Microbiota Modulating Nutritional Approaches to Countering the Effects of Viral Respiratory Infections Including SARS-CoV-2 through Promoting Metabolic and Immune Fitness with Probiotics and Plant Bioactives
Abstract
1. Introduction
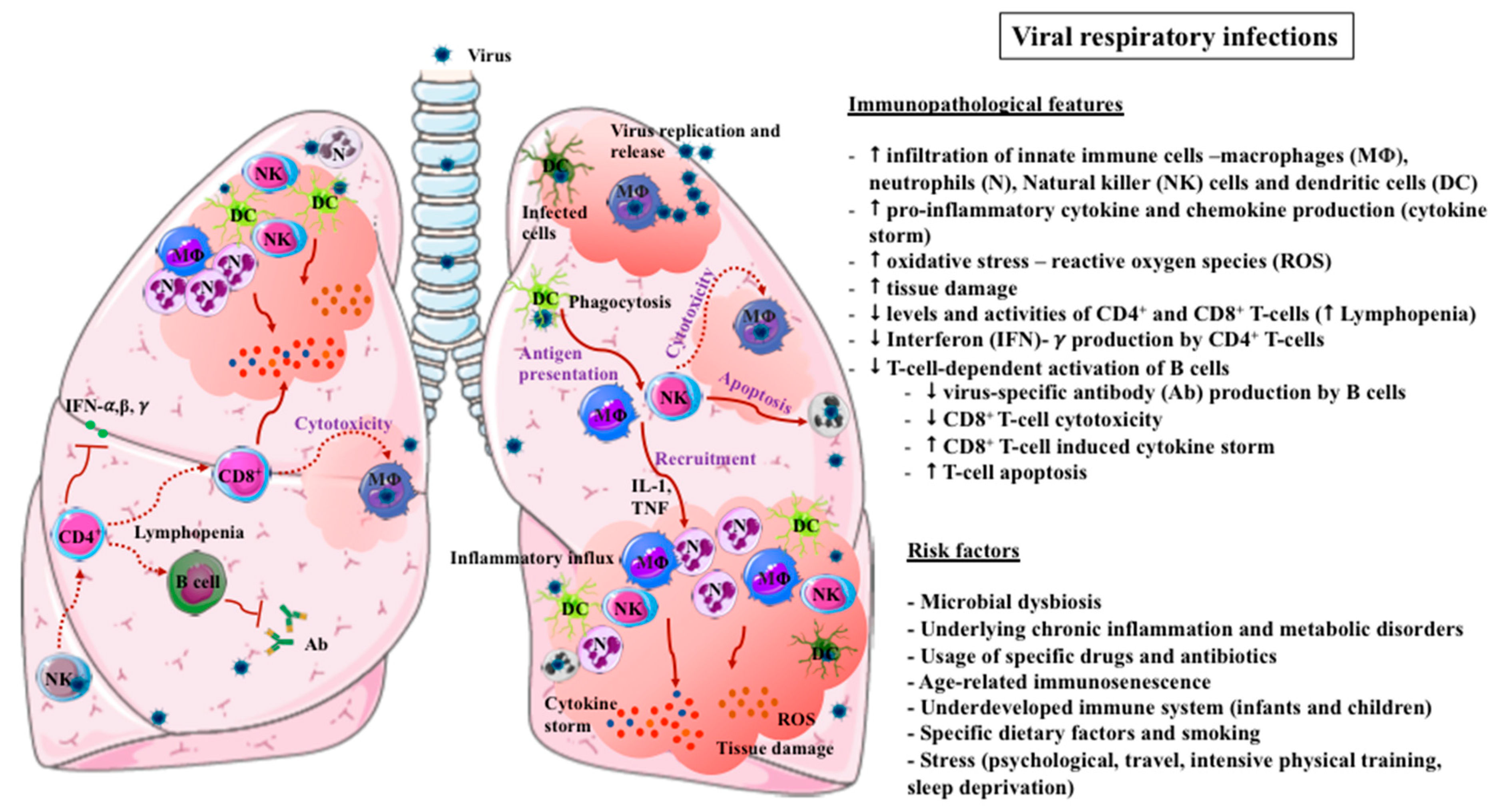
2. Immunopathogenesis of VRIs
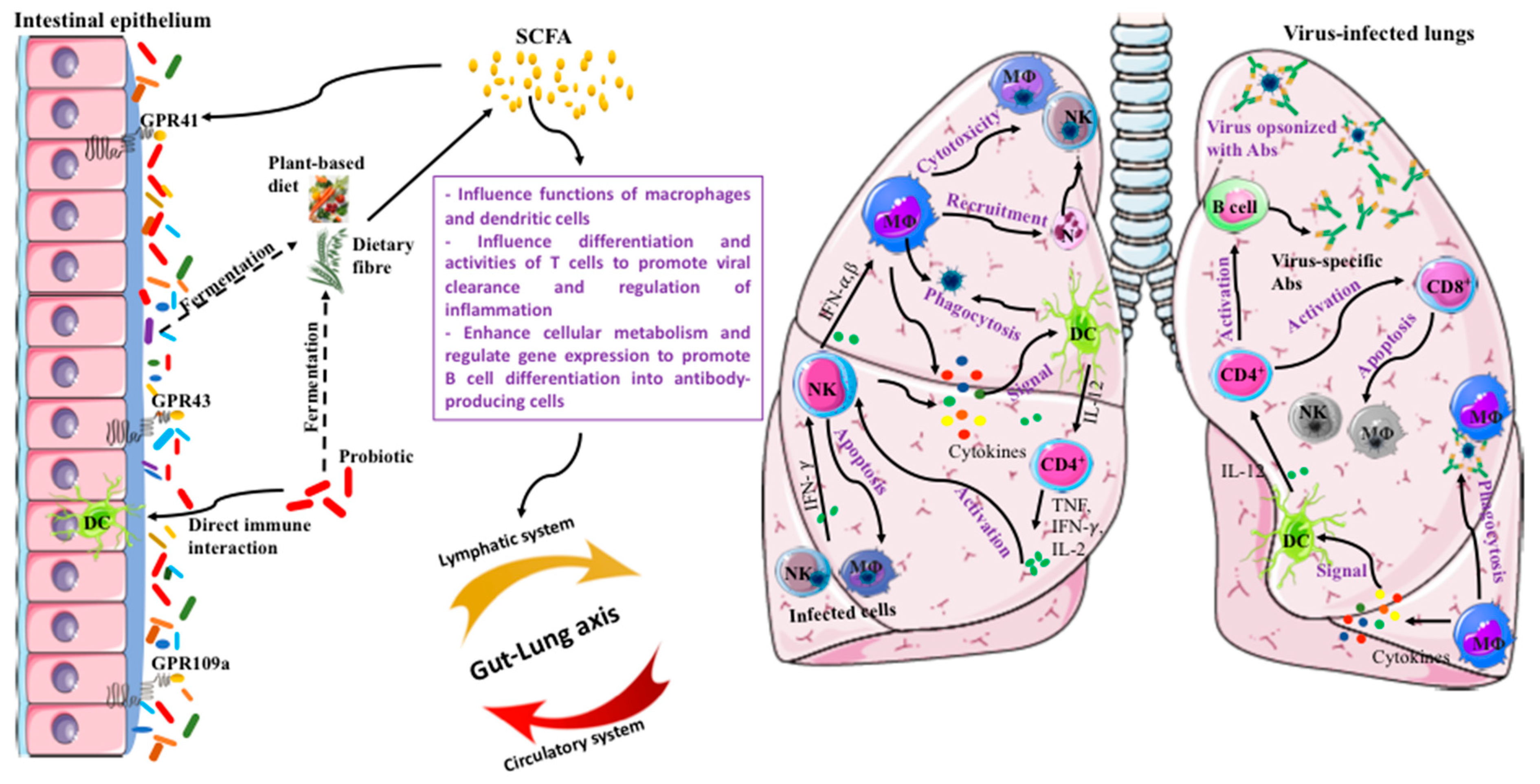
3. Sub-Optimal Immunity Driven by Microbial Dysbiosis
4. Functional Foods for Immune Fitness
5. Probiotics
6. Prebiotic DF
7. Synbiotics
8. Polyphenolic Plant Bioactives
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, N.; Wang, L.; Deng, X.; Liang, R.; Su, M.; He, C.; Hu, L.; Su, Y.; Ren, J.; Yu, F. Recent advances in the detection of respiratory virus infection in humans. J. Med. Virol. 2020, 92, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Iuliano, A.D.; Roguski, K.M.; Chang, H.H.; Muscatello, D.J.; Palekar, R.; Tempia, S.; Cohen, C.; Gran, J.M.; Schanzer, D.; Cowling, B.J.; et al. Estimates of global seasonal influenza-associated respiratory mortality: A modelling study. Lancet 2018, 391, 1285–1300. [Google Scholar] [CrossRef]
- WHO. Coronavirus disease 2019 (Covid-19): Situation report, 80. Available online: https://www.Who.Int/docs/default-source/coronaviruse/situation-reports/20200409-sitrep-80-covid-19.Pdf?Sfvrsn=1b685d64_4 (accessed on 9 April 2020).
