Listeria monocytogenes Survey in Cubed Cooked Ham Packaged in Modified Atmosphere and Bioprotective Effect of Selected Lactic Acid Bacteria
Abstract
1. Introduction
2. Materials and Methods
2.1. L. monocytogenes Monitoring in Cooked Cubed Ham Packages
2.2. Cooked Cubed Ham Package Preparation for a Bioprotective Study
2.3. Preparation of the Inoculum of Listeria monocytogenes and Bioprotective Starter Cultures
2.4. Inoculation Design
- (A)
- Control trial (not inoculated, NI);
- (B)
- Samples inoculated with the Sacco BOX-74 bioprotective starter culture and L. monocytogenes (BOX74 + LM);
- (C)
- Samples inoculated with only the Sacco BOX-74 bioprotective starter culture (BOX74);
- (D)
- Samples inoculated with only L. monocytogenes (LM);
- (E)
- Samples inoculated with the Sacco BOX-57 bioprotective starter culture and L. monocytogenes (BOX57 + LM);
- (F)
- Samples inoculated with only the Sacco BOX-57 bioprotective starter culture (BOX57).
2.5. Microbiological Analysis
2.6. Physicochemical Analysis
2.7. Sensory Analysis
2.8. Statistical Analysis
3. Results
3.1. Epidemiology of L. monocytogenes in Cooked Cubed Ham Packages
3.2. Physicochemical Characteristics of Cooked Cubed Ham
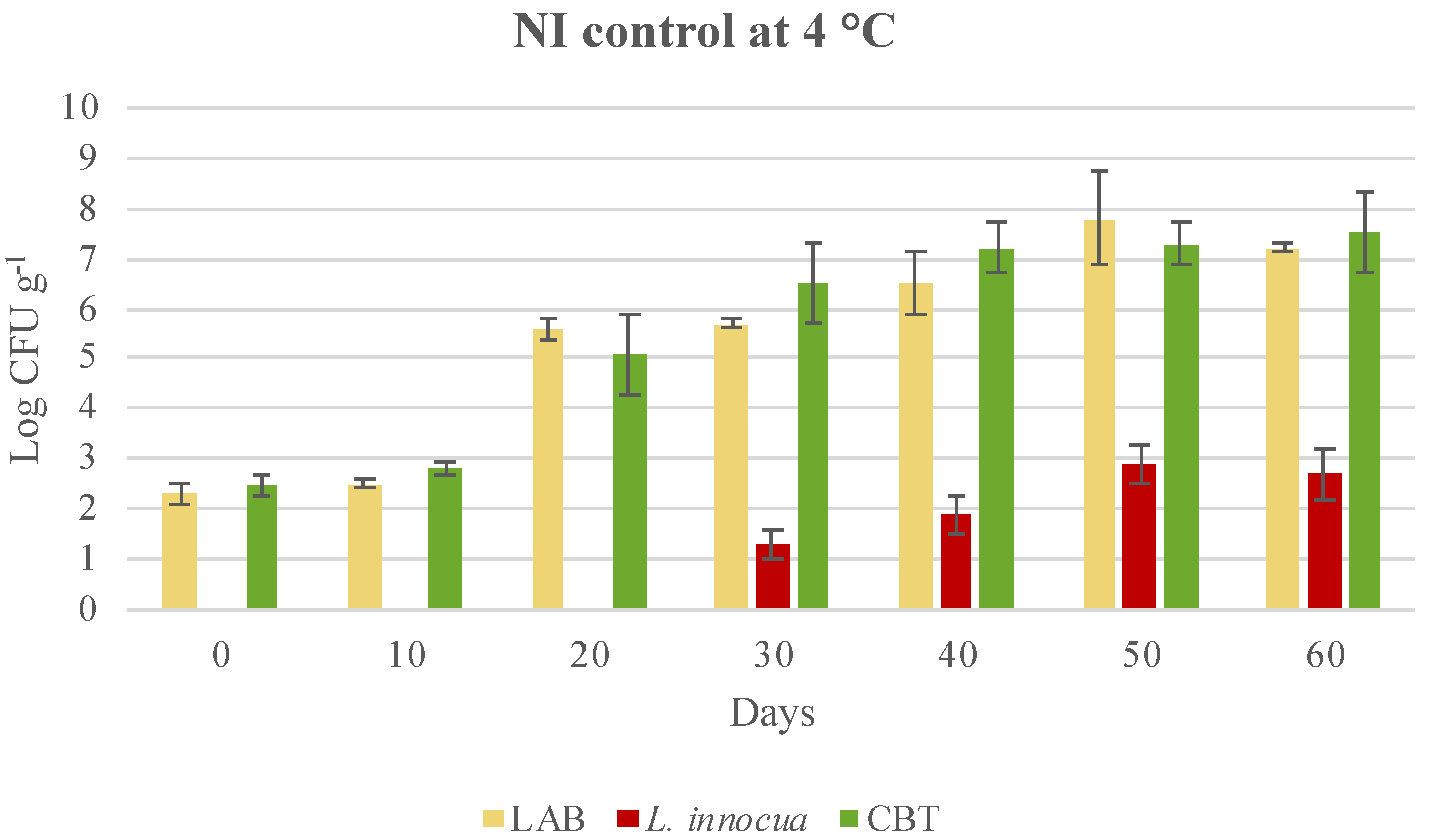
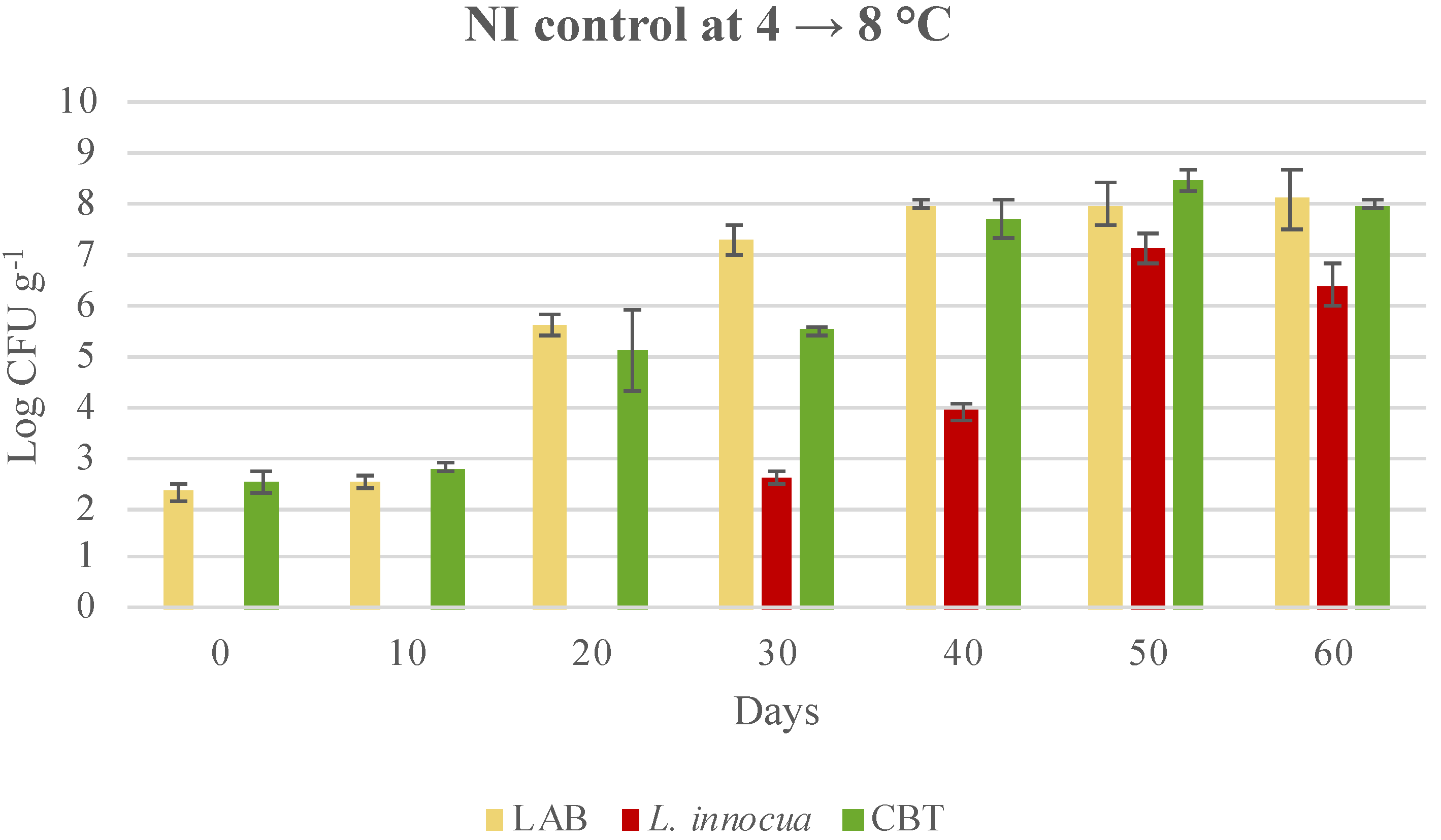
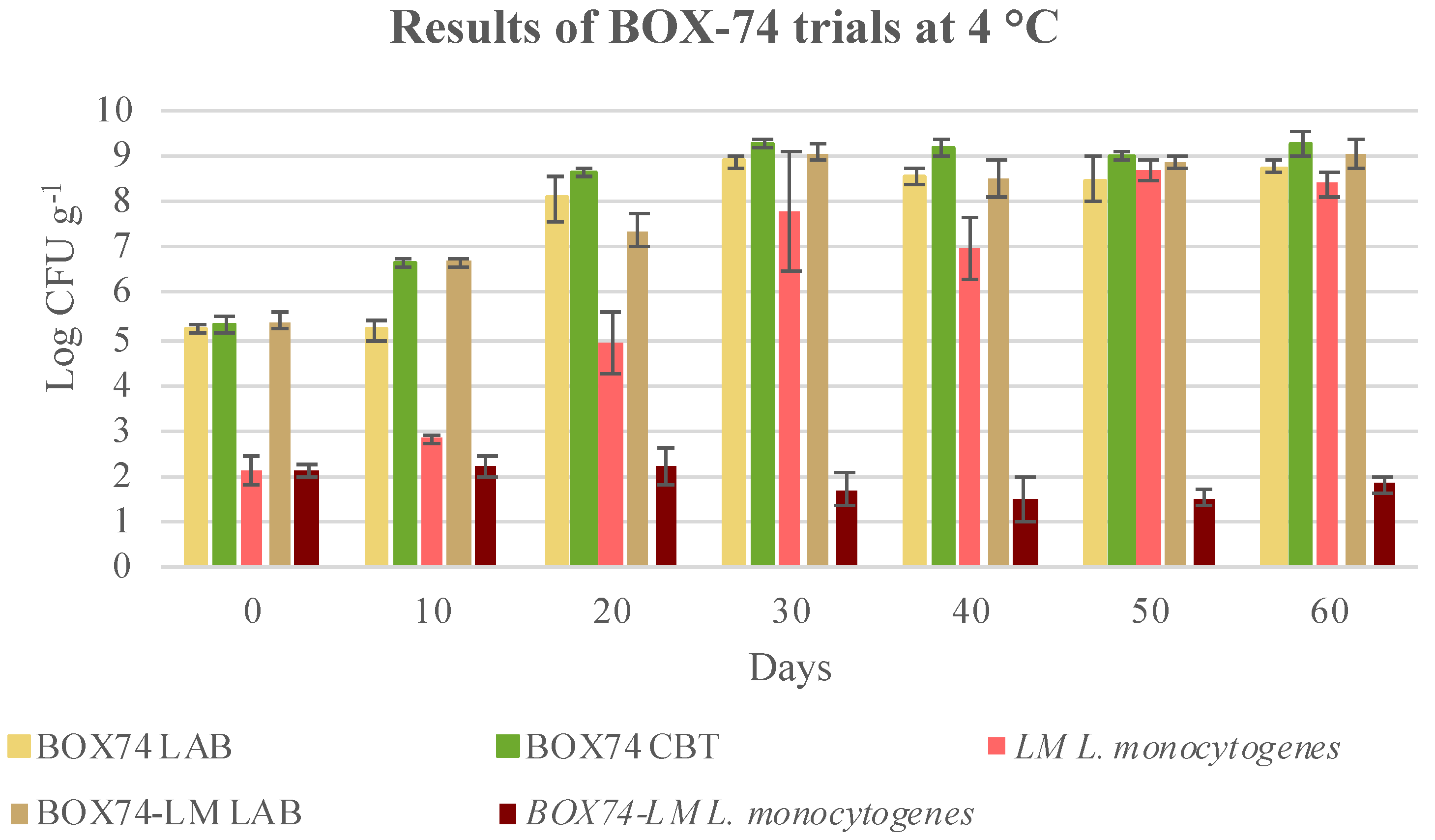
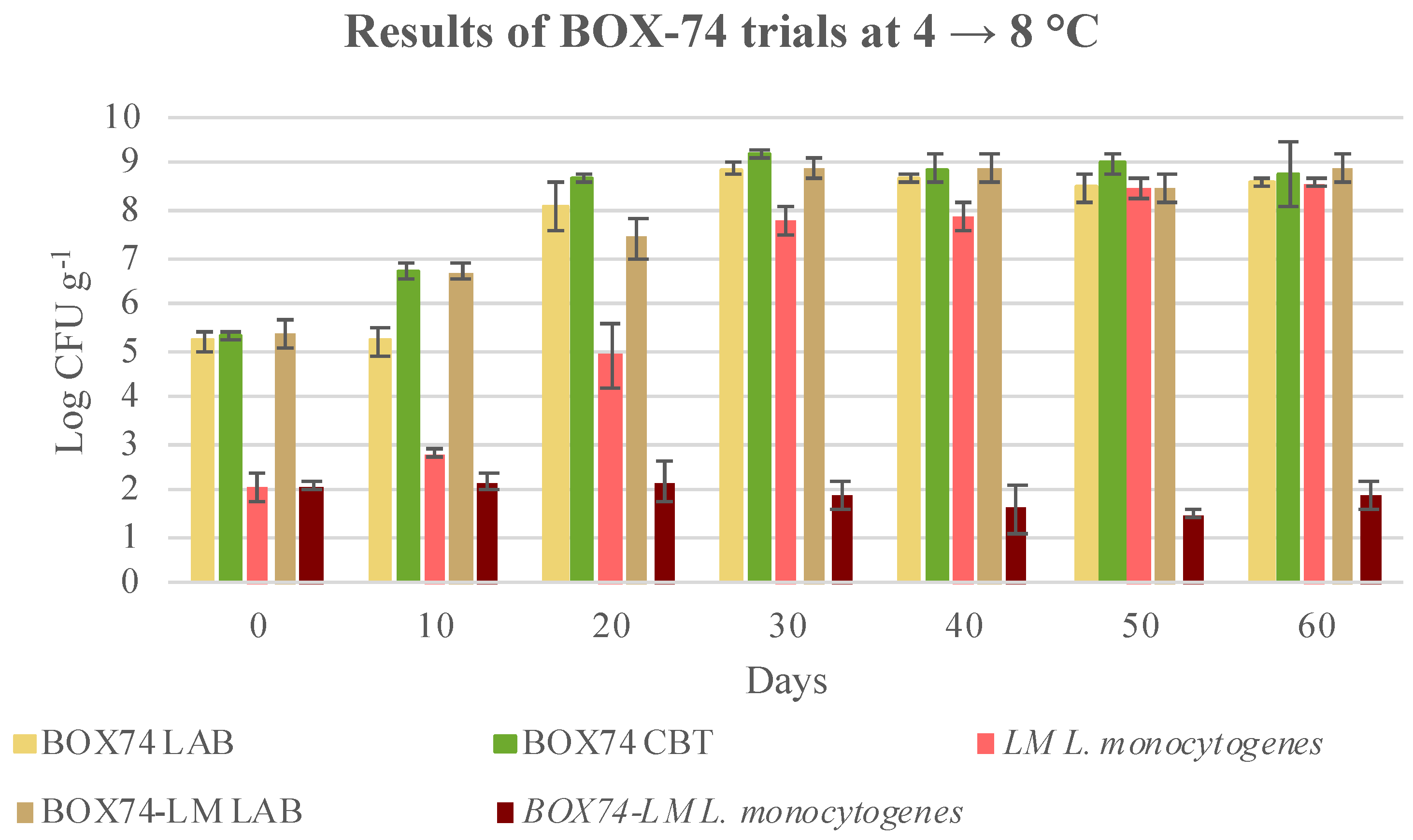
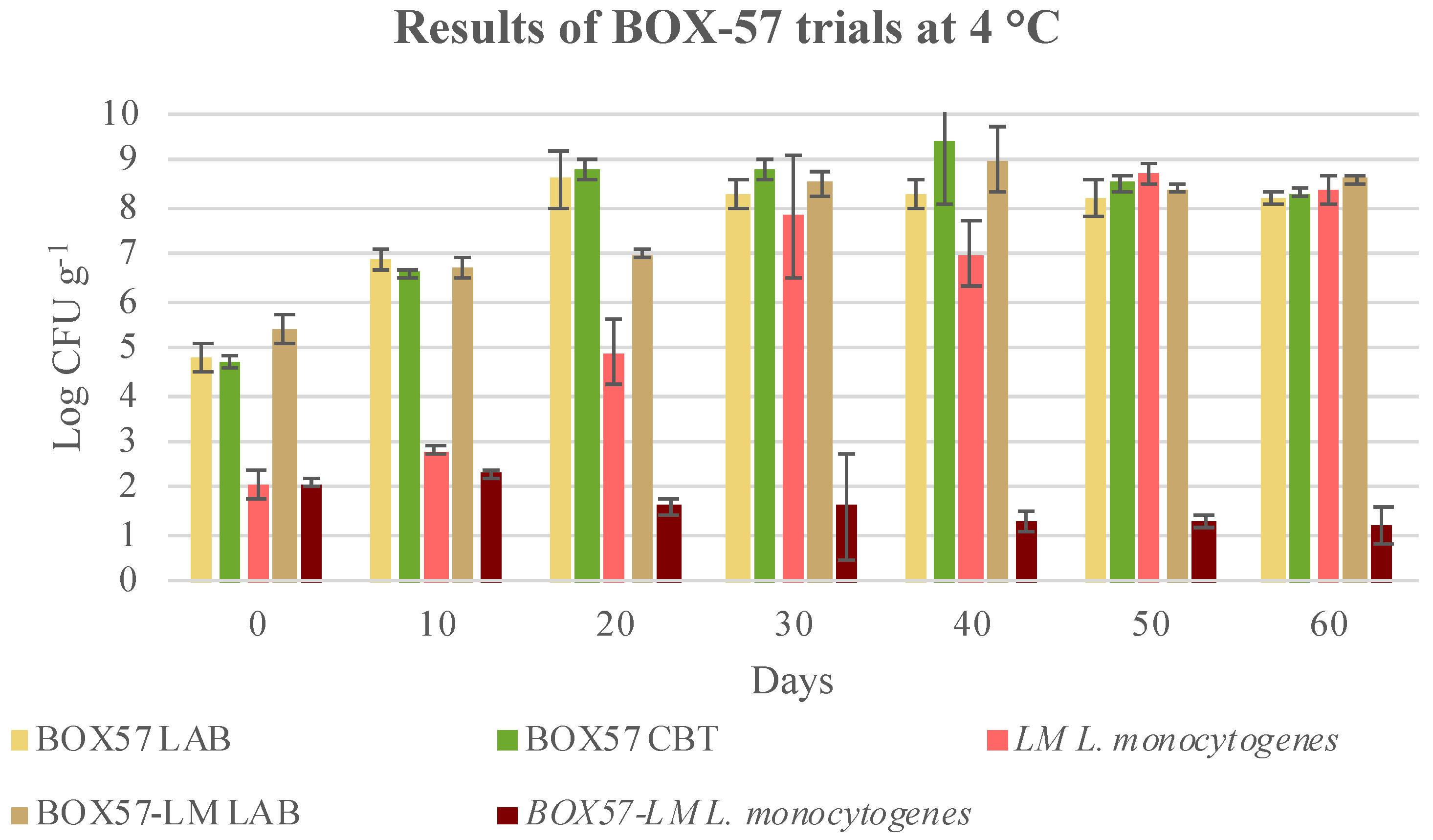
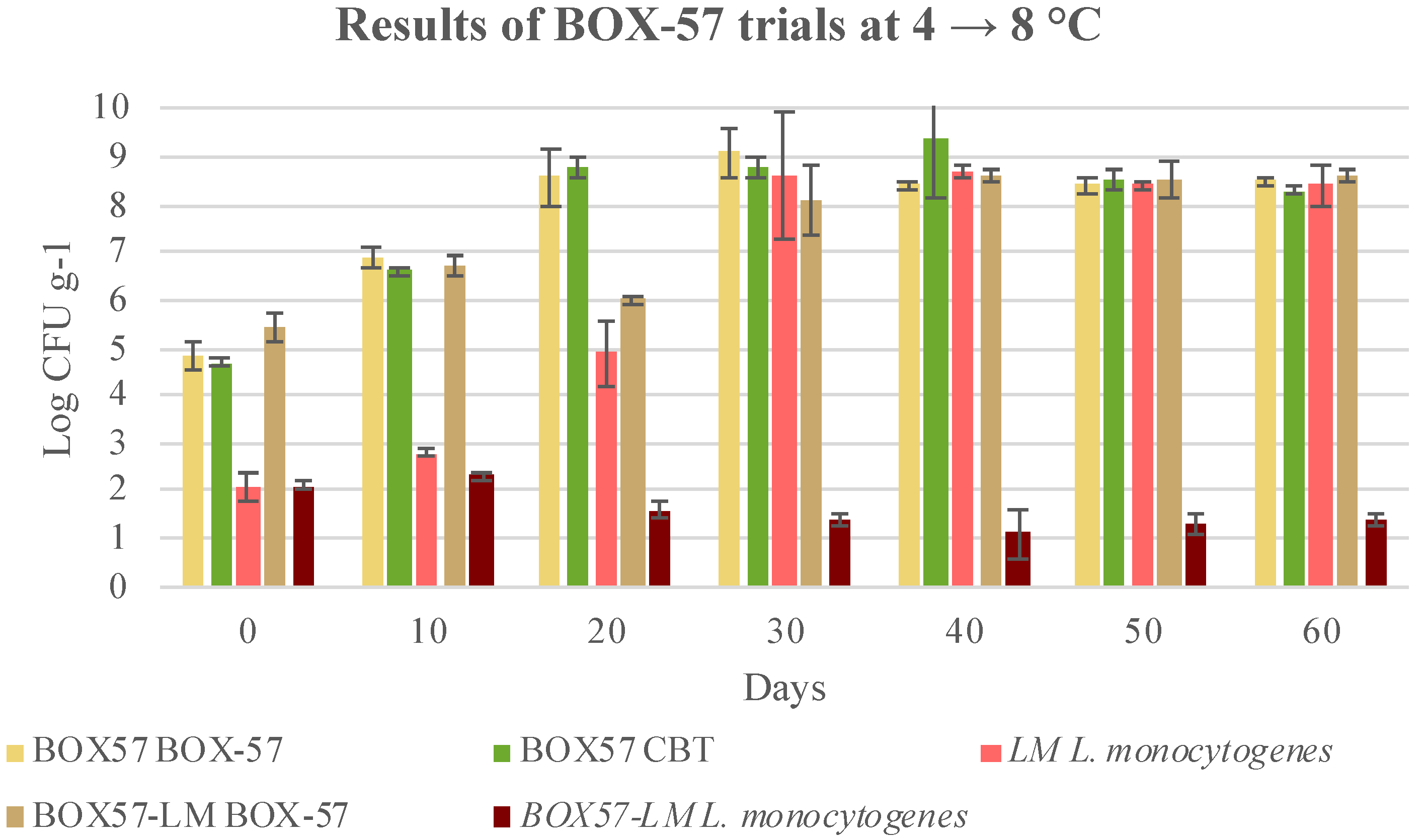
3.3. Microbial Evolution and Interaction between Bioprotective Culture and L. monocytogenes
3.4. Sensorial Analysis of Cubed Cooked Ham Samples Treated or not with BOX-74 and BOX-57 Starter Cultures
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Berger, S. Listeriosis: Global Status; GIDEON Informatics. Inc.: Los Angeles, CA, USA, 2016. [Google Scholar]
- Posfay-Barbe, K.M.; Wald, E.R. Listeriosis. Semin. Fetal Neonatal Med. 2009, 14, 228–233. [Google Scholar] [CrossRef]
- Szczawinński, J.; Szczawinńska, E.M.; Łobacz, A.; Tracz, M.; Jackowska-Tracz, A. Modelling the growth rate of Listeria monocytogenes in cooked ham stored at different temperatures. Vet. Res. 2017, 61, 45–51. [Google Scholar] [CrossRef]
- EFSA: European Food Safety Authority. Analysis of the baseline survey on the prevalence of Listeria monocytogenes in certain ready-to-eat (RTE) foods in the EU, 2010–2011 Part A: Listeria monocytogenes prevalence estimates. EFSA J. 2013, 11, 3241. [Google Scholar] [CrossRef]
