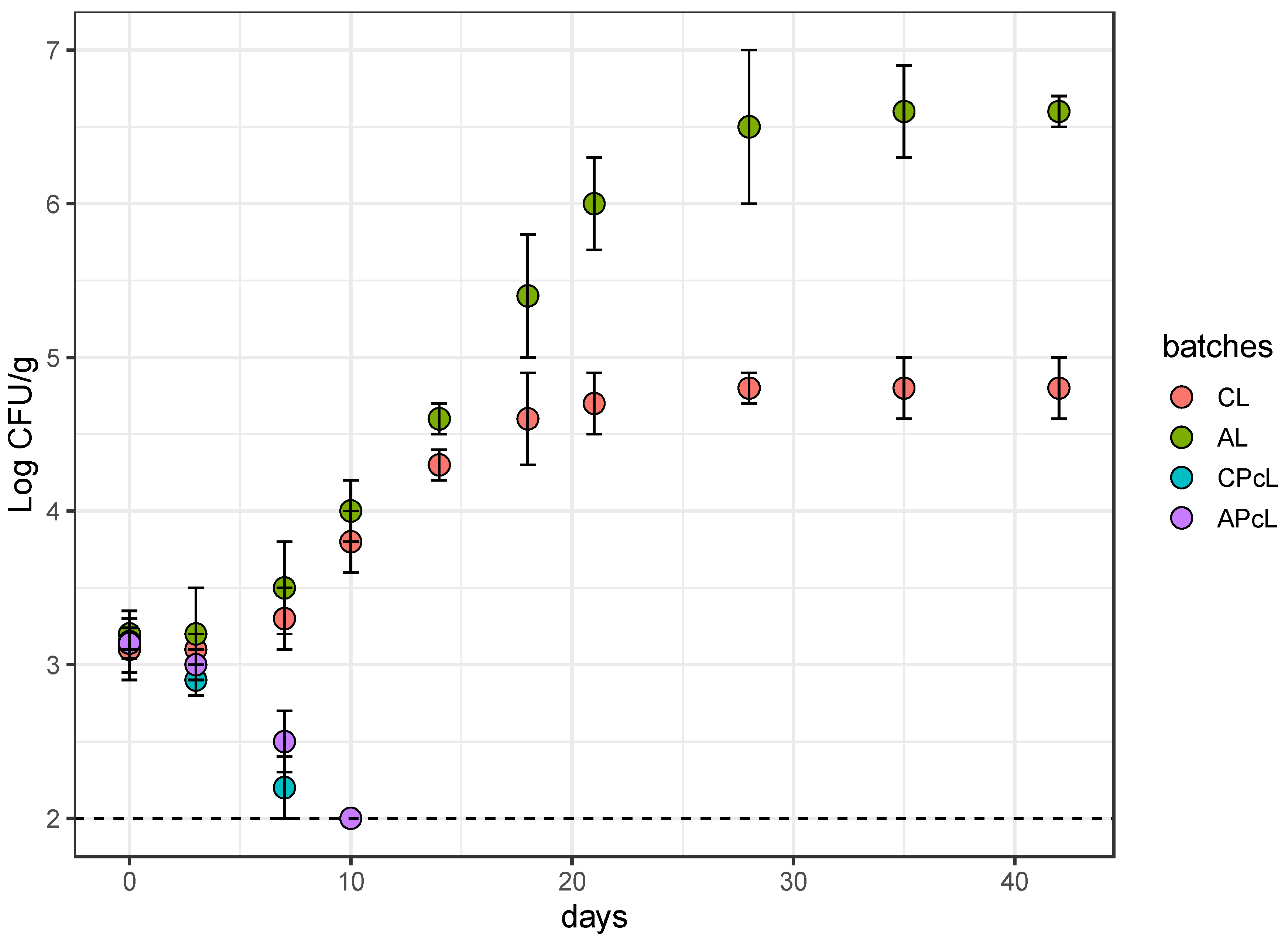
3.3. Anti-Listeria Action in Low-Fat Fermented Sausages
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Heileson, J.L. Dietary saturated fat and heart disease: A narrative review. Nutr. Rev. 2020, 78, 474–485. [Google Scholar] [CrossRef]
- World Health Organization. Thirteenth General Programme of Work (GPW13) 2019–2023; World Health Organization: Geneva, Switzeralnd, 2018. [Google Scholar]
- Siri-Tarino, P.W.; Sun, Q.; Hu, F.B.; Krauss, R.M. Meta-analysis of prospective cohort studies evaluating the association of saturated fat with cardiovascular disease. Am. J. Clin. Nutr. 2010, 91, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Ramsden, C.E.; Zamora, D.; Leelarthaepin, B.; Majchrzak-Hong, S.F.; Faurot, K.R.; Suchindran, C.M.; Ringel, A.; Davis, J.M.; Hibbeln, J.R.. Use of dietary linoleic acid for secondary prevention of coronary heart disease and death: Evaluation of recovered data from the Sydney Diet Heart Study and updated meta-analysis. BMJ 2013, 346, e8707. [Google Scholar] [CrossRef] [PubMed]
- Sacks, F.M.; Lichtenstein, A.H.; Wu, J.H.; Appel, L.J.; Creager, M.A.; Kris-Etherton, P.M.; Miller, M.; Rimm, E.B.; Rudel, L.L.; Robinson, J.G.; et al. Dietary fats and cardiovascular disease: A presidential advisory from the American Heart Association. Circulation 2017, 136, 1–23. [Google Scholar] [CrossRef]
- Hooper, L.; Martin, N.; Jimoh, O.F.; Kirk, C.; Foster, E.; Abdelhamid, A.S. Reduction in saturated fat intake for cardiovascular disease. Cochrane Database Syst. Rev. 2020. [Google Scholar] [CrossRef] [PubMed]
- WHO. Diet, Nutrition and the Prevention of Chronic Diseases: Report of a Joint WHO/FAO Expert Consultation; WHO Technical Report Series, No. 916; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- FAO. Fats and Fatty Acids in Human Nutrition: Report of an Expert Consultation; FAO Food and Nutrition Paper 91; Food and Agriculture Organization of the United Nations: Rome, Italy, 2010. [Google Scholar]
- Cengiz, E.; Gokoglu, N. Changes in energy and cholesterol contents of frankfurter-type sausages with fat reduction and fat replacer addition. Food Chem. 2005, 91, 443–447. [Google Scholar] [CrossRef]
- Vlassopoulos, A.; Masset, G.; Leroy, F.; Hoover, C.; Chesneau-Guillemont, C.; Leroy, F.; Lehmann, U.; Spieldenner, J.; Tee, E.-S.; Gibney, M.; et al. A nutrient profiling system for the (re)formulation of a global food & beverage portfolio. Eur. J. Nutr. 2017, 53, 1105–1122. [Google Scholar]
- Mendoza, E.; García, M.L.; Casas, C.; Seglas, M.D. Inulin as fat substitute in low fat, dry fermented sausages. Meat Sci. 2001, 57, 387–393. [Google Scholar] [CrossRef]
- Tremonte, P.; Gambacorta, G.; Pannella, G.; Trani, A.; Succi, M.; La Gatta, B.; Tipaldi, L.; Grazia, L.; Sorrentino, E.; Coppola, R.; et al. NaCl replacement with KCl affects lipolysis, microbiological and sensorial features of soppressata molisana. Eur. J. Lipid Sci. Technol. 2018, 120, 1700449. [Google Scholar] [CrossRef]
