Impact of High-Hydrostatic Pressure Treatments Applied Singly or in Combination with Moderate Heat on the Microbial Load, Antimicrobial Resistance, and Bacterial Diversity of Guacamole
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Guacamole Samples
2.2. High-Hydrostatic Pressure Treatments
2.3. Microbiological Analysis
2.4. DNA Extraction
2.5. DNA Sequencing and Analysis
2.6. Statistical Analysis
3. Results
3.1. Effect of Pressure Treatments on Microbial Load and Antimicrobial Resistance
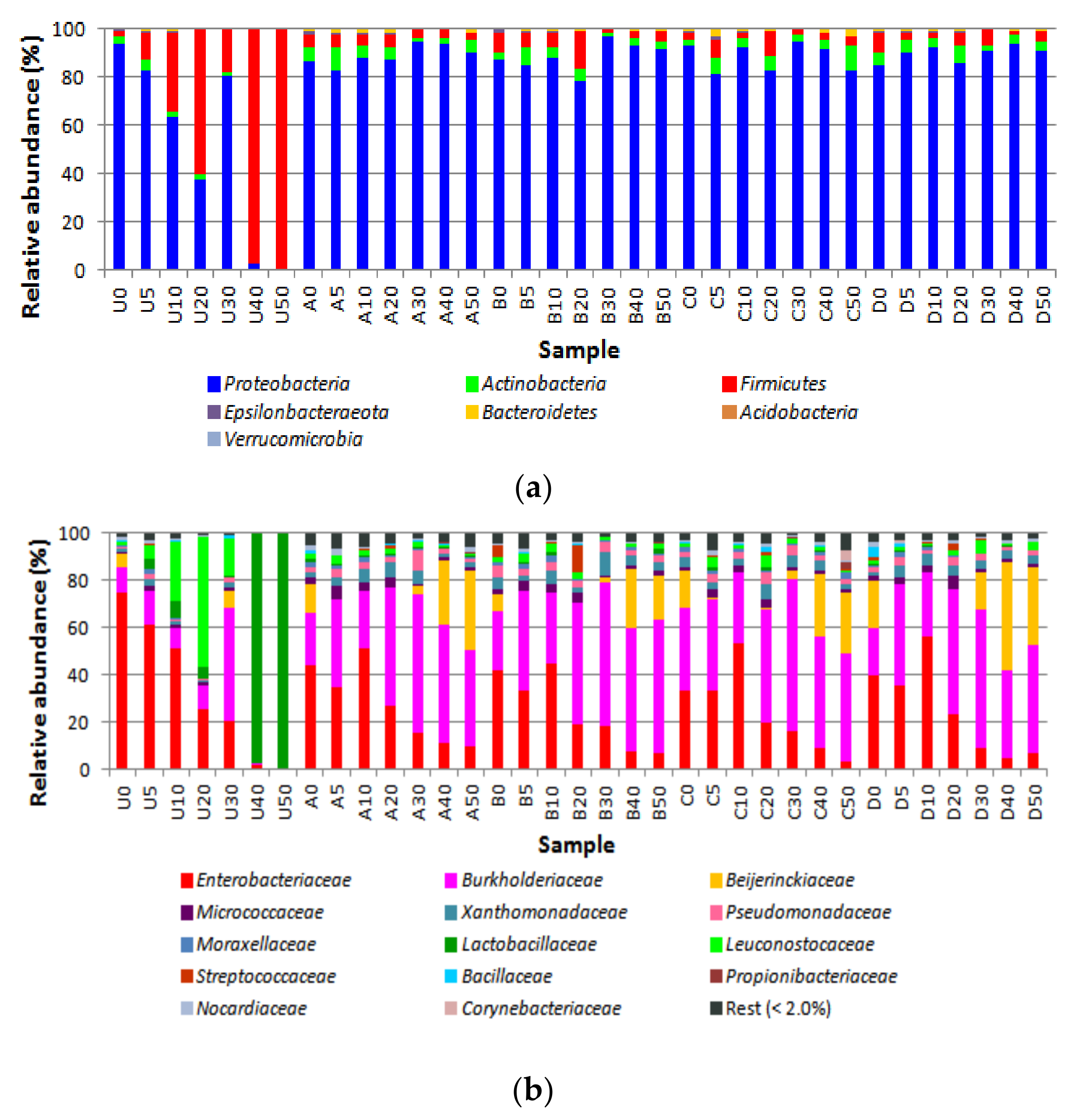
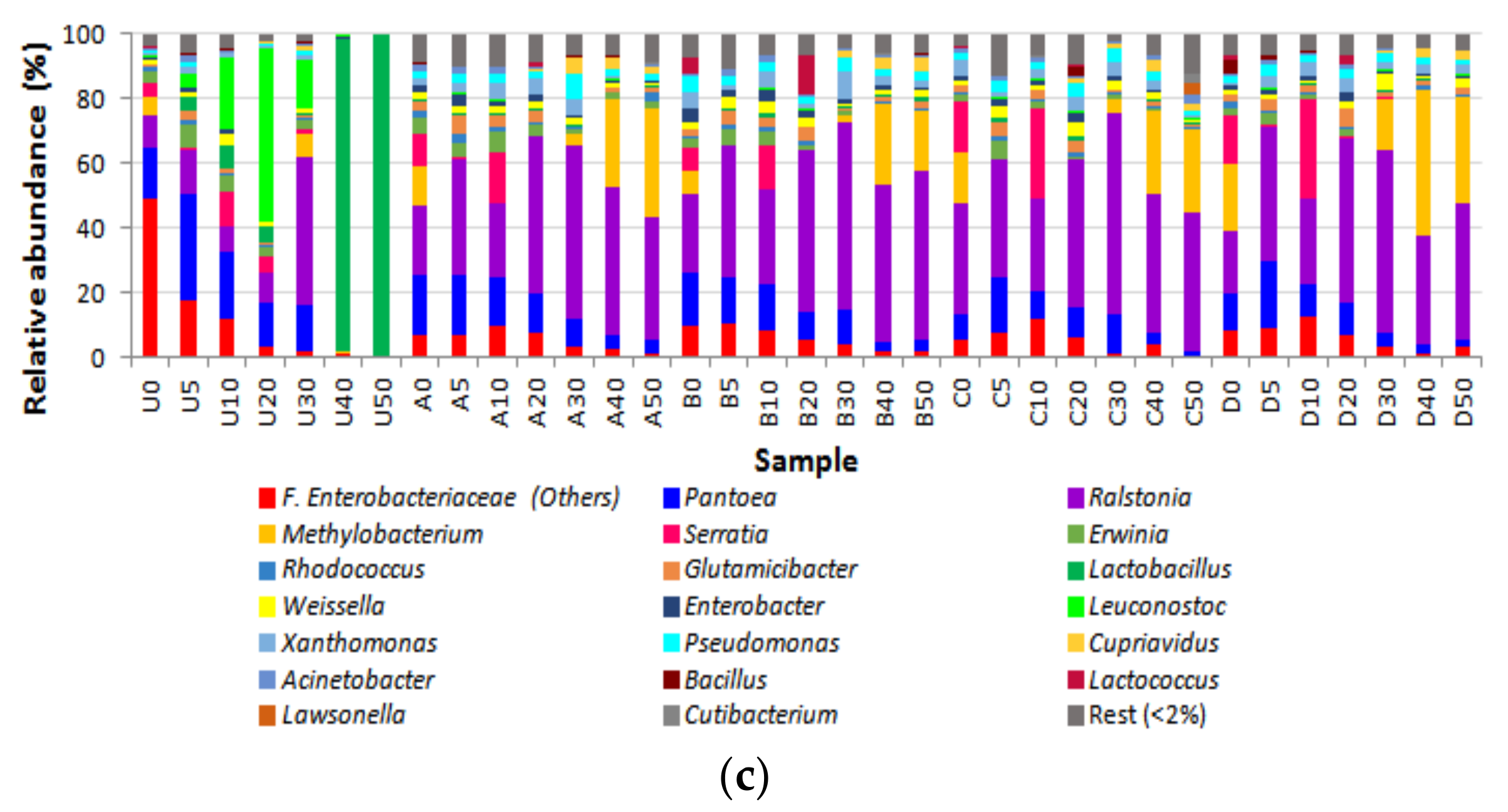
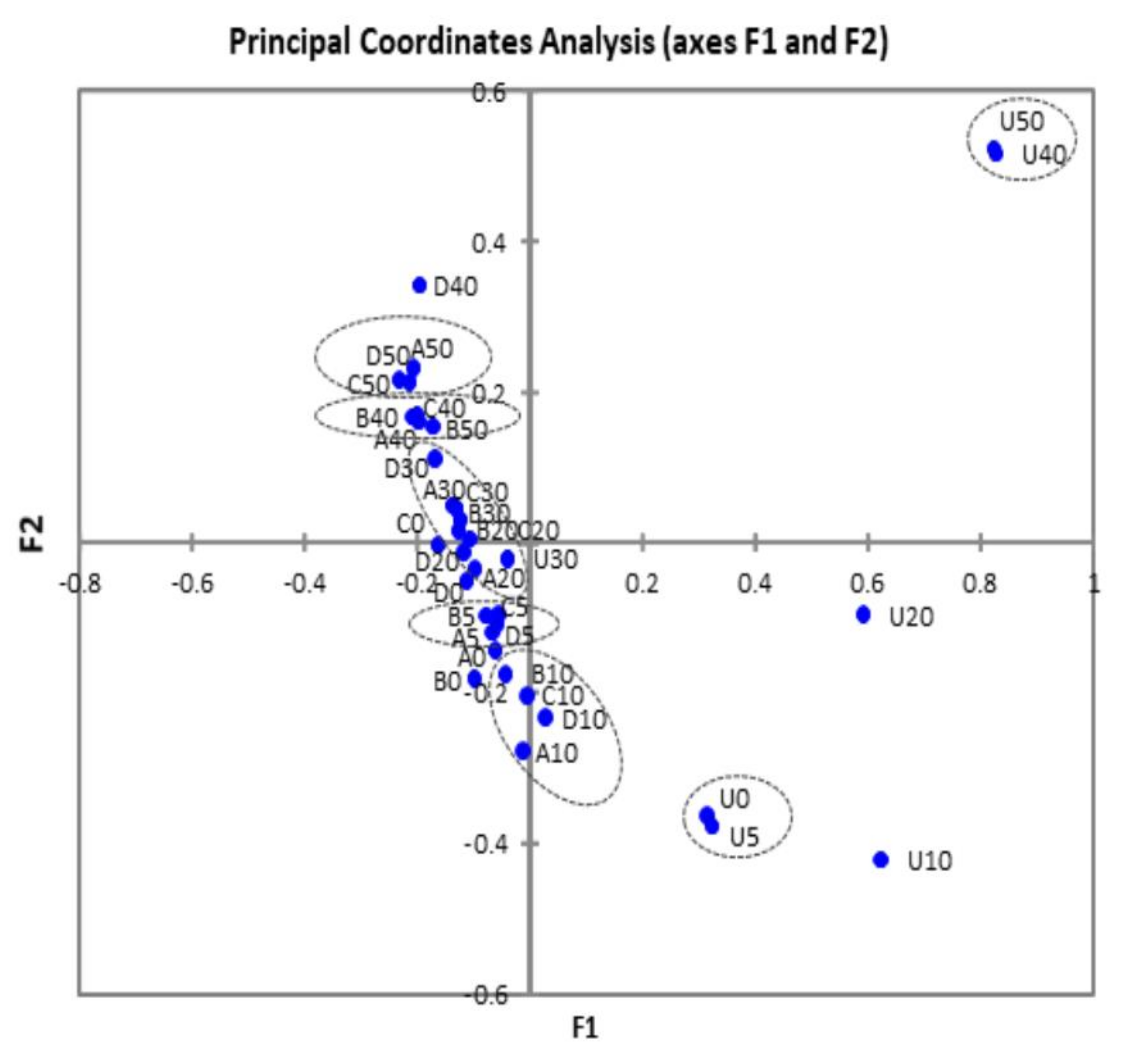
3.2. Bacterial Diversity