- EFSA: European Food Safety Authority. The European Union Summary Report on Trends and Sources of Zoonoses, Zoonotic Agents and Food-borne Outbreaks in 2017. EFSA J. 2018, 16, 5500. [Google Scholar]
- Ahmed, O.; Pangloli, P.; Hwang, C.; Zivanovic, S.; Wu, T.; D’Souza, D.; Draughon, F.A. The occurrence of Listeria monocytogens in retail ready-to-eat meat and poultry products related to the levels of acetate and lactate in the products. Food Control 2015, 52, 43–48. [Google Scholar] [CrossRef]
- Angelidis, S.; Koutsoumanis, K. Prevalence and concentration of Listeria monocytogenes in sliced ready-to-eat meat products in the Hellenic retail market. J. Food Prot. 2006, 69, 938–942. [Google Scholar] [CrossRef] [PubMed]
- Dalgaard, P.; Jorgensen, L.V. Predicted and observed growth of Listeria monocytogenes in seafood challenge tests and in naturally contaminated cold-smoked salmon. Int. J. Food Microbiol. 1998, 40, 105–115. [Google Scholar] [CrossRef]
- Gómez, D.; Iguácel, L.P.; Rota, M.C.; Carramiñana, J.J.; Ariño, A.; Yangüela, J. Occurrence of Listeria monocytogenes in ready-to- eat meat products and meat processing plants in Spain. Foods 2015, 4, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Hoelzer, K.; Pouillot, R.; Gallagher, D.; Silverman, M.B.; Kause, J.; Dennis, S. Estimation of Listeria monocytogenes transfer coefficients and efficacy of bacterial removal through cleaning and sanitation. Int. J. Food Microbiol. 2012, 157, 267–277. [Google Scholar] [CrossRef]
- Kushwaha, K.; Muriana, P.M. Adherence characteristics of Listeria strains isolated from three ready-to-eat meat processing plants. J. Food Prot. 2009, 72, 2125–2131. [Google Scholar] [CrossRef]
- Lunden, J.M.; Miettinen, M.K.; Autio, T.J.; Korkeala, H.J. Persistent Listeria monocytogenes strains show enhanced adherence to food contact surface after short contact times. J. Food Protect. 2000, 63, 1204–1207. [Google Scholar] [CrossRef] [PubMed]
- Mafu, A.A.; Roy, D.; Goulet, J.; Savoie, L. Characterization of physic—Chemical forces involved in adhesion of Listeria monocytogenes to surfaces. Appl. Environ. Microbiol. 1991, 57, 1969–1973. [Google Scholar] [CrossRef] [PubMed]
- Cox, L.J.; Kleiss, T.; Cordier, J.L.; Cordellana, C.; Konkel, P.; Pedrazzini, C.; Beumer, R.; Siebenga, A. Listeria spp. in food processing, non-food and domestic environments. Food Microbiol. 1989, 6, 49–61. [Google Scholar] [CrossRef]