- Li, Q.; Guan, X.; Wu, P.; Wang, X.; Zhou, L.; Tong, Y.; Ren, R.; Leung, K.S.M.; Lau, E.H.Y.; Wong, J.Y.; et al. Early transmission dynamics in wuhan, china, of novel coronavirus–infected pneumonia. N. Engl. J. Med. 2020, 382, 1199–1207. [Google Scholar] [CrossRef] [PubMed]
- Paules, C.I.; Sullivan, S.G.; Subbarao, K.; Fauci, A.S. Chasing seasonal influenza—the need for a universal influenza vaccine. N. Engl. J. Med. 2018, 378, 7–9. [Google Scholar] [CrossRef] [PubMed]
- Belongia, E.A.; Simpson, M.D.; King, J.P.; Sundaram, M.E.; Kelley, N.S.; Osterholm, M.T.; McLean, H.Q. Variable influenza vaccine effectiveness by subtype: A systematic review and meta-analysis of test-negative design studies. Lancet Infect. Dis. 2016, 16, 942–951. [Google Scholar] [CrossRef]
- Chen-Yu Hsu, A.; Starkey, M.R.; Hanish, I.; Parsons, K.; Haw, T.J.; Howland, L.J.; Barr, I.; Mahony, J.B.; Foster, P.S.; Knight, D.A. Targeting pi3k-p110α suppresses influenza virus infection in chronic obstructive pulmonary disease. Am. J. Resp. Crit. Care Med. 2015, 191, 1012–1023. [Google Scholar] [CrossRef]
- Starkey, M.R.; Jarnicki, A.G.; Essilfie, A.-T.; Gellatly, S.L.; Kim, R.Y.; Brown, A.C.; Foster, P.S.; Horvat, J.C.; Hansbro, P.M. Murine models of infectious exacerbations of airway inflammation. Curr. Opin. Pharmacol. 2013, 13, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Aiello, A.; Farzaneh, F.; Candore, G.; Caruso, C.; Davinelli, S.; Gambino, C.M.; Ligotti, M.E.; Zareian, N.; Accardi, G. Immunosenescence and its hallmarks: How to oppose aging strategically? A review of potential options for therapeutic intervention. Front. Immunol. 2019, 10, 2247. [Google Scholar] [CrossRef]
- Hijano, D.R.; Maron, G.; Hayden, R.T. Respiratory viral infections in patients with cancer or undergoing hematopoietic cell transplant. Front. Microbiol. 2018, 9, 3097. [Google Scholar] [CrossRef]
- Honce, R.R.; Schultz-Cherry, S. Impact of obesity on influenza a virus pathogenesis, immune response, and evolution. Front. Immunol. 2019, 10, 1071. [Google Scholar] [CrossRef]
- Tiollier, E.; Chennaoui, M.; Gomez-Merino, D.; Drogou, C.; Filaire, E.; Guezennec, C.Y. Effect of a probiotics supplementation on respiratory infections and immune and hormonal parameters during intense military training. Mil. Med. 2007, 172, 1006–1011. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, Y.; Xiang, P.; Pu, L.; Xiong, H.; Li, C.; Zhang, M.; Tan, J.; Xu, Y.; Song, R.; et al. Neutrophil-to-lymphocyte ratio predicts severe illness patients with 2019 novel coronavirus in the early stage. medRxiv 2020. [Google Scholar] [CrossRef]
- Ranney, M.L.; Griffeth, V.; Jha, A.K. Critical supply shortages—the need for ventilators and personal protective equipment during the covid-19 pandemic. N. Engl. J. Med. 2020, 382, e41. [Google Scholar] [CrossRef]
- Emanuel, E.J.; Persad, G.; Upshur, R.; Thome, B.; Parker, M.; Glickman, A.; Zhang, C.; Boyle, C.; Smith, M.; Phillips, J.P. Fair allocation of scarce medical resources in the time of covid-19. N. Engl. J. Med. 2020, 382, 2049–2055. [Google Scholar] [CrossRef] [PubMed]
- Chotirmall, S.H.; Gellatly, S.L.; Budden, K.F.; Mac Aogáin, M.; Shukla, S.D.; Wood, D.L.A.; Hugenholtz, P.; Pethe, K.; Hansbro, P.M. Microbiomes in respiratory health and disease: An asia-pacific perspective. Respirology 2017, 22, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Budden, K.F.; Shukla, S.D.; Rehman, S.F.; Bowerman, K.L.; Keely, S.; Hugenholtz, P.; Armstrong-James, D.P.; Adcock, I.M.; Chotirmall, S.H.; Chung, K.F.; et al. Functional effects of the microbiota in chronic respiratory disease. Lancet Resp. Med. 2019, 7, 907–920. [Google Scholar] [CrossRef]
- Lee, K.H.; Gordon, A.; Shedden, K.; Kuan, G.; Ng, S.; Balmaseda, A.; Foxman, B. The respiratory microbiome and susceptibility to influenza virus infection. PLoS ONE 2019, 14, e0207898. [Google Scholar] [CrossRef]
- Li, N.; Ma, W.-T.; Pang, M.; Fan, Q.-L.; Hua, J.-L. The commensal microbiota and viral infection: A comprehensive review. Front. Immunol. 2019, 10, 1551. [Google Scholar] [CrossRef]
- Wilks, J.; Golovkina, T. Influence of microbiota on viral infections. PLoS Pathog. 2012, 8, e1002681. [Google Scholar] [CrossRef] [PubMed]
- Ichinohe, T.; Pang, I.K.; Kumamoto, Y.; Peaper, D.R.; Ho, J.H.; Murray, T.S.; Iwasaki, A. Microbiota regulates immune defense against respiratory tract influenza a virus infection. Proc. Natl. Acad. Sci. USA 2011, 108, 5354–5359. [Google Scholar] [CrossRef]
- Damjanovic, D.; Small, C.-L.; Jeyananthan, M.; McCormick, S.; Xing, Z. Immunopathology in influenza virus infection: Uncoupling the friend from foe. Clin. Immunol. 2012, 144, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Dandekar, A.A.; Perlman, S. Immunopathogenesis of coronavirus infections: Implications for sars. Nat. Rev. Immunol. 2005, 5, 917–927. [Google Scholar] [CrossRef] [PubMed]
- Atto, B.; Eapen, M.S.; Sharma, P.; Frey, U.; Ammit, A.J.; Markos, J.; Chia, C.; Larby, J.; Haug, G.; Weber, H.C. New therapeutic targets for the prevention of infectious acute exacerbations of copd: Role of epithelial adhesion molecules and inflammatory pathways. Clin. Sci. 2019, 133, 1663–1703. [Google Scholar] [CrossRef] [PubMed]
- Eapen, M.S.; Sohal, S.S. Understanding novel mechanisms of microbial pathogenesis in chronic lung disease: Implications for new therapeutic targets. Clin. Sci. 2018, 132, 375–379. [Google Scholar] [CrossRef]
- Narasaraju, T.; Yang, E.; Samy, R.P.; Ng, H.H.; Poh, W.P.; Liew, A.-A.; Phoon, M.C.; van Rooijen, N.; Chow, V.T. Excessive neutrophils and neutrophil extracellular traps contribute to acute lung injury of influenza pneumonitis. Am. J. Pathol. 2011, 179, 199–210. [Google Scholar] [CrossRef]
- Shi, H.; Han, X.; Jiang, N.; Cao, Y.; Alwalid, O.; Gu, J.; Fan, Y.; Zheng, C. Radiological findings from 81 patients with covid-19 pneumonia in wuhan, china: A descriptive study. Lancet Infect. Dis. 2020, 20, 425–434. [Google Scholar] [CrossRef]
- Barnes, B.J.; Adrover, J.M.; Baxter-Stoltzfus, A.; Borczuk, A.; Cools-Lartigue, J.; Crawford, J.M.; Daßler-Plenker, J.; Guerci, P.; Huynh, C.; Knight, J.S.; et al. Targeting potential drivers of covid-19: Neutrophil extracellular traps. J. Exp. Med. 2020, 217. [Google Scholar] [CrossRef]
- Chen, G.; Wu, D.; Guo, W.; Cao, Y.; Huang, D.; Wang, H.; Wang, T.; Zhang, X.; Chen, H.; Yu, H.; et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Investig. 2020, 130. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, E.; López-Sobaler, A.M.; Ortega, R.M.; Delgado-Losada, M.L.; López-Parra, A.M.; Aparicio, A. Association between neutrophil-to-lymphocyte ratio with abdominal obesity and healthy eating index in a representative older Spanish population. Nutrients 2020, 12, 855. [Google Scholar] [CrossRef]