- Samapundo, S.; Xhaferi, R.; Szczcepaniak, S.; Goemare, O.; Steen, L.; Paelinck, H.; Devlieghere, F. The effect of water soluble fat replacers and fat reduction on the growth of Lactobacillus sakei and Listeria monocytogenes in broth and pork liver paté. LWT-Food Sci. Technol. 2015, 61, 316–321. [Google Scholar] [CrossRef]
- Muguerza, E.; Gimeno, O.; Ansorena, D.; Astiasarán, I. New formulations for healthier dry fermented sausages: A review. Trends Food Sci. Technol. 2004, 15, 452–457. [Google Scholar] [CrossRef]
- Jiménez-Colmenero, F.; Salcedo-Sandoval, L.; Bou, R.; Cofrades, S.; Herrero, A.M.; Ruiz-Capillas, C. Novel applications of oil-structuring methods as a strategy to improve the fat content of meat products. Trends Food Sci. Technol. 2015, 44, 177–188. [Google Scholar] [CrossRef]
- Yalınkılıç, B.; Kaban, G.; Kaya, M. The effects of different levels of orange fiber and fat on microbiological, physical, chemical and sensorial properties of sucuk. Food Microbiol. 2012, 29, 255–259. [Google Scholar] [CrossRef]
- Fernandez-Lopez, J.; Sendra, E.; Sayas-Barbera, E.; Navarro, C.; Perez-Alvarez, J.A. Physico-chemical and microbiological profiles of “salchichon” (Spanish dry-fermented sausage) enriched with orange fiber. Meat Sci. 2008, 80, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, H.; Czajkowska, K.; Cichowska, J.; Lenart, A. What’s new in biopotential of fruits and vegetable by-products applied in the food processing industry. Trends Food Sci. Technol. 2017, 67, 150–159. [Google Scholar] [CrossRef]
- Aleson-Carbonell, L.; Fernández-López, J.; Pérez-Alvarez, J.A.; Kuri, V. Functional and sensory effects of fibre-rich ingredients on breakfast fresh sausages manufacture. Food Sci. Technol. Int. 2005, 11, 89–97. [Google Scholar] [CrossRef]
- Calderón-Oliver, M.; López-Hernández, L.H. Food vegetable and fruit waste used in meat products. Food Rev. Int. 2020, 1–27. [Google Scholar] [CrossRef]
- Caggia, C.; Palmeri, R.; Russo, N.; Timpone, R.; Randazzo, C.L.; Todaro, A.; Barbagallo, S. Employ of citrus by-product as fat replacer ingredient for bakery confectionery products. Front. Nutr. 2020, 7, 46. [Google Scholar] [CrossRef]
- Mirabella, N.; Castellani, V.; Sala, S. Current options for the valorization of food manufacturing waste: A review. J. Clean. Prod. 2014, 65, 28–41. [Google Scholar] [CrossRef]
- Dhingra, D.; Michael, M.; Rajput, H.; Patil, R.T. Dietary fiber in foods: A review. J. Food Sci. Technol. 2011, 49, 255–266. [Google Scholar] [CrossRef]
- Lücke, F.K. Utilization of microbes to process and preserve meat. Meat Sci. 2000, 56, 105–115. [Google Scholar] [CrossRef]
- Iacumin, L.; Manzano, M.; Comi, G. Phage inactivation of Listeria monocytogenes on San Daniele dry-cured ham and elimination of biofilms from equipment and working environments. Microorganisms 2016, 4, 4. [Google Scholar] [CrossRef] [PubMed]