- Norwood, D.E.; Gilmour, A. Adherence of Listeria monocytogenes strains to stainless steel coupons. J. Appl. Microbiol. 1999, 86, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Ray, B.; Bhunia, A. Control by low pH and organic acids. In Fundamental Food Microbiology; CRC Press by Taylor & Francis Group: Boca Raton, FL, USA; London, UK; New York, NY, USA, 2008; pp. 394–410. [Google Scholar]
- Laguerre, O.; Derens, E.; Palagos, B. Study of domestic refrigerator temperature and analysis of factors affecting temperature: A French survey. Int. J. Refrig. 2002, 25, 653–659. [Google Scholar] [CrossRef]
- Likar, K.; Jevsnik, M. Cold chain maintaining in food trade. Food Control 2006, 17, 108–113. [Google Scholar] [CrossRef]
- Bortolussi, N.; Sponchiado, A.; Gottardo, F.; Iacumin, L.; Comi, G. Controllo e sopravvivenza di Listeria innocua in fase di cottura e pastorizzazione di prosciutti cotti. Ing. Aliment. 2010, 32, 27–34. [Google Scholar]
- Comi, G.; Ottaviani, S.; Patthey, C.; Mitri, E.; Menardi, G.; Andyanto, D.; Iacumin, L. Miglioramento della qualità di prosciutti cotti affettati e confezionati in MAP. Ing. Aliment. 2011, 12, 14–16. [Google Scholar]
- Comi, G.; Urso, R.; Vailati, E.; Ottaviani, S. Controllo e sopravvivenza di Listeria monocytogenes in fase di cottura e pastorizzazione di prosciutti cotti. Ind. Aliment. 2005, 446, 368–373. [Google Scholar]
- Aarnisalo, K.; Sheen, S.; Raaska, L.; Tamplin, M. Modelling transfer of Listeria monocytogenes during slicing of ‘gravad’ salmon. Int. J. Food Microbiol. 2007, 118, 76–78. [Google Scholar] [CrossRef]
- Chaitiemwong, H.; Hazeleger, W.C.; Beumer, R.R.; Zwietering, M.H. Quantification of transfer of Listeria monocytogenes between cooked ham and slicing machine surfaces. Food Control 2014, 44, 177–184. [Google Scholar] [CrossRef]
- Frustoli, M.A.; Cigarini, M.; Garritani, A.; Garulli, S.; Bovis, N.; Schivazzappa, C.; Barbuti, S. Fate of Listeria monocytogenes during shelf-life of pre-sliced bresaola packaged under modified atmosphere. Ind. Conserve 2007, 82, 325–330. [Google Scholar]
- Hoelzer, K.; Sauders, B.D.; Sanchez, M.D.; Olsen, P.T.; Pickett, M.M.; Mangione, K.J.; Rice, D.H.; Corby, J.; Stich, S.; Fortes, E.D.; et al. Prevalence, distribution, and diversity of Listeria monocytogenes in retail environments, focusing on small establishments and establishments with a history of failed inspections. J. Food Prot. 2011, 74, 1083–1095. [Google Scholar] [CrossRef] [PubMed]