- Hosakote, Y.M.; Rayavara, K. Respiratory syncytial virus-induced oxidative stress in lung pathogenesis. In Oxidative Stress in Lung Diseases; Chakraborti, S., Parinandi, N., Ghosh, R., Ganguly, N., Chakraborti, T., Eds.; Springer: Singapore, 2020; pp. 297–330. [Google Scholar]
- Gonzalez-Juarbe, N.; Riegler, A.N.; Jureka, A.S.; Gilley, R.P.; Brand, J.; Trombley, J.E.; Scott, N.R.; Dube, P.H.; Petit, C.M.; Harrod, K.S.; et al. Influenza-induced oxidative stress sensitizes lung cells to bacterial toxin-mediated necroptosis. bioRxiv 2020. [Google Scholar] [CrossRef]
- Xu, X.; Gao, X.-M. Immunological responses against SARS-coronavirus infection in humans. Cell. Mol. Immunol. 2004, 1, 119–122. [Google Scholar] [PubMed]
- Frasca, L.; Piazza, C.; Piccolella, E. CD4+ T cells orchestrate both amplification and deletion of CD8+ T cells. Crit. Rev. Immunol. 1998, 18, 569–594. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.; Zhou, J.; Wong, B.H.-Y.; Li, C.; Chan, J.F.-W.; Cheng, Z.-S.; Yang, D.; Wang, D.; Lee, A.C.-Y.; Li, C. Middle east respiratory syndrome coronavirus efficiently infects human primary t lymphocytes and activates the extrinsic and intrinsic apoptosis pathways. J. Infect. Dis. 2016, 213, 904–914. [Google Scholar] [CrossRef]
- He, Z.; Zhao, C.; Dong, Q.; Zhuang, H.; Song, S.; Peng, G.; Dwyer, D.E. Effects of severe acute respiratory syndrome (sars) coronavirus infection on peripheral blood lymphocytes and their subsets. Int. J. Infect. Dis. 2005, 9, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Sompayrac, L.M. How the Immune System Work; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Domínguez-Díaz, C.; García-Orozco, A.; Riera-Leal, A.; Padilla-Arellano, J.R.; Fafutis-Morris, M. Microbiota and its role on viral evasion: Is it with us or against us? Front. Cell Infect. Microbiol. 2019, 9, 256. [Google Scholar] [CrossRef] [PubMed]
- Budden, K.F.; Gellatly, S.L.; Wood, D.L.; Cooper, M.A.; Morrison, M.; Hugenholtz, P.; Hansbro, P.M. Emerging pathogenic links between microbiota and the gut–lung axis. Nat. Rev. Microbiol. 2017, 15, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.D.; Budden, K.F.; Neal, R.; Hansbro, P.M. Microbiome effects on immunity, health and disease in the lung. Clin. Transl. Immunol. 2017, 6, e133. [Google Scholar] [CrossRef]
- Fricker, M.; Goggins, B.J.; Mateer, S.; Jones, B.; Kim, R.Y.; Gellatly, S.L.; Jarnicki, A.G.; Powell, N.; Oliver, B.G.; Radford-Smith, G.; et al. Chronic cigarette smoke exposure induces systemic hypoxia that drives intestinal dysfunction. JCI Insight 2018, 3, e94040. [Google Scholar] [CrossRef]
- Mateer, S.W.; Mathe, A.; Bruce, J.; Liu, G.; Maltby, S.; Fricker, M.; Goggins, B.J.; Tay, H.L.; Marks, E.; Burns, G.; et al. Il-6 drives neutrophil-mediated pulmonary inflammation associated with bacteremia in murine models of colitis. Am. J. Pathol. 2018, 188, 1625–1639. [Google Scholar] [CrossRef]
- Liu, G.; Mateer, S.W.; Hsu, A.; Goggins, B.J.; Tay, H.; Mathe, A.; Fan, K.; Neal, R.; Bruce, J.; Burns, G.; et al. Platelet activating factor receptor regulates colitis-induced pulmonary inflammation through the nlrp3 inflammasome. Mucosal Immunol. 2019, 12, 862–873. [Google Scholar] [CrossRef]
- Hanada, S.; Pirzadeh, M.; Carver, K.Y.; Deng, J.C. Respiratory viral infection-induced microbiome alterations and secondary bacterial pneumonia. Front. Immunol. 2018, 9, 2640. [Google Scholar] [CrossRef] [PubMed]
- Hansbro, P.M.; Kim, R.Y.; Starkey, M.R.; Donovan, C.; Dua, K.; Mayall, J.R.; Liu, G.; Hansbro, N.G.; Simpson, J.L.; Wood, L.G. Mechanisms and treatments for severe, steroid-resistant allergic airway disease and asthma. Immunol. Rev. 2017, 278, 41–62. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Chen, X.; Cai, Y.; Xia, J.a.; Zhou, X.; Xu, S.; Huang, H.; Zhang, L.; Zhou, X.; Du, C.; et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern. Med. 2020, e200994. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Sun, J.; Cai, J.; Miao, Z.; Lu, M.; Qin, S.; Wang, X.; Lv, H.; Yu, Z.; Amer, S. Epidemiological, clinical and viral characteristics of fatal cases of human avian influenza a (h7n9) virus in Zhejiang province, China. J. Infect. 2013, 67, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.; Kolodziejczyk, A.A.; Thaiss, C.A.; Elinav, E. Dysbiosis and the immune system. Nat. Rev. Immunol. 2017, 17, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Salazar, N.; Arboleya, S.; Fernández-Navarro, T.; de los Reyes-Gavilán, C.G.; Gonzalez, S.; Gueimonde, M. Age-associated changes in gut microbiota and dietary components related with the immune system in adulthood and old age: A cross-sectional study. Nutrients 2019, 11, 1765. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.P.; Sikder, M.A.A.; Curren, B.F.; Werder, R.B.; Simpson, J.; Cuív, P.Ó.; Dennis, P.G.; Everard, M.L.; Phipps, S. The influence of the microbiome on early-life severe viral lower respiratory infections and asthma—food for thought? Front. Immunol. 2017, 8, 156. [Google Scholar] [CrossRef]
- Lawrence, H.; Hunter, A.; Murray, R.; Lim, W.S.; McKeever, T. Cigarette smoking and the occurrence of influenza -systematic review. J. Infect. 2019, 79, 401–406. [Google Scholar] [CrossRef]
- Bauer, C.M.T.; Morissette, M.C.; Stämpfli, M.R. The influence of cigarette smoking on viral infections: Translating bench science to impact copd pathogenesis and acute exacerbations of copd clinically. Chest 2013, 143, 196–206. [Google Scholar] [CrossRef]
- Brake, S.J.; Barnsley, K.; Lu, W.; McAlinden, K.D.; Eapen, M.S.; Sohal, S.S. Smoking upregulates angiotensin-converting enzyme-2 receptor: A potential adhesion site for novel coronavirus SARS-CoV-2 (Covid-19). J. Clin. Med. 2020, 9, 841. [Google Scholar] [CrossRef]
- Kim, M.; Qie, Y.; Park, J.; Kim, C.H. Gut microbial metabolites fuel host antibody responses. Cell Host Microbe 2016, 20, 202–214. [Google Scholar] [CrossRef] [PubMed]
- Trompette, A.; Gollwitzer, E.S.; Pattaroni, C.; Lopez-Mejia, I.C.; Riva, E.; Pernot, J.; Ubags, N.; Fajas, L.; Nicod, L.P.; Marsland, B.J. Dietary fiber confers protection against flu by shaping ly6− patrolling monocyte hematopoiesis and CD8+ T cell metabolism. Immunity 2018, 48, 992–1005. [Google Scholar] [CrossRef] [PubMed]