- Comi, G.; Cocolin, L.; Cantoni, C.; Manzano, M. A RE-PCR method to distinguish Listeria monocytogenes serovars. FEMS Immunol. Med. Microbiol. 1997, 18, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Comi, G.; Andyanto, D.; Manzano, M.; Iacumin, L. Lactococcus lactis and Lactobacillus sakei as bio-protective culture to eliminate Leuconostoc mesenteroides spoilage and improve the shelf life and sensorial characteristics of commercial cooked bacon. Food Microbiol. 2016, 58, 16–22. [Google Scholar] [CrossRef]
- Tremonte, P.; Succi, M.; Coppola, R.; Sorrentino, E.; Tipaldi, L.; Picariello, G.; Pannella, G.; Fraternali, F. Homology-based modeling of universal stress protein from Listeria innocua up-regulated under acid stress conditions. Front. Microbiol. 2016, 7, 1998. [Google Scholar] [CrossRef]
- Giello, M.; La Storia, A.; De Filippis, F.; Ercolini, D.; Villani, F. Impact of Lactobacillus curvatus 54M16 on microbiota composition and growth of Listeria monocytogenes in fermented sausages. Food Microbiol. 2018, 72, 1–15. [Google Scholar] [CrossRef]
- Sorrentino, E.; Succi, M.; Tipaldi, L.; Pannella, G.; Maiuro, L.; Sturchio, M.; Coppola, R.; Tremonte, P. Antimicrobial activity of gallic acid against food-related Pseudomonas strains and its use as biocontrol tool to improve the shelf life of fresh black truffles. Int. J. Food Microbiol. 2018, 266, 183–189. [Google Scholar] [CrossRef]
- Abdulhussain Kareem, R.; Razavi, S.H. Plantaricin bacteriocins: As safe alternative antimicrobial peptides in food preservation—A review. J. Food Safety 2020, 40, e12735. [Google Scholar] [CrossRef]
- Oliveira, M.; Ferreira, V.; Magalhães, R.; Teixeira, P. Biocontrol strategies for Mediterranean-style fermented sausages. Food Res. Int. 2018, 103, 438–449. [Google Scholar] [CrossRef]
- Fernandez-Gines, J.M.; Fernandez-Lopez, J.; Sayas-Barbera, E.; Sendra, E.; Perez- Alvarez, J.A. Effect of storage conditions on quality characteristics of Bologna sausages made with citrus fiber. J. Food Sci. 2003, 68, 710–715. [Google Scholar] [CrossRef]
- Di Luccia, A.; Tremonte, P.; Trani, A.; Loizzo, P.; La Gatta, B.; Succi, M.; Sorrentino, E.; Coppola, R. Influence of starter cultures and KCl on some biochemical, microbiological and sensory features of soppressata molisana, an Italian fermented sausage. Eur. Food Res. Technol. 2016, 242, 855–867. [Google Scholar] [CrossRef]
- Tremonte, P.; Reale, A.; Di Renzo, T.; Tipaldi, L.; Di Luccia, A.; Coppola, R.; Sorrentino, E.; Succi, M. Interactions between Lactobacillus sakei and CNC (Staphylococcus xylosus and Kocuria varians) and their influence on proteolytic activity. Lett. Appl. Microbiol. 2010, 51, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Tremonte, P.; Succi, M.; Reale, A.; Di Renzo, T.; Sorrentino, E.; Coppola, R. Interactions between strains of Staphylococcus xylosus and Kocuria varians isolated from fermented meats. J. Appl. Microbiol. 