- Little, C.L.; de Louvois, J. The microbiological examination of butchery products and butcheries’ premises in the United Kingdom. J. Appl. Microbiol. 1998, 85, 177–186. [Google Scholar] [CrossRef]
- Uyttendaele, M.; De Troy, P.; Debevere, J. Incidence of Listeria monocytogenes in different types of meat products on the Belgian retail market. Int. J. Food Microbiol. 1999, 53, 75–80. [Google Scholar] [CrossRef]
- Humphrey, T.J.; Worthington, D.M. Listeria monocytogenes of retail meat slicers. PHLS Microbiol. Dig. 1990, 7, 57. [Google Scholar]
- Hudson, J.A.; Mott, S.J. Presence of Listeria monocytogenes, motile aeromonads and Yersinia enterocolitica in environmental samples taken from a supermarket delicatessen. Int. J. Food Microbiol. 1993, 18, 333–337. [Google Scholar] [CrossRef]
- Pérez-Rodríguez, F.; Valero, A.; Carrasco, E.; García, R.M.; Zurera, G. Understanding and modelling bacterial transfer to foods: A review. Trends Food Sci. Technol. 2008, 19, 131–144. [Google Scholar] [CrossRef]
- Vorst, K.L.; Todd, E.C.D.; Ryser, E.T. Transfer of Listeria monocytogenes during mechanical slicing of turkey breast, bologna, and salami. J. Food Prot. 2006, 69, 619–626. [Google Scholar] [CrossRef]
- Sheen, S. Modelling surface transfer of Listeria monocytogenes on salami during slicing. J. Food Sci. 2008, 73, E304–E311. [Google Scholar] [CrossRef]
- Vorst, K.L.; Todd, E.C.D.; Ryser, E.T. Transfer of Listeria monocytogenes during slicing of turkey breast, bologna, and salami with simulated kitchen knives. J. Food Prot. 2006, 69, 2939–2946. [Google Scholar] [CrossRef] [PubMed]
- Montville, R.; Schaffner, D.W. Inoculum size influences bacterial cross contamination between surfaces. Appl. Environ. Microbiol. 2003, 69, 7188–7193. [Google Scholar] [CrossRef] [PubMed]
- Dickson, J.S. Transfer of Listeria monocytogenes and Salmonella typhimurium between beef tissue surfaces. J. Food Protect. 1990, 53, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Kusumaningrum, H.D.; Riboldi, G.; Hazeleger, W.C.; Beumer, R.R. Survival of foodborne pathogens on stainless steel surfaces and cross-contamination to foods. Int. J. Food Microbiol. 2003, 85, 227–236. [Google Scholar] [CrossRef]
- Lin, C.M.; Takeuchi, K.; Zhang, L.; Dohm, C.B.; Meyer, J.D.; Hall, P.A.; Doyle, M.P. Cross-contamination between processing equipment and deli meats by Listeria monocytogenes. J. Food Prot. 2006, 69, 71–79. [Google Scholar] [CrossRef]