- Kosaraju, R.; Guesdon, W.; Crouch, M.J.; Teague, H.L.; Sullivan, E.M.; Karlsson, E.A.; Schultz-Cherry, S.; Gowdy, K.; Bridges, L.C.; Reese, L.R. B cell activity is impaired in human and mouse obesity and is responsive to an essential fatty acid upon murine influenza infection. J. Immunol. 2017, 198, 4738–4752. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitsche, A.; et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020, 181, 271–280.e278. [Google Scholar] [CrossRef]
- Crucian, B.; Babiak-Vazquez, A.; Johnston, S.; Pierson, D.L.; Ott, C.M.; Sams, C. Incidence of clinical symptoms during long-duration orbital spaceflight. Int. J. Gen. Med. 2016, 9, 383–391. [Google Scholar] [CrossRef]
- Colbey, C.; Cox, A.J.; Pyne, D.B.; Zhang, P.; Cripps, A.W.; West, N.P. Upper respiratory symptoms, gut health and mucosal immunity in athletes. Sports Med. 2018, 48, 65–77. [Google Scholar] [CrossRef]
- Trimble, A.; Moffat, V.; Collins, A.M. Pulmonary infections in the returned traveller. Pneumonia 2017, 9, 1. [Google Scholar] [CrossRef]
- Hughes, D.A. Dietary antioxidants and human immune function. Nutr. Bull. 2000, 25, 35–41. [Google Scholar] [CrossRef]
- Gombart, A.F.; Pierre, A.; Maggini, S. A review of micronutrients and the immune system–working in harmony to reduce the risk of infection. Nutrients 2020, 12, 236. [Google Scholar] [CrossRef] [PubMed]
- Maslowski, K.M.; Mackay, C.R. Diet, gut microbiota and immune responses. Nat. Immunol. 2011, 12, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Sgarbanti, R.; Amatore, D.; Celestino, I.; Elena Marcocci, M.; Fraternale, A.; Ciriolo, M.R.; Magnani, M.; Saladino, R.; Garaci, E.; Teresa Palamara, A. Intracellular redox state as target for anti-influenza therapy: Are antioxidants always effective? Curr. Top. Med. Chem. 2014, 14, 2529–2541. [Google Scholar] [CrossRef] [PubMed]
- De Clercq, E.; Li, G. Approved antiviral drugs over the past 50 years. Clin. Microbiol. Rev. 2016, 29, 695–747. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S. Expert consensus document: The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, N.; Saito, T.; Uematsu, T.; Kishi, K.; Toba, M.; Kohda, N.; Suzuki, T. Oral administration of heat-killed lactobacillus pentosus strain b240 augments protection against influenza virus infection in mice. Int. Immunopharmacol. 2011, 11, 199–203. [Google Scholar] [CrossRef]
- Nagai, T.; Makino, S.; Ikegami, S.; Itoh, H.; Yamada, H. Effects of oral administration of yogurt fermented with lactobacillus delbrueckii ssp. Bulgaricus oll1073r-1 and its exopolysaccharides against influenza virus infection in mice. Int. Immunopharmacol. 2011, 11, 2246–2250. [Google Scholar] [CrossRef]
- Jung, Y.-J.; Lee, Y.-T.; Le Ngo, V.; Cho, Y.-H.; Ko, E.-J.; Hong, S.-M.; Kim, K.-H.; Jang, J.-H.; Oh, J.-S.; Park, M.-K. Heat-killed lactobacillus casei confers broad protection against influenza a virus primary infection and develops heterosubtypic immunity against future secondary infection. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef]
- Tonetti, F.R.; Islam, M.A.; Vizoso-Pinto, M.G.; Takahashi, H.; Kitazawa, H.; Villena, J. Nasal priming with immunobiotic lactobacilli improves the adaptive immune response against influenza virus. Int. Immunopharmacol. 2020, 78, 106115. [Google Scholar] [CrossRef]
- Baron, M. A patented strain of Bacillus coagulans increased immune response to viral challenge. Postgrad. Med. 2009, 121, 114–118. [Google Scholar] [CrossRef]
- Shinde, T.; Vemuri, R.; Shastri, M.D.; Perera, A.P.; Tristram, S.; Stanley, R.; Eri, R. Probiotic Bacillus coagulans mtcc 5856 spores exhibit excellent in-vitro functional efficacy in simulated gastric survival, mucosal adhesion and immunomodulation. J. Funct. Foods 2019, 52, 100–108. [Google Scholar] [CrossRef]
- Galdeano, C.M.; Cazorla, S.I.; Dumit, J.M.L.; Vélez, E.; Perdigón, G. Beneficial effects of probiotic consumption on the immune system. Ann. Nutr. Metab. 2019, 74, 115–124. [Google Scholar] [CrossRef]
- Wieërs, G.; Belkhir, L.; Enaud, R.; Leclercq, S.; Philippart de Foy, J.-M.; Dequenne, I.; de Timary, P.; Cani, P.D. How probiotics affect the microbiota. Front. Cell Infect. Microbiol. 2020, 9, 454. [Google Scholar] [CrossRef] [PubMed]
- Smelt, M.J.; de Haan, B.J.; Bron, P.A.; van Swam, I.; Meijerink, M.; Wells, J.M.; Faas, M.M.; de Vos, P. Probiotics can generate FoxP3 T-cell responses in the small intestine and simultaneously inducing CD4 and CD8 T cell activation in the large intestine. PLoS ONE 2013, 8, e68952. [Google Scholar] [CrossRef] [PubMed]
- de LeBlanc, A.d.M.; Dogi, C.A.; Galdeano, C.M.; Carmuega, E.; Weill, R.; Perdigón, G. Effect of the administration of a fermented milk containing lactobacillus casei dn-114001 on intestinal microbiota and gut associated immune cells of nursing mice and after weaning until immune maturity. BMC Immunol. 2008, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Shinde, T.; Perera, A.P.; Vemuri, R.; Gondalia, S.V.; Karpe, A.V.; Beale, D.J.; Shastri, S.; Southam, B.; Eri, R.; Stanley, R. Synbiotic supplementation containing whole plant sugar cane fibre and probiotic spores potentiates protective synergistic effects in mouse model of ibd. Nutrients 2019, 11, 818. [Google Scholar] [CrossRef]
- Shinde, T.; Perera, A.P.; Vemuri, R.; Gondalia, S.V.; Beale, D.J.; Karpe, A.V.; Shastri, S.; Basheer, W.; Southam, B.; Eri, R.; et al. Synbiotic supplementation with prebiotic green banana resistant starch and probiotic bacillus coagulans spores ameliorates gut inflammation in mouse model of inflammatory bowel diseases. Eur. J. Nutr. 2020. [Google Scholar] [CrossRef]
- Kristensen, N.B.; Bryrup, T.; Allin, K.H.; Nielsen, T.; Hansen, T.H.; Pedersen, O. Alterations in fecal microbiota composition by probiotic supplementation in healthy adults: A systematic review of randomized controlled trials. Genome Med. 2016, 8, 52. [Google Scholar] [CrossRef]
- McNulty, N.P.; Yatsunenko, T.; Hsiao, A.; Faith, J.J.; Muegge, B.D.; Goodman, A.L.; Henrissat, B.; Oozeer, R.; Cools-Portier, S.; Gobert, G.; et al. The impact of a consortium of fermented milk strains on the gut microbiome of gnotobiotic mice and monozygotic twins. Sci. Transl. Med. 2011, 3, 106ra106. [Google Scholar] [CrossRef]
- Larsen, N.; Vogensen, F.K.; Gøbel, R.; Michaelsen, K.F.; Al-Soud, W.A.; Sørensen, S.J.; Hansen, L.H.; Jakobsen, M. Predominant genera of fecal microbiota in children with atopic dermatitis are not altered by intake of probiotic bacteria Lactobacillus acidophilus ncfm and Bifidobacterium animalis subsp. Lactis bi-07. FEMS Microbiol. Ecol. 2011, 75, 482–496. [Google Scholar] [CrossRef]
- Vemuri, R.; Gundamaraju, R.; Shinde, T.; Perera, A.P.; Basheer, W.; Southam, B.; Gondalia, S.V.; Karpe, A.V.; Beale, D.J.; Tristram, S.; et al. Lactobacillus acidophilus dds-1 modulates intestinal-specific microbiota, short-chain fatty acid and immunological profiles in aging mice. Nutrients 2019, 11, 1297. [Google Scholar] [CrossRef]