2007, 103, 743–751. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, S.J.; Pannella, G.; Iorizzo, M.; Testa, B.; Succi, M.; Tremonte, P.; Sorrentino, E.; Di Renzo, M.; Strollo, D.; Coppola, R. Inoculum strategies and performances of malolactic starter Lactobacillus plantarum M10: Impact on chemical and sensorial characteristics of Fiano Wine. Microorganisms 2020, 8, 516. [Google Scholar] [CrossRef]
- Succi, M.; Pannella, G.; Tremonte, P.; Tipaldi, L.; Coppola, R.; Iorizzo, M.; Lombardi, S.J.; Sorrentino, E. Sub-optimal pH preadaptation improves the survival of Lactobacillus plantarum strains and the malic acid consumption in wine-like medium. Front. Microbiol. 2017, 8, 470. [Google Scholar] [CrossRef]
- Tremonte, P.; Pannella, G.; Succi, M.; Tipaldi, L.; Sturchio, M.; Coppola, R.; Luongo, D.; Sorrentino, E. Antimicrobial activity of Lactobacillus plantarum strains isolated from different environments: A preliminary study. Int. Food Res. J. 2017, 21, 852–859. [Google Scholar]
- Tremonte, P.; Sorrentino, E.; Pannella, G.; Tipaldi, L.; Sturchio, M.; Masucci, A.; Maiuro, L.; Coppola, R.; Succi, M. Detection of different microenvironments and Lactobacillus sakei biotypes in Ventricina, a traditional fermented sausage from central Italy. Int. J. Food Microbiol. 2017, 242, 132–140. [Google Scholar] [CrossRef]
- De Leonardis, A.; Testa, B.; Macciola, V.; Lombardi, S.J.; Iorizzo, M. Exploring enzyme and microbial technology for the preparation of green table olives. Eur. Food Res. Technol. 2016, 242, 363–370. [Google Scholar] [CrossRef]
- Iorizzo, M.; Lombardi, S.J.; Macciola, V.; Testa, B.; Lustrato, G.; Lopez, F.; De Leonardis, A. Technological potential of Lactobacillus strains isolated from fermented green olives: In vitro studies with emphasis on oleuropein-degrading capability. Sci. World J. 2016. [Google Scholar] [CrossRef]
- Iorizzo, M.; Testa, B.; Lombardi, S.J.; García-Ruiz, A.; Muñoz-González, C.; Bartolomé, B.; Moreno-Arribas, M.V. Selection and technological potential of Lactobacillus plantarum bacteria suitable for wine malolactic fermentation and grape aroma release. LWT 2016, 73, 557–566. [Google Scholar] [CrossRef]
- Sorrentino, E.; Reale, A.; Tremonte, P.; Maiuro, L.; Succi, M.; Tipaldi, L.; Renzo, T.; Pannella, G.; Coppola, R. Lactobacillus plantarum 29 inhibits Penicillium spp. involved in the spoilage of black truffles (Tuber aestivum). J. Food Sci. 2013, 78, M1188–M1194. [Google Scholar] [CrossRef] [PubMed]
- Succi, M.; Tremonte, P.; Reale, A.; Sorrentino, E.; Coppola, R. Preservation by freezing of potentially probiotic strains of Lactobacillus rhamnosus. Ann. Microbiol. 2007, 57, 537–544. [Google Scholar] [CrossRef]
- Pannella, G.; Messia, M.C.; Tremonte, P.; Tipaldi, L.; La Gatta, B.; Lombardi, S.J.; Succi, M.; Marconi, E.; Coppola, R.; Sorrentino, E. Concerns and solutions for raw milk from vending machines. J. Food Process. Pres. 2019, 43, e14140. [Google Scholar] [CrossRef]