- Atkins, A.G.; Xu, X. Slicing of soft flexible solids with industrial applications. Intern. J. Mech. Sci. 2005, 47, 479–492. [Google Scholar] [CrossRef]
- Dawson, P.; Han, I.; Cox, M.; Black, C.; Simmons, L. Residence time and food contact time effects on transfer of Salmonella Typhimurium from tile, wood and carpet: Testing the five-second rule. J. Appl. Microbiol. 2007, 102, 945–953. [Google Scholar] [CrossRef]
- Cao, Y.; Gu, W.; Zhang, J.; Chu, Y.; Ye, X.; Hu, Y.; Chen, J. Effects of chitosan, aqueous extract of ginger, onion and garlic on quality and shelf life of stewed-pork during refrigerated storage. Food Chem. 2013, 141, 1655–1660. [Google Scholar] [CrossRef]
- Jasour, M.S.; Ehsani, A.; Mehryar, L.; Naghibi, S.S. Chitosan coating incorporated with the lactoperoxidase system: An active edible coating for fish preservation. J. Sci. Food Agric. 2015, 95, 1373–1378. [Google Scholar] [CrossRef]
- Luo, W.; Lin, H.S.; Ren, F.; Wu, L.; Chen, L.; Sun, Y. Antioxidant and antimicrobial capacity of Chinese medicinal herb extracts in raw sheep meat. J. Food Prot. 2007, 70, 1440–1445. [Google Scholar] [CrossRef]
- Pattanayaiying, R.; Kittikun, A.H.; Cutter, C.N. Incorporation of nisin Z and lauric arginate into pullulan films to inhibit foodborne pathogens associated with fresh and ready-to-eat muscle foods. Int. J. Food Microbiol. 2015, 207, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Zhang, H.; Zhou, H.; Junhua, J.; Xie, Y. Synergistic Effect of Plantaricin BM-1 Combined with Physicochemical Treatments on the Control of Listeria monocytogenes in Cooked Ham. J. Food Prot. 2017, 80, 976–981. [Google Scholar] [CrossRef]
- Woraprayote, W.; Malila, W.Y.; Sorapukdee, S.; Swetwiwathana, A.; Benjakul, S.; Visessanguan, W. Bacteriocins from lactic acid bacteria and their applications in meat and meat products. Meat Sci. 2016, 120, 118–132. [Google Scholar] [CrossRef] [PubMed]
- Gàlvez, A.; Abriouel, H.; Lòpez, R.L.; Ben Omar, N. Bacteriocin-based strategies for food biopreservation. Int. J. Food Microbiol. 2007, 120, 51–70. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Xie, Y.; Liu, H.; Jin, J.; Duan, H.; Zhang, H. Effects of two application methods of plantaricin BM-1 on control of Listeria monocytogenes and background spoilage bacteria in sliced vacuum-packaged cooked ham stored at 4 °C. J. Food Prot. 2015, 78, 1835–1841. [Google Scholar] [CrossRef] [PubMed]