- Tankou, S.K.; Regev, K.; Healy, B.C.; Tjon, E.; Laghi, L.; Cox, L.M.; Kivisäkk, P.; Pierre, I.V.; Hrishikesh, L.; Gandhi, R.; et al. A probiotic modulates the microbiome and immunity in multiple sclerosis. Ann. Neurol. 2018, 83, 1147–1161. [Google Scholar] [CrossRef]
- Korpela, K.; Salonen, A.; Vepsäläinen, O.; Suomalainen, M.; Kolmeder, C.; Varjosalo, M.; Miettinen, S.; Kukkonen, K.; Savilahti, E.; Kuitunen, M.; et al. Probiotic supplementation restores normal microbiota composition and function in antibiotic-treated and in caesarean-born infants. Microbiome 2018, 6, 182. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, B.; Delgado, S.; Blanco-Míguez, A.; Lourenço, A.; Gueimonde, M.; Margolles, A. Probiotics, gut microbiota, and their influence on host health and disease. Mol. Nutr. Food Res. 2017, 61, 1600240. [Google Scholar] [CrossRef] [PubMed]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Venegas, D.P.; Marjorie, K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.; Faber, K.N.; Hermoso, M.A. Short chain fatty acids (scfas)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef]
- Lehtoranta, L.; Pitkäranta, A.; Korpela, R. Probiotics in respiratory virus infections. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 1289–1302. [Google Scholar] [CrossRef]
- Perdigón, G.; Fuller, R.; Raya, R. Lactic acid bacteria and their effect on the immune system. Curr. Issues Intest. Microbiol. 2001, 2, 27–42. [Google Scholar]
- Yasui, H.; Kiyoshima, J.; Hori, T. Reduction of influenza virus titer and protection against influenza virus infection in infant mice fed Lactobacillus casei shirota. Clin. Vaccine Immunol. 2004, 11, 675–679. [Google Scholar] [CrossRef]
- Takeda, S.; Takeshita, M.; Kikuchi, Y.; Dashnyam, B.; Kawahara, S.; Yoshida, H.; Watanabe, W.; Muguruma, M.; Kurokawa, M. Efficacy of oral administration of heat-killed probiotics from mongolian dairy products against influenza infection in mice: Alleviation of influenza infection by its immunomodulatory activity through intestinal immunity. Int. Immunopharmacol. 2011, 11, 1976–1983. [Google Scholar] [CrossRef]
- Park, M.-K.; Vu, N.; Kwon, Y.-M.; Lee, Y.-T.; Yoo, S.; Cho, Y.-H.; Hong, S.-M.; Hwang, H.S.; Ko, E.-J.; Jung, Y.-J. Lactobacillus plantarum dk119 as a probiotic confers protection against influenza virus by modulating innate immunity. PLoS ONE 2013, 8, e75368. [Google Scholar] [CrossRef]
- Kawase, M.; He, F.; Kubota, A.; Yoda, K.; Miyazawa, K.; Hiramatsu, M. Heat-killed Lactobacillus gasseri tmc0356 protects mice against influenza virus infection by stimulating gut and respiratory immune responses. FEMS Immunol. Med. Microbiol. 2012, 64, 280–288. [Google Scholar] [CrossRef]
- Waki, N.; Yajima, N.; Suganuma, H.; Buddle, B.; Luo, D.; Heiser, A.; Zheng, T. Oral administration of Lactobacillus brevis kb 290 to mice alleviates clinical symptoms following influenza virus infection. Lett. Appl. Microbiol. 2014, 58, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Goto, H.; Sagitani, A.; Ashida, N.; Kato, S.; Hirota, T.; Shinoda, T.; Yamamoto, N. Anti-influenza virus effects of both live and non-live lactobacillus acidophilus l-92 accompanied by the activation of innate immunity. Br. J. Nutr. 2013, 110, 1810–1818. [Google Scholar] [CrossRef]
- Iwabuchi, N.; Xiao, J.-Z.; Yaeshima, T.; Iwatsuki, K. Oral administration of Bifidobacterium longum ameliorates influenza virus infection in mice. Biol. Pharm. Bull. 2011, 34, 1352–1355. [Google Scholar] [CrossRef] [PubMed]
- Harata, G.; He, F.; Hiruta, N.; Kawase, M.; Kubota, A.; Hiramatsu, M.; Yausi, H. Intranasal administration of lactobacillus rhamnosus gg protects mice from h1n1 influenza virus infection by regulating respiratory immune responses. Lett. Appl. Microbiol. 2010, 50, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Izumo, T.; Maekawa, T.; Ida, M.; Noguchi, A.; Kitagawa, Y.; Shibata, H.; Yasui, H.; Kiso, Y. Effect of intranasal administration of lactobacillus pentosus s-pt84 on influenza virus infection in mice. Int. Immunopharmacol. 2010, 10, 1101–1106. [Google Scholar] [CrossRef] [PubMed]
- Gabryszewski, S.J.; Bachar, O.; Dyer, K.D.; Percopo, C.M.; Killoran, K.E.; Domachowske, J.B.; Rosenberg, H.F. Lactobacillus-mediated priming of the respiratory mucosa protects against lethal pneumovirus infection. J. Immunol. 2011, 186, 1151–1161. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Crespo, K.E.; Chan, C.C.; Gabryszewski, S.J.; Percopo, C.M.; Rigaux, P.; Dyer, K.D.; Domachowske, J.B.; Rosenberg, H.F. Lactobacillus priming of the respiratory tract: Heterologous immunity and protection against lethal pneumovirus infection. Antivir. Res. 2013, 97, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Hori, T.; Kiyoshima, J.; Shida, K.; Yasui, H. Augmentation of cellular immunity and reduction of influenza virus titer in aged mice fed lactobacillus casei strain shirota. Clin. Vaccine Immunol. 2002, 9, 105–108. [Google Scholar] [CrossRef]
- Yasui, H.; Kiyoshima, J.; Hori, T.; Shida, K. Protection against influenza virus infection of mice fed bifidobacterium breve yit4064. Clin. Vaccine Immunol. 1999, 6, 186–192. [Google Scholar] [CrossRef]
- Lee, Y.-N.; Youn, H.-N.; Kwon, J.-H.; Lee, D.-H.; Park, J.-K.; Yuk, S.-S.; Erdene-Ochir, T.-O.; Kim, K.-T.; Lee, J.-B.; Park, S.-Y. Sublingual administration of Lactobacillus rhamnosus affects respiratory immune responses and facilitates protection against influenza virus infection in mice. Antivir. Res. 2013, 98, 284–290. [Google Scholar] [CrossRef]
- Nguyen, T.L.A.; Vieira-Silva, S.; Liston, A.; Raes, J. How informative is the mouse for human gut microbiota research? Dis. Model. Mech. 2015, 8, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Tiderencel, K.A.; Hutcheon, D.A.; Ziegler, J. Probiotics for the treatment of type 2 diabetes: A review of randomized controlled trials. Diabetes Metab. Res. Rev. 2020, 36, e3213. [Google Scholar] [CrossRef] [PubMed]
- Cerdó, T.; García-Santos, J.A.; Bermúdez, M.G.; Campoy, C. The role of probiotics and prebiotics in the prevention and treatment of obesity. Nutrients 2019, 11, 635. [Google Scholar] [CrossRef] [PubMed]
- Celiberto, L.S.; Bedani, R.; Rossi, E.A.; Cavallini, D.C.U. Probiotics: The scientific evidence in the context of inflammatory bowel disease. Crit. Rev. Food Sci. Nutr. 2017, 57, 1759–1768. [Google Scholar] [CrossRef] [PubMed]
- Helmink, B.A.; Khan, M.W.; Hermann, A.; Gopalakrishnan, V.; Wargo, J.A. The microbiome, cancer, and cancer therapy. Nat. Med. 2019, 25, 377–388. [Google Scholar] [CrossRef]