- Basso, A.L.; Picariello, G.; Coppola, R.; Tremonte, P.; Spagnamusso, S.; Di Luccia, A. Proteolytic activity of Lactobacillus sakei, Lactobacillus farciminis and Lactobacillus plantarum on sarcoplasmic proteins of pork lean. J. Food Biochem. 2004, 28, 195–212. [Google Scholar] [CrossRef]
- Patarata, L.; Novais, M.; Fraqueza, M.J.; Silva, J.A. Influence of Meat Spoilage Microbiota Initial Load on the Growth and Survival of Three Pathogens on a Naturally Fermented Sausage. Foods 2020, 9, 676. [Google Scholar] [CrossRef]
- Tremonte, P.; Sorrentino, E.; Succi, M.; Tipaldi, L.; Pannella, G.; Ibañez, E.; Mendiola, J.A.; Di Renzo, T.; Reale, A.; Coppola, R. Antimicrobial effect of Malpighia punicifolia and extension of water buffalo steak shelf-life. J. Food Sci. 2016, 81, M97–M105. [Google Scholar] [CrossRef]
- Tremonte, P.; Sorrentino, E.; Succi, M.; Reale, A.; Maiorano, G.; Coppola, R. Shelf life of fresh sausages stored under modified atmospheres. J. Food Prot. 2005, 68, 2686–2692. [Google Scholar] [CrossRef]
- Testa, B.; Lombardi, S.J.; Macciola, E.; Succi, M.; Tremonte, P.; Iorizzo, M. Efficacy of olive leaf extract (Olea europaea L. cv Gentile di Larino) in marinated anchovies (Engraulis encrasicolus, L.) process. Heliyon 2019, 5, e01727. [Google Scholar] [CrossRef]
- Testa, B.; Lombardi, S.J.; Tremonte, P.; Succi, M.; Tipaldi, L.; Pannella, G.; Sorrentino, E.; Iorizzo, M.; Coppola, R. Biodiversity of Lactobacillus plantarum from traditional Italian wines. World J. Microbiol. Biotechnol. 2014, 30, 2299–2305. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane-Stanley, G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar]
- International Organization for Standardization. ISO 8589: Sensory Analysis—General Guidance for the Design of Test Rooms; International Organization for Standardization: Geneva, Switzeralnd, 1998; pp. 1–9. [Google Scholar]
- Iorizzo, M.; Lombardi, S.J.; Ganassi, S.; Testa, B.; Ianiro, M.; Letizia, F.; Succi, M.; Tremonte, P.; Vergalito, F.; Cozzolino, A.; et al. Antagonistic Activity against Ascosphaera apis and Functional Properties of Lactobacillus kunkeei Strains. Antibiotics 2020, 9, 262. [Google Scholar] [CrossRef] [PubMed]
- Vinicius De Melo Pereira, G.; De Carvalho Neto, D.P.; Junqueira, A.C.D.O.; Karp, S.G.; Letti, L.A.; Magalhães Júnior, A.I.; Soccol, C.R. A review of selection criteria for starter culture development in the food fermentation industry. Food Rev. Int. 2020, 36, 135–167. [Google Scholar] [CrossRef]
- Laranjo, M.; Potes, M.E.; Elias, M. Role of starter cultures on the safety of fermented meat products. Front. Microbiol. 2019, 10, 853. [Google Scholar] [CrossRef] [PubMed]
- Zagorec, M.; Champomier-Vergès, M.C. Lactobacillus sakei: A starter for sausage fermentation, a protective culture for meat products. Microorganisms 2017, 5, 56. [Google Scholar] [CrossRef] [PubMed]