- Leroy, F.; Verluyten, J.; De Vuyst, L. Functional meat starter cultures for improved sausage fermentation. Int. J. Food Microbiol. 2005, 106, 270–285. [Google Scholar] [CrossRef]
- Schillinger, U.; Kaya, M.; Lücke, F.K. Behaviour of Listeria monocytogenes in meat and its control by a bacteriocin-producing strain of Lactobacillus sake. J. Appl. Bacteriol. 1991, 70, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Hugas, M.; Neumeyer, B.; Pagés, F.; Garriga, M.; Hammes, W.P. Antimicrobial activity of bacteriocin-producing cultures in meat products: 2. Comparison of bacteriocin producing lactobacilli on Listeria growth in fermented sausages. Fleischwirtschaft 1996, 76, 649–652. [Google Scholar]
- Kotzekidou, P.; Bloukas, J.G. Effect of protective cultures and packaging film permeability on shelf-life of sliced vacuum-packed cooked ham. Meat Sci. 1996, 42, 333–345. [Google Scholar] [CrossRef]
- Bredholt, S.; Nesbakken, T.; Holck, A. Protective cultures inhibit growth of Listeria monocytogenes and Escherichia coli O157:H7 in cooked, sliced, vacuum- and gas-packaged meat. Int. J. Food Microbiol. 1999, 53, 43–52. [Google Scholar] [CrossRef]
- Bredholt, S.; Nesbakken, T.; Holck, A. Industrial application of an antilisterial strain of Lactobacillus sakei as a protective culture and its effect on the sensory acceptability of cooked, sliced, vacuum-packaged meats. Int. J. Food Microbiol. 2001, 66, 191–196. [Google Scholar] [CrossRef]
- ISO 11290-1,2:1996 Adm.1. 2004 Microbiology of Food and Animal Feeding Stuffs e Horizontal Method for the Detection of Listeria Monocytogenes; ISO: Geneva, Switzerland, 2004. [Google Scholar]
- Mokrzycki, W.S.; Tatol, M. Colour difference ΔE—A Survey, Mach. Gr. Vis. 2012, 20, 383–411. [Google Scholar]
- AOAC. Official Methods of Analysis, 16th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1995. [Google Scholar]
- Baublis, R.T.; Meullenet, J.F.; Sawyer, J.T.; Mehaffey, J.M.; Saha, A. Pump rate and cooked temperature effects on pork loin instrumental, sensory descriptive and consumer rated characteristics. Meat Sci. 2005, 72, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Vàlkovà, V.; Salàkovà, A.; Buchtovà, H.; Tremlovà, B. Chemical, instrumental and sensory characteristics of cooked pork ham. Meat Sci. 2007, 77, 608–615. [Google Scholar] [CrossRef] [PubMed]
- Regolamento (CE) n. 2073/2005. Available online: http://europa.eu.int/eurex/lex/LexUriServ/site/it/oj/2005/l_338/l_33820051222it00010026.pd (accessed on 25 April 2020).