- Miller, L.E.; Lehtoranta, L.; Lehtinen, M.J. The effect of bifidobacterium animalis ssp. Lactis hn019 on cellular immune function in healthy elderly subjects: Systematic review and meta-analysis. Nutrients 2017, 9, 191. [Google Scholar] [CrossRef]
- Shaw, A.C.; Joshi, S.; Greenwood, H.; Panda, A.; Lord, J.M. Aging of the innate immune system. Curr. Opin. Immunol. 2010, 22, 507–513. [Google Scholar] [CrossRef]
- Mohr, A.E.; Basile, A.J.; Crawford, M.s.S.; Sweazea, K.L.; Carpenter, K.C. Probiotic supplementation has a limited effect on circulating immune and inflammatory markers in healthy adults: A systematic review of randomized controlled trials. J. Acad. Nutr. Dietet. 2020, 120, 548–564. [Google Scholar] [CrossRef]
- Khalesi, S.; Bellissimo, N.; Vandelanotte, C.; Williams, S.; Stanley, D.; Irwin, C. A review of probiotic supplementation in healthy adults: Helpful or hype? Eur. J. Clin. Nutr. 2019, 73, 24–37. [Google Scholar] [CrossRef]
- King, S.; Glanville, J.; Sanders, M.E.; Fitzgerald, A.; Varley, D. Effectiveness of probiotics on the duration of illness in healthy children and adults who develop common acute respiratory infectious conditions: A systematic review and meta-analysis. Br. J. Nutr. 2014, 112, 41–54. [Google Scholar] [CrossRef]
- Hazeldine, J.; Lord, J.M. The impact of ageing on natural killer cell function and potential consequences for health in older adults. Ageing Res. Rev. 2013, 12, 1069–1078. [Google Scholar] [CrossRef] [PubMed]
- Rukavina, D.; Laskarin, G.; Rubesa, G.; Strbo, N.; Bedenicki, I.; Manestar, D.; Glavas, M.; Christmas, S.E.; Podack, E.R. Age-related decline of perforin expression in human cytotoxic T lymphocytes and natural killer cells. Blood 1998, 92, 2410–2420. [Google Scholar] [CrossRef] [PubMed]
- Hayhoe, R.P.G.; Henson, S.M.; Akbar, A.N.; Palmer, D.B. Variation of human natural killer cell phenotypes with age: Identification of a unique klrg1-negative subset. Hum. Immunol. 2010, 71, 676–681. [Google Scholar] [CrossRef]
- Ouwehand, A.C.; Bergsma, N.; Parhiala, R.; Lahtinen, S.; Gueimonde, M.; Finne-Soveri, H.; Strandberg, T.; Pitkälä, K.; Salminen, S. Bifidobacterium microbiota and parameters of immune function in elderly subjects. FEMS Immunol. Med. Microbiol. 2008, 53, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Nyangale, E.P.; Farmer, S.; Cash, H.A.; Keller, D.; Chernoff, D.; Gibson, G.R. Bacillus coagulans gbi-30, 6086 modulates Faecalibacterium prausnitzii in older men and women. J. Nutr. 2015, 145, 1446–1452. [Google Scholar] [CrossRef] [PubMed]
- Alameddine, J.; Godefroy, E.; Papargyris, L.; Sarrabayrouse, G.; Tabiasco, J.; Bridonneau, C.; Yazdanbakhsh, K.; Sokol, H.; Altare, F.; Jotereau, F. Faecalibacterium prausnitzii skews human dc to prime il10-producing t cells through tlr2/6/jnk signaling and il-10, il-27, cd39, and ido-1 induction. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef]
- Boge, T.; Rémigy, M.; Vaudaine, S.; Tanguy, J.; Bourdet-Sicard, R.; van der Werf, S. A probiotic fermented dairy drink improves antibody response to influenza vaccination in the elderly in two randomised controlled trials. Vaccine 2009, 27, 5677–5684. [Google Scholar] [CrossRef]
- Bosch, M.; Méndez, M.; Pérez, M.; Farran, A.; Fuentes, M.C.; Cuñé, J. Lactobacillus plantarum cect7315 and cect7316 stimulate immunoglobulin production after influenza vaccination in elderly. Nutr. Hosp. 2012, 27, 504–509. [Google Scholar] [CrossRef]
- Gutierrez-Castrellon, P.; Weizman, Z.; Cruchet, S.; Dinleyci, E.C.; Jimenez-Gutierrez, C.; Lopez-Velazquez, G. Role of probiotics to prevent and reduce the duration of upper respiratory infections in ambulatory children: Systematic review with network-meta analysis. Preprints 2018. [Google Scholar] [CrossRef]
- Hojsak, I.; Snovak, N.; Abdović, S.; Szajewska, H.; Mišak, Z.; Kolaček, S. Lactobacillus gg in the prevention of gastrointestinal and respiratory tract infections in children who attend day care centers: A randomized, double-blind, placebo-controlled trial. Clin. Nutr. 2010, 29, 312–316. [Google Scholar] [CrossRef]
- King, S.; Tancredi, D.; Lenoir-Wijnkoop, I.; Gould, K.; Vann, H.; Connors, G.; Sanders, M.E.; Linder, J.A.; Shane, A.L.; Merenstein, D. Does probiotic consumption reduce antibiotic utilization for common acute infections? A systematic review and meta-analysis. Eur. J. Public Health 2018, 29, 494–499. [Google Scholar] [CrossRef] [PubMed]
- Laursen, R.P.; Larnkjær, A.; Ritz, C.; Hauger, H.; Michaelsen, K.F.; Mølgaard, C. Probiotics and child care absence due to infections: A randomized controlled trial. Pediatrics 2017, 140, e20170735. [Google Scholar] [CrossRef] [PubMed]
- Laursen, R.P.; Hojsak, I. Probiotics for respiratory tract infections in children attending day care centers—A systematic review. Eur. J. Pediatr. 2018, 177, 979–994. [Google Scholar] [CrossRef] [PubMed]
- Skórka, A.; Piescik-Lech, M.; Kolodziej, M.; Szajewska, H. To add or not to add probiotics to infant formulae? An updated systematic review. Benef. Microbes 2017, 8, 717–725. [Google Scholar] [CrossRef]
- Xiao, L.; Gong, C.; Ding, Y.; Ding, G.; Xu, X.; Deng, C.; Ze, X.; Malard, P.; Ben, X. Probiotics maintain intestinal secretory immunoglobulin a levels in healthy formula-fed infants: A randomised, double-blind, placebo-controlled study. Benef. Microbes 2019, 10, 729–739. [Google Scholar] [CrossRef]
- Cacho, N.T.; Lawrence, R.M. Innate immunity and breast milk. Front. Immunol. 2017, 8. [Google Scholar] [CrossRef]
- Moore, L.V.; Thompson, F.E. Adults meeting fruit and vegetable intake recommendations—United States, 2013. MMWR Morb. Mortal Wkly. Rep. 2015, 64, 709–713. [Google Scholar]
- Nour, M.; Sui, Z.; Grech, A.; Rangan, A.; McGeechan, K.; Allman-Farinelli, M. The fruit and vegetable intake of young australian adults: A population perspective. Public Health Nutr. 2017, 20, 2499–2512. [Google Scholar] [CrossRef]
- Wood, L.G.; Li, Q.; Scott, H.A.; Rutting, S.; Berthon, B.S.; Gibson, P.G.; Hansbro, P.M.; Williams, E.; Horvat, J.; Simpson, J.L. Saturated fatty acids, obesity, and the nucleotide oligomerization domain–like receptor protein 3 (nlrp3) inflammasome in asthmatic patients. J. Allergy Clin. Immunol. 2019, 143, 305–315. [Google Scholar] [CrossRef]