- Biscola, V.; Todorov, S.D.; Capuano, V.S.C.; Abriouel, H.; Gálvez, A.; Franco, B.D. Isolation and characterization of a nisin-like bacteriocin produced by a Lactococcus lactis strain isolated from charqui, a Brazilian fermented, salted and dried meat product. Meat Sci. 2013, 93, 607–613. [Google Scholar] [CrossRef] [PubMed]
- de Souza Barbosa, M.; Todorov, S.D.; Ivanova, I.; Chobert, J.M.; Haertlé, T.; De Melo Franco, B.D.G. Improving safety of salami by application of bacteriocins produced by an autochthonous Lactobacillus curvatus isolate. Food Microbiol. 2015, 46, 254–262. [Google Scholar] [CrossRef]
- Girardin, H.; Morris, C.E.; Albagnac, C.; Dreux, N.; Glaux, C.; Nguyen-The, C. Behaviour of the pathogen surrogates Listeria innocua and Clostridium sporogenes during production of parsley in fields fertilized with contaminated amendments. FEMS Microbiol. Ecol. 2005, 54, 287–295. [Google Scholar] [CrossRef]
- Castellano, P.; Pérez Ibarreche, M.; Blanco Massani, M.; Fontana, C.; Vignolo, G.M. Strategies for pathogen biocontrol using lactic acid bacteria and their metabolites: A focus on meat ecosystems and industrial environments. Microorganisms 2017, 5, 38. [Google Scholar] [CrossRef]
- Iacumin, L.; Osualdini, M.; Bovolenta, S.; Boscolo, D.; Chiesa, L.; Panseri, S.; Comi, G. Microbial, chemico-physical and volatile aromatic compounds characterization of Pitina PGI, a peculiar sausage-like product of North East Italy. Meat Sci. 2020, 163, 108081. [Google Scholar] [CrossRef]
- Mainar, M.S.; Stavropoulou, D.A.; Leroy, F. Exploring the metabolic heterogeneity of coagulase-negative staphylococci to improve the quality and safety of fermented meats: A review. Int. J. Food Microbiol. 2017, 247, 24–37. [Google Scholar] [CrossRef]
- Corbo, M.R.; Bevilacqua, A.; Speranza, B.; Gallo, M.; Campaniello, D.; Sinigaglia, M. Selection of wild lactic acid bacteria for sausages: Design of a selection protocol combining statistic tools, technological and functional properties. LWT-Food Sci. Technol. 2017, 81, 144–152. [Google Scholar] [CrossRef]
- Sorrentino, E.; Tremonte, P.; Capobianco, F.; Succi, M.; Reale, A.; Di Renzo, T.; Coppola, R. Rapporti di interazione tra microrganismi di interesse tecnologico isolati da soppressata molisana. Ind. Aliment. 2007, 46, 633–636. [Google Scholar]
- Urso, R.; Comi, G.; Cocolin, L. Ecology of lactic acid bacteria in Italian fermented sausages: Isolation, identification and molecular characterization. Syst. Appl. Microbiol. 2006, 29, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Cardinali, F.; Milanović, V.; Osimani, A.; Aquilanti, L.; Taccari, M.; Garofalo, C.; Polverigiani, S.; Clementi, F.; Franciosi, E.; Tuohy, K.; et al. Microbial dynamics of model Fabriano-like fermented sausages as affected by starter cultures, nitrates and nitrites. Int. J. Food Microbiol. 2018, 278, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Pasini, F.; Soglia, F.; Petracci, M.; Caboni, M.F.; Marziali, S.; Montanari, C.; Gardini, F.; Grazia, L.; Tabanelli, G. Effect of fermentation with different lactic acid bacteria starter cultures on biogenic amine content and ripening patterns in dry fermented sausages. Nutrients 2018, 10, 1497. [Google Scholar] [CrossRef] [PubMed]