- Hwang, C.A.; Sheen, S. Growth characteristics of Listeria monocytogenes as affected by a native microflora in cooked ham under refrigerated and temperature abuse conditions. Food Microbiol. 2011, 28, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Amezquita, A.; Brashears, M.M. Competitive inhibition of Listeria monocytogenes in ready-to-eat meat products by lactic acid bacteria. J. Food Prot. 2002, 65, 315–325. [Google Scholar] [CrossRef]
- Hwang, C.A.; Sheen, S.; Juneja, V.; Hwang, C.-F.; Yin, T.-C.; Chang, N.-Y. The influence of acid stress on the growth of Listeria monocytogenes and Escherichia coli O157:H7 on cooked ham. Food Control 2016, 37, 245–250. [Google Scholar] [CrossRef]
- Luchansky, J.B.; Call, J.E.; Hristov, B.; Rumery, L.; Yoder, L.; Oser, A. Viability of Listeria monocytogenes on commercially-prepared hams surface treated with acidic calcium sulfate and lauric arginate and stored at 4 °C. Meat Sci. 2006, 71, 92–99. [Google Scholar] [CrossRef]
- Samelis, J.; Bedie, G.K.; Sofos, J.N.; Belk, K.E.; Scanga, J.A.; Smith, G.C. Combinations of nisin with organic acids or salts to control Listeria monocytogenes on sliced pork bologna stored at 4 °C in vacuum packages. Lebensm. Wiss. Technol.-Food Sci. Technol. 2005, 38, 21–28. [Google Scholar] [CrossRef]
- Samelis, J.; Sofos, J.; Kain, J.; Scanga, M.; Belk, K.; Smith, G. Organic acids and their salts as dipping solutions to control Listeria monocytogenes inoculated following processing of sliced pork bologna stored at 4 °C in vacuum packages. J. Food Prot. 2001, 64, 1722–1729. [Google Scholar] [CrossRef] [PubMed]
- Stekelenburg, F.K. Enhanced inhibition of Listeria monocytogenes in frankfurter sausage by the addition of potassium lactate and sodium diacetate mixtures. Food Microbiol. 2003, 20, 133–137. [Google Scholar] [CrossRef]
- Hwang, C.A.; Tamplin, M.L. Modelling the lag phase and growth rate of Listeria monocytogenes in ground ham containing sodium lactate and sodium diacetate at various storage temperatures. J. Food Sci. 2007, 72, M246–M253. [Google Scholar] [CrossRef] [PubMed]
- Legan, J.D.; Seman, D.L.; Milkowski, A.L.; Hirschey, J.A.; Vandeven, M.H. Modelling the growth boundary of Listeria monocytogenes in ready-to-eat cooked meat products as a function of the product salt, moisture, potassium lactate, and sodium diacetate concentrations. J. Food Prot. 2004, 67, 2195–2204. [Google Scholar] [CrossRef] [PubMed]
- Comi, G.; Iacumin, L. The use of bioprotective cultures. In Strategies to Obtaining Healthier Foods; Nova Science Publishers, Inc.: New York, NY, USA, 2017; pp. 1–40. [Google Scholar]
- Schobitz, R.; Zaror, T.; Leon, O.; Costa, M. A bacteriocin from Carnobacterium piscicola for the control of Listeria monocytogenes in vacuum-packaged meat. Food Microbiol. 1999, 16, 249–255. [Google Scholar] [CrossRef]
- Vermeiren, L.; Devlieghere, F.; Debevere, J. Evaluation of meat borne lactic acid bacteria as protective cultures for the biopreservation of cooked meat products. Int. J. Food Microbiol. 2004, 96, 149–164. [Google Scholar] [CrossRef] [PubMed]
| Parameters | % |
|---|---|
| Moisture | 73.4 ± 0.4 |
| Proteins | 18.5± 1.0 |
| Lipids | 3.7 ± 0.3 |
| Ash | 3.2 ± 0.2 |
| Sugar | 1.2 ±1.1 |
| aw | 0.992 ± 0.002 |
| pH | 6.35 ± 0.11 |
| Brands | 1 | 2 | 3 | |||
|---|---|---|---|---|---|---|
| Days | 0 | 60 | 0 | 60 | 0 | 60 |
| Lot 1 | 0/5 | 0/5 | 0/5 | 1/5 | 0/5 | 0/5 |
| Lot 2 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 1/5 |
| Lot 3 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 1/5 |
| Lot 4 | 0/5 | 1/5 | 1/5 | 0/5 | 1/5 | 0/5 |
| Lot 5 | 0/5 | 0/5 | 0/5 | 1/5 | 0/5 | 0/5 |
| Lot 6 | 0/5 | 1/5 * | 0/5 | 0/5 | 0/5 | 0/5 |
| Total | 0/30 | 2/30 | 1/30 | 2/30 | 1/30 | 2/30 |
| Days | aw (4 °C) | aw (4 → 8 °C) * |
|---|---|---|
| 0 | 0.986 ± 0.001 aw | 0.986 ± 0.001 aw |
| 10 | 0.992 ± 0.002 av | 0.992 ± 0.002 av |
| 20 | 0.994 ± 0.003 av | 0.994 ± 0.003 av |
| 30 | 0.990 ± 0.001 av | 0.986 ± 0.001 bw |
| 40 | 0.990 ± 0.003 av | 0.997 ± 0.001 bv |
| 50 | 0.993 ± 0.004 av | 0.983 ± 0.003 bw |
| 60 | 0.993 ± 0.001 av | 0.992 ± 0.001 av |
| Control NI | BOX-74 | BOX-57 | ||||