- Peeri, N.C.; Shrestha, N.; Rahman, M.S.; Zaki, R.; Tan, Z.; Bibi, S.; Baghbanzadeh, M.; Aghamohammadi, N.; Zhang, W.; Haque, U. The SARS, MERS and novel coronavirus (Covid-19) epidemics, the newest and biggest global health threats: What lessons have we learned? Int. J. Epidemiol. 2020. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with covid-19 in wuhan, china: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Kim, H.; Caulfield Laura, E.; Garcia-Larsen, V.; Steffen Lyn, M.; Coresh, J.; Rebholz Casey, M. Plant-based diets are associated with a lower risk of incident cardiovascular disease, cardiovascular disease mortality, and all-cause mortality in a general population of middle-aged adults. J. Am. Heart Assoc. 2019, 8, e012865. [Google Scholar] [CrossRef] [PubMed]
- Kopf, J.C.; Suhr, M.J.; Clarke, J.; Eyun, S.-i.; Riethoven, J.-J.M.; Ramer-Tait, A.E.; Rose, D.J. Role of whole grains versus fruits and vegetables in reducing subclinical inflammation and promoting gastrointestinal health in individuals affected by overweight and obesity: A randomized controlled trial. Nutr. J. 2018, 17, 72. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Liu, X.; Wang, W.; Zhang, D. Consumption of vegetables and fruit and the risk of inflammatory bowel disease: A meta-analysis. Eur. J. Gastroenterol. Hepatol. 2015, 27, 623–630. [Google Scholar] [CrossRef]
- Cui, J.; Lian, Y.; Zhao, C.; Du, H.; Han, Y.; Gao, W.; Xiao, H.; Zheng, J. Dietary fibers from fruits and vegetables and their health benefits via modulation of gut microbiota. Compr. Rev. Food Sci. F. 2019, 18, 1514–1532. [Google Scholar] [CrossRef]
- Park, Y.; Subar, A.F.; Hollenbeck, A.; Schatzkin, A. Dietary fiber intake and mortality in the nih-aarp diet and health study. Arch. Intern. Med. 2011, 171, 1061–1068. [Google Scholar] [CrossRef]
- Hanson, C.; Lyden, E.; Rennard, S.; Mannino, D.M.; Rutten, E.P.; Hopkins, R.; Young, R. The relationship between dietary fiber intake and lung function in the national health and nutrition examination surveys. Ann. Am. Thorac. Soc. 2016, 13, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Langkamp-Henken, B.; Wood, S.M.; Herlinger-Garcia, K.A.; Thomas, D.J.; Stechmiller, J.K.; Bender, B.S.; Gardner, E.M.; DeMichele, S.J.; Schaller, J.P.; Murasko, D.M. Nutritional formula improved immune profiles of seniors living in nursing homes. J. Am. Geriatr. Soc. 2006, 54, 1861–1870. [Google Scholar] [CrossRef]
- Langkamp-henken, B.; Bender, B.S.; Gardner, E.M.; Herrlinger-garcia, K.A.; Kelley, M.J.; Murasko, D.M.; Schaller, J.P.; Stechmiller, J.K.; Thomas, D.J.; Wood, S.M. Nutritional formula enhanced immune function and reduced days of symptoms of upper respiratory tract infection in seniors. J. Am. Geriatr. Soc. 2004, 52, 3–12. [Google Scholar] [CrossRef]
- Bunout, D.; Hirsch, S.; de la Maza, M.; Munoz, C.; Haschke, F.; Steenhout, P.; Klassen, P.; Barrera, G.; Gattas, V.; Petermann, M. Effects of prebiotics on the immune response to vaccination in the elderly. J. Parenter. Enteral Nutr. 2002, 26, 372–376. [Google Scholar] [CrossRef]
- Lomax, A.R.; Cheung, L.V.Y.; Noakes, P.S.; Miles, E.A.; Calder, P.C. Inulin-type β2-1 fructans have some effect on the antibody response to seasonal influenza vaccination in healthy middle-aged humans. Front. Immunol. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Trompette, A.; Gollwitzer, E.S.; Yadava, K.; Sichelstiel, A.K.; Sprenger, N.; Ngom-Bru, C.; Blanchard, C.; Junt, T.; Nicod, L.P.; Harris, N.L. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat. Med. 2014, 20, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Kamp, M.E.; Shim, R.; Nicholls, A.J.; Oliveira, A.C.; Mason, L.J.; Binge, L.; Mackay, C.R.; Wong, C.H.Y. G protein-coupled receptor 43 modulates neutrophil recruitment during acute inflammation. PLoS ONE 2016, 11, e0163750. [Google Scholar] [CrossRef]
- Ang, Z.; Ding, J.L. Gpr41 and gpr43 in obesity and inflammation - protective or causative? Front. Immunol. 2016, 7, 28. [Google Scholar] [CrossRef]
- Baxter, N.T.; Schmidt, A.W.; Venkataraman, A.; Kim, K.S.; Waldron, C.; Schmidt, T.M. Dynamics of human gut microbiota and short-chain fatty acids in response to dietary interventions with three fermentable fibers. MBio 2019, 10, e02566–02518. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.A.; Grant, L.J.; Gidley, M.J.; Mikkelsen, D. Gut fermentation of dietary fibres: Physico-chemistry of plant cell walls and implications for health. Int. J. Mol. Sci. 2017, 18, 2203. [Google Scholar] [CrossRef]
- Kolida, S.; Gibson, G.R. Synbiotics in health and disease. Annu. Rev. Food Sci. Technol. 2011, 2, 373–393. [Google Scholar] [CrossRef]
- Weiss, G.; Christensen, H.R.; Zeuthen, L.H.; Vogensen, F.K.; Jakobsen, M.; Frøkiær, H. Lactobacilli and bifidobacteria induce differential interferon-β profiles in dendritic cells. Cytokine 2011, 56, 520–530. [Google Scholar] [CrossRef]
- Donohoe, D.R.; Holley, D.; Collins, L.B.; Montgomery, S.A.; Whitmore, A.C.; Hillhouse, A.; Curry, K.P.; Renner, S.W.; Greenwalt, A.; Ryan, E.P.; et al. A gnotobiotic mouse model demonstrates that dietary fiber protects against colorectal tumorigenesis in a microbiota- and butyrate-dependent manner. Cancer Discov. 2014, 4, 1387–1397. [Google Scholar] [CrossRef] [PubMed]
- Akatsu, H.; Nagafuchi, S.; Kurihara, R.; Okuda, K.; Kanesaka, T.; Ogawa, N.; Kanematsu, T.; Takasugi, S.; Yamaji, T.; Takami, M.; et al. Enhanced vaccination effect against influenza by prebiotics in elderly patients receiving enteral nutrition. Geriat. Gerontol. Int. 2016, 16, 205–213. [Google Scholar] [CrossRef]
- Przemska-Kosicka, A.; Childs, C.E.; Maidens, C.; Dong, H.; Todd, S.; Gosney, M.A.; Tuohy, K.M.; Yaqoob, P. Age-related changes in the natural killer cell response to seasonal influenza vaccination are not influenced by a synbiotic: A randomised controlled trial. Front. Immunol. 2018, 9, 591. [Google Scholar] [CrossRef] [PubMed]
- Enani, S.; Przemska-Kosicka, A.; Childs, C.E.; Maidens, C.; Dong, H.; Conterno, L.; Tuohy, K.; Todd, S.; Gosney, M.; Yaqoob, P. Impact of ageing and a synbiotic on the immune response to seasonal influenza vaccination; a randomised controlled trial. Clin. Nutr. 2018, 37, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Przemska-Kosicka, A.; Childs, C.E.; Enani, S.; Maidens, C.; Dong, H.; Dayel, I.B.; Tuohy, K.; Todd, S.; Gosney, M.A.; Yaqoob, P. Effect of a synbiotic on the response to seasonal influenza vaccination is strongly influenced by degree of immunosenescence. Immun. Ageing 2016, 13. [Google Scholar] [CrossRef]
- Hickson, L.J.; Langhi Prata, L.G.P.; Bobart, S.A.; Evans, T.K.; Giorgadze, N.; Hashmi, S.K.; Herrmann, S.M.; Jensen, M.D.; Jia, Q.; Jordan, K.L.; et al. Senolytics decrease senescent cells in humans: Preliminary report from a clinical trial of dasatinib plus quercetin in individuals with diabetic kidney disease. EBioMedicine 2019, 47, 446–456. [Google Scholar] [CrossRef] [PubMed]