- Coloretti, F.; Chiavari, C.; Poeta, A.; Succi, M.; Tremonte, P.; Grazia, L. Hidden sugars in the mixture: Effects on microbiota and the sensory characteristics of horse meat sausage. LWT-Food Sci. Technol. 2019, 106, 22–28. [Google Scholar] [CrossRef]
- Coksever, E.; Saricoban, C. Effects of bitter orange albedo addition on the quality characteristics of naturally fermented Turkish style sausages (sucuks). J. Food Agric. Environ. 2010, 8, 34–37. [Google Scholar]
- Aleson-Carbonell, L.; Fernández-López, J.; Sayas-Barberá, E.; Sendra, E.; Pérez-Alvarez, J.A. Utilization of lemon albedo in dry-cured sausages. J. Food Sci. 2003, 68, 1826–1830. [Google Scholar] [CrossRef]
- Pini, F.; Aquilani, C.; Giovannetti, L.; Viti, C.; Pugliese, C. Characterization of the microbial community composition in Italian Cinta Senese sausages dry-fermented with natural extracts as alternatives to sodium nitrite. Food Microbiol. 2020, 89, 103417. [Google Scholar] [CrossRef]
- Comi, G.; Muzzin, A.; Corazzin, M.; Iacumin, L. Lactic acid bacteria: Variability due to different pork breeds, breeding systems and fermented sausage production technology. Foods 2020, 9, 338. [Google Scholar] [CrossRef]
- Dias, I.; Laranjo, M.; Potes, M.E.; Agulheiro-Santos, A.C.; Ricardo-Rodrigues, S.; Fialho, A.R.; Véstia, J.; Fraqueza, M.J.; Oliveira, M.; Elias, M. Autochthonous starter cultures are able to reduce biogenic amines in a traditional portuguese smoked fermented sausage. Microorganisms 2020, 8, 686. [Google Scholar] [CrossRef] [PubMed]
- De Filippis, F.; La Storia, A.; Villani, F.; Ercolini, D. Exploring the sources of bacterial spoilers in beefsteaks by culture-independent high-throughput sequencing. PLoS ONE 2013, 8, e70222. [Google Scholar] [CrossRef] [PubMed]
- Stellato, G.; Utter, D.R.; Voorhis, A.; De Angelis, M.; Eren, A.M.; Ercolini, D. A few Pseudomonas oligotypes dominate in the meat and dairy processing environment. Front. Microbiol. 2017, 8, 264. [Google Scholar] [CrossRef] [PubMed]
- Bonomo, M.G.; Cafaro, C.; Salzano, G. Application of molecular methods in fermented meat microbiota: Biotechnological and food safety benefits. In Fermented Meat Products: Health Aspects, 1st ed.; Zdolec, N., Ed.; CRC Press: Boca Raton, FL, USA, 2016; pp. 167–195. [Google Scholar]
- Iacumin, L.; Manzano, M.; Panseri, S.; Chiesa, L.; Comi, G. A new cause of spoilage in goose sausages. Food Microbiol. 2016, 58, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Stanborough, T.; Fegan, N.; Powell, S.M.; Tamplin, M.; Chandry, P.S. Insight into the genome of Brochothrix thermosphacta, a problematic meat spoilage bacterium. Appl. Environ. Microbiol. 2017, 83, e02786-16. [Google Scholar] [CrossRef]