|---|---|---|---|---|---|---|
| Days | pH 4 °C | * pH 4 → 8 °C | pH 4 °C | * pH 4 → 8 °C | pH 4 °C | * pH 4 → 8 °C |
| 0 | 6.35 ± 0.11 av | 6.35 ± 0.11 av | 6.35 ± 0.11 av | 6.35 ± 0.11 av | 6.35 ± 0.11 av | 6.35 ± 0.11 av |
| 10 | 6.35 ± 0.20 av | 6.35 ± 0.20 av | 6.21 ± 0.31 av | 6.21 ± 0.31 av | 6.18 ± 0.20 av | 6.25 ± 0.10 av |
| 20 | 6.06 ± 0.11 aw | 6.06 ± 0.11 aw | 6.01 ± 0.21 av | 6.01 ± 0.21 av | 6.00 ± 0.11 av | 5.90 ± 0.25 aw |
| 30 | 5.82 ± 0.06 ax | 5.94 ± 0.08 bw | 5.82 ± 0.06 aw | 5.94 ± 0.08 bv | 5.80 ± 0.06 aw | 5.75 ± 0.03 cx |
| 40 | 6.12 ± 0.06 aw | 6.06 ± 0.06 aw | 6.02 ± 0.06 av | 6.06 ± 0.03 av | 5.80 ± 0.10 bw | 5.70 ± 0.01 cx |
| 50 | 6.33 ± 0.05 av | 6.27 ± 0.01 bv | 5.59 ± 0.25 cw | 5.49 ± 0.07 cw | 5.48 ± 0.20 cx | 5.65 ± 0.22 dx |
| 60 | 6.18 ± 0.05 ax | 6.17 ± 0.02 ax | 5.69 ± 0.10 bw | 5.14 ± 004 cx | 5.65 ± 0.30 bx | 5.33 ± 0.20 dy |
| L* (4 °C) | L* (4 → 8 °C) | |||||
| Days | NI | BOX-74 | BOX-57 * | NI | BOX-74 | BOX-57 |
| 0 | 62.86 ± 2.90 av | 65.71 ± 1.43 av | 65.97 ± 0.72 av | 62.86 ± 2.90 av | 63.40 ± 5.39 av | 65.31 ± 0.79 av |
| 60 | 65.02 ± 2.81 av | 64.66 ± 2.77 av | 66.83 ± 0.58 av | 65.72 ± 1.03 av | 65.74 ± 2.03 av | 67.22 ± 1.03 av |
| a* (4 °C) | a* (4 → 8 °C) | |||||
| NI | BOX-74 | BOX-57 * | NI | BOX-74 | BOX-57 | |
| 0 | 14.20 ± 0.23 av | 14.84 ± 0.32 av | 14.24 ± 0.40 av | 14.20 ± 0.23 av | 13.98 ±1.15 av | 14.65 ± 0.18 av |
| 60 | 14.23 ± 0.65 av | 14.29 ± 0.31 av | 14.65 ± 0.11 av | 14.26 ± 0.35 av | 14.57 ±0.22 av | 13.23 ± 0.61 av |
| b* (4 °C) | b* (4 → 8 °C) | |||||
| NI | BOX-57 | BOX-57 | NI | BOX-57 | BOX-57 | |
| 0 | 3.60 ± 0.50 av | 3.66 ± 0.13 av | 3.51 ± 0.20 av | 3.60 ± 0.50 av | 3.07 ± 0.74 av | 3.62 ± 0.10 av |
| 60 | 3.59 ± 0.28 av | 3.36 ± 0.12 av | 3.51 ± 0.26 av | 4.36 ± 0.56 av | 3.76 ± 0.23 av | 3.48 ± 0.45 av |
| Temperature 4 °C | |||||
|---|---|---|---|---|---|
| 4 → 8 °C | |||||
| Days | * LM | BOX-74 + LM | BOX-57 + LM | BOX-74 + LM | BOX-57 + LM |
| 0 | 2.1 ± 0.3 av | 2.1 ± 0.1 av | 2.1 ± 0.1 av | 2.1 ± 0.1 av | 2.1 ± 0.1 av |
| 10 | 2.8 ± 0.1 bw | 2.2 ± 0.2 av | 2.3 ± 0.1 av | 2.2 ± 0.2 av | 2.3 ± 0.1 av |
| 20 | 4.9 ± 0.7 ax | 2.2 ± 0.4 bv | 1.6 ± 0.2 cw | 2.2 ± 0.4 bv | 1.6 ± 0.2 cw |
| 30 | 8.2 ± 1.3 ay | 1.7 ± 0.4 bv | 1.6 ± 0.1 bw | 1.9 ± 0.3 bv | 1.4 ± 0.1 bcx |
| 40 | 7.9 ± 0.5 ay | 1.5 ± 0.5 bv | 1.3 ± 0.2 bw | 1.6 ± 0.5 bv | 1.1 ± 0.5 by |
| 50 | 8.5 ± 0.2 ay | 1.5 ± 0.2 bv | 1.3 ± 0.1 bw | 1.5 ± 0.1 bv | 1.3 ± 0.2 bx |
| 60 | 8.4 ± 0.3 ay | 1.8 ± 0.2 bv | 1.2 ± 0.4 cw | 1.9 ± 0.3 bv | 1.4 ± 0.1 cx |
| Parameter | Control | Starter 1 | Starter 2 |
|---|---|---|---|
| Fermented | 0/12 | 0/12 | 0/12 |
| Rancidity | 0/12 | 0/12 | 0/12 |
| Sweet | 10/12 | 9/12 | 9/12 |
| Acid | 0/12 | 1/12 | 1/12 |
| Meaty | 12/12 | 12/12 | 12/12 |
| Fresh | 6/12 | 6/12 | 7/12 |
| Bitter | 0/12 | 0/12 | 0/12 |
| Ammoniacal | 0/12 | 0/12 | 0/12 |
| Slime | 0/12 | 0/12 | 0/12 |
| Final value * | 2 | 2 | 2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iacumin, L.; Cappellari, G.; Colautti, A.; Comi, G. Listeria monocytogenes Survey in Cubed Cooked Ham Packaged in Modified Atmosphere and Bioprotective Effect of Selected Lactic Acid Bacteria. Microorganisms 2020, 8, 898. https://doi.org/10.3390/microorganisms8060898
Iacumin L, Cappellari G, Colautti A, Comi G. Listeria monocytogenes Survey in Cubed Cooked Ham Packaged in Modified Atmosphere and Bioprotective Effect of Selected Lactic Acid Bacteria. Microorganisms. 2020; 8(6):898. https://doi.org/10.3390/microorganisms8060898
Chicago/Turabian StyleIacumin, Lucilla, Giorgia Cappellari, Andrea Colautti, and Giuseppe Comi. 2020. "Listeria monocytogenes Survey in Cubed Cooked Ham Packaged in Modified Atmosphere and Bioprotective Effect of Selected Lactic Acid Bacteria" Microorganisms 8, no. 6: 898. https://doi.org/10.3390/microorganisms8060898
APA StyleIacumin, L., Cappellari, G., Colautti, A., & Comi, G. (2020). Listeria monocytogenes Survey in Cubed Cooked Ham Packaged in Modified Atmosphere and Bioprotective Effect of Selected Lactic Acid Bacteria. Microorganisms, 8(6), 898. https://doi.org/10.3390/microorganisms8060898