- Thoppil, H.; Riabowol, K. Senolytics: A translational bridge between cellular senescence and organismal aging. Front. Cell Dev. Biol. 2020, 7, 367. [Google Scholar] [CrossRef]
- Li, W.; Qin, L.; Feng, R.; Hu, G.; Sun, H.; He, Y.; Zhang, R. Emerging senolytic agents derived from natural products. Mech. Ageing Dev. 2019, 181, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Park, J.; Kim, M. Gut microbiota-derived short-chain fatty acids, T cells, and inflammation. Immune Netw. 2014, 14, 277–288. [Google Scholar] [CrossRef]
- Schulthess, J.; Pandey, S.; Capitani, M.; Rue-Albrecht, K.C.; Arnold, I.; Franchini, F.; Chomka, A.; Ilott, N.E.; Johnston, D.G.; Pires, E. The short chain fatty acid butyrate imprints an antimicrobial program in macrophages. Immunity 2019, 50, 432–445.e437. [Google Scholar] [CrossRef]
- Li, N.; Liu, X.-X.; Hong, M.; Huang, X.-Z.; Chen, H.; Xu, J.-H.; Wang, C.; Zhang, Y.-X.; Zhong, J.-X.; Nie, H. Sodium butyrate alleviates lps-induced acute lung injury in mice via inhibiting hmgb1 release. Int. Immunopharmacol. 2018, 56, 242–248. [Google Scholar] [CrossRef]
- Majeed, M.; Nagabhushanam, K.; Arumugam, S.; Natarajan, S.; Majeed, S.; Pande, A.; Beede, K.; Ali, F. Cranberry seed fibre: A promising prebiotic fibre and its fermentation by the probiotic bacillus coagulans mtcc 5856. Int. J. Food Sci. Technol. 2018, 53, 1640–1647. [Google Scholar] [CrossRef]
- Majeed, M.; Majeed, S.; Nagabhushanam, K.; Arumugam, S.; Natarajan, S.; Beede, K.; Ali, F. Galactomannan from trigonella foenum-graecum l. Seed: Prebiotic application and its fermentation by the probiotic bacillus coagulans strain mtcc 5856. Food Sci. Nutr. 2018, 6, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Walton, S.L.; Bischoff, K.M.; van Heiningen, A.R.; van Walsum, G.P. Production of lactic acid from hemicellulose extracts by Bacillus coagulans MXL-9. J. Ind. Microbiol. Biotechnol. 2010, 37, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Nyangale, E.P.; Farmer, S.; Keller, D.; Chernoff, D.; Gibson, G.R. Effect of prebiotics on the fecal microbiota of elderly volunteers after dietary supplementation of bacillus coagulans gbi-30, 6086. Anaerobe 2014, 30, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Trachsel, J.; Briggs, C.; Gabler, N.K.; Allen, H.K.; Loving, C.L. Dietary resistant potato starch alters intestinal microbial communities and their metabolites, and markers of immune regulation and barrier function in swine. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef]
- Denaro, M.; Smeriglio, A.; Barreca, D.; De Francesco, C.; Occhiuto, C.; Milano, G.; Trombetta, D. Antiviral activity of plants and their isolated bioactive compounds: An update. Phytother. Res. 2020, 34, 742–768. [Google Scholar] [CrossRef]
- Calder, P.C.; Carr, A.C.; Gombart, A.F.; Eggersdorfer, M. Optimal nutritional status for a well-functioning immune system is an important factor to protect against viral infections. Nutrients 2020, 12, 1181. [Google Scholar] [CrossRef]
- Bahramsoltani, R.; Sodagari, H.R.; Farzaei, M.H.; Abdolghaffari, A.H.; Gooshe, M.; Rezaei, N. The preventive and therapeutic potential of natural polyphenols on influenza. Expert Rev. Anti. Infect. Ther. 2016, 14, 57–80. [Google Scholar] [CrossRef]
- Mohammadi Pour, P.; Fakhri, S.; Asgary, S.; Farzaei, M.H.; Echeverría, J. The signaling pathways, and therapeutic targets of antiviral agents: Focusing on the antiviral approaches and clinical perspectives of anthocyanins in the management of viral diseases. Front. Pharmacol. 2019, 10, 1207. [Google Scholar] [CrossRef]
- Kawabata, K.; Yoshioka, Y.; Terao, J. Role of intestinal microbiota in the bioavailability and physiological functions of dietary polyphenols. Molecules 2019, 24, 370. [Google Scholar] [CrossRef]
- Pae, M.; Wu, D. Immunomodulating effects of epigallocatechin-3-gallate from green tea: Mechanisms and applications. Food Funct. 2013, 4, 1287–1303. [Google Scholar] [CrossRef]
- Xu, M.-J.; Liu, B.-J.; Wang, C.-L.; Wang, G.-H.; Tian, Y.; Wang, S.-H.; Li, J.; Li, P.-Y.; Zhang, R.-H.; Wei, D.; et al. Epigallocatechin-3-gallate inhibits tlr4 signaling through the 67-kda laminin receptor and effectively alleviates acute lung injury induced by h9n2 swine influenza virus. Int. Immunopharmacol. 2017, 52, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Won, Y.-S.; Yang, X.; Kumazoe, M.; Yamashita, S.; Hara, A.; Takagaki, A.; Goto, K.; Nanjo, F.; Tachibana, H. Green tea catechin metabolites exert immunoregulatory effects on cd4+ t cell and natural killer cell activities. J. Agric. Food Chem. 2016, 64, 3591–3597. [Google Scholar] [CrossRef] [PubMed]
- Ozdal, T.; Sela, D.A.; Xiao, J.; Boyacioglu, D.; Chen, F.; Capanoglu, E. The reciprocal interactions between polyphenols and gut microbiota and effects on bioaccessibility. Nutrients 2016, 8, 78. [Google Scholar] [CrossRef] [PubMed]
- Nash, V.; Ranadheera, C.S.; Georgousopoulou, E.N.; Mellor, D.D.; Panagiotakos, D.B.; McKune, A.J.; Kellett, J.; Naumovski, N. The effects of grape and red wine polyphenols on gut microbiota—A systematic review. Food Res. Int. 2018, 113, 277–287. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shinde, T.; Hansbro, P.M.; Sohal, S.S.; Dingle, P.; Eri, R.; Stanley, R. Microbiota Modulating Nutritional Approaches to Countering the Effects of Viral Respiratory Infections Including SARS-CoV-2 through Promoting Metabolic and Immune Fitness with Probiotics and Plant Bioactives. Microorganisms 2020, 8, 921. https://doi.org/10.3390/microorganisms8060921
Shinde T, Hansbro PM, Sohal SS, Dingle P, Eri R, Stanley R. Microbiota Modulating Nutritional Approaches to Countering the Effects of Viral Respiratory Infections Including SARS-CoV-2 through Promoting Metabolic and Immune Fitness with Probiotics and Plant Bioactives. Microorganisms. 2020; 8(6):921. https://doi.org/10.3390/microorganisms8060921
Chicago/Turabian StyleShinde, Tanvi, Philip M Hansbro, Sukhwinder Singh Sohal, Peter Dingle, Rajaraman Eri, and Roger Stanley. 2020. "Microbiota Modulating Nutritional Approaches to Countering the Effects of Viral Respiratory Infections Including SARS-CoV-2 through Promoting Metabolic and Immune Fitness with Probiotics and Plant Bioactives" Microorganisms 8, no. 6: 921. https://doi.org/10.3390/microorganisms8060921
APA StyleShinde, T., Hansbro, P. M., Sohal, S. S., Dingle, P., Eri, R., & Stanley, R. (2020). Microbiota Modulating Nutritional Approaches to Countering the Effects of Viral Respiratory Infections Including SARS-CoV-2 through Promoting Metabolic and Immune Fitness with Probiotics and Plant Bioactives. Microorganisms, 8(6), 921. https://doi.org/10.3390/microorganisms8060921