- Corral, S.; Salvador, A.; Flores, M. Salt reduction in slow fermented sausages affects the generation of aroma active compounds. Meat Sci. 2013, 93, 776–785. [Google Scholar] [CrossRef]
- Fonseca, S.; Gómez, M.; Domínguez, R.; Lorenzo, J.M. Physicochemical and sensory properties of Celta dry-ripened “salchichón” as affected by fat content. Grasas y Aceites 2015, 66, 059. [Google Scholar]
- Kumar, P.; Chatli, M.K.; Verma, A.K.; Mehta, N.; Malav, O.P.; Kumar, D.; Sharma, N. Quality, functionality, and shelf life of fermented meat and meat products: A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 2844–2856. [Google Scholar] [CrossRef]
- Wood, J.D.; Enser, M.; Fisher, A.V.; Nute, G.R.; Sheard, P.R.; Richardson, R.I.; Hughes, S.I.; Whittington, F.M. Fat deposition, fatty acid composition and meat quality: A review. Meat Sci. 2008, 78, 343–358. [Google Scholar] [CrossRef]
- Weiss, J.; Gibis, M.; Schuh, V.; Salminen, H. Advances in ingredient and processing systems for meat and meat products. Meat Sci. 2010, 86, 196–213. [Google Scholar] [CrossRef]
- Mataragas, M.; Bellio, A.; Rovetto, F.; Astegiano, S.; Decastelli, L.; Cocolin, L. Risk-based control of food-borne pathogens Listeria monocytogenes and Salmonella enterica in the Italian fermented sausages Cacciatore and Felino. Meat Sci. 2015, 103, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Gounadaki, A.; Skandamis, P.; Drosinos, E.H.; Nychas, G.J.E. Effect of packaging and storage temperature on the survival of Listeria monocytogenes inoculated postprocessing on sliced salami. J. Food Prot. 2007, 70, 2313–2320. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tremonte, P.; Pannella, G.; Lombardi, S.J.; Iorizzo, M.; Vergalito, F.; Cozzolino, A.; Maiuro, L.; Succi, M.; Sorrentino, E.; Coppola, R. Low-Fat and High-Quality Fermented Sausages. Microorganisms 2020, 8, 1025. https://doi.org/10.3390/microorganisms8071025
Tremonte P, Pannella G, Lombardi SJ, Iorizzo M, Vergalito F, Cozzolino A, Maiuro L, Succi M, Sorrentino E, Coppola R. Low-Fat and High-Quality Fermented Sausages. Microorganisms. 2020; 8(7):1025. https://doi.org/10.3390/microorganisms8071025
Chicago/Turabian StyleTremonte, Patrizio, Gianfranco Pannella, Silvia Jane Lombardi, Massimo Iorizzo, Franca Vergalito, Autilia Cozzolino, Lucia Maiuro, Mariantonietta Succi, Elena Sorrentino, and Raffaele Coppola. 2020. "Low-Fat and High-Quality Fermented Sausages" Microorganisms 8, no. 7: 1025. https://doi.org/10.3390/microorganisms8071025
APA StyleTremonte, P., Pannella, G., Lombardi, S. J., Iorizzo, M., Vergalito, F., Cozzolino, A., Maiuro, L., Succi, M., Sorrentino, E., & Coppola, R. (2020). Low-Fat and High-Quality Fermented Sausages. Microorganisms, 8(7), 1025. https://doi.org/10.3390/microorganisms8071025