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Slater, G.G.; Shankman, S.; Shepherd, J.S.; Alfin-Slater, R.B. Seasonal variation in the composition of California avocados. J. Agric. Food Chem. 1975, 23, 468–474. [Google Scholar] [CrossRef] [PubMed]
- USDA (U.S. Department of Agriculture). Avocado, Almond, Pistachio and Walnut Composition; Nutrient Data Laboratory, USDA National Nutrient Database for Standard Reference, Release 24; U.S. Department of Agriculture: Washington, DC, USA, 2011.
- Alvarez, L.D.; Moreno, A.O.; Ochoa, F.G. Avocado. In Tropical and Subtropical Fruits: Postharvest Physiology, Processing and Packaging; Wiley-Blackwell: Hoboken, NJ, USA, 2012; Volume 23, pp. 437–454. ISBN 978-0-813-81142-0. [Google Scholar]
- Duarte, P.F.; Chaves, M.A.; Borges, C.D.; Mendonça, C.R.B. Avocado: Characteristics, health benefits and uses. Ciência Rural 2016, 46, 747–754. [Google Scholar] [CrossRef]
- Araújo, R.G.; Rodriguez-Jasso, R.; Ruíz, H.A.; Pintado, M.M.E.; Aguilar, C.N. Avocado by-products: Nutritional and functional properties. Trends Food Sci. Technol. 2018, 80, 51–60. [Google Scholar] [CrossRef]
- Lee, J.; Koo, N.; Min, D.B. Reactive Oxygen Species, Aging, and Antioxidative Nutraceuticals. Compr. Rev. Food Sci. Food Saf. 2004, 3, 21–33. [Google Scholar] [CrossRef]
- Ortiz, J.; Rodriguez, A.; Vega, C.; Osorio, F.; Defillipi, B.; Ferreira, R.; Saavedra, J. Textural, flow and viscoelastic properties of Hass avocado (Persea americana Mill.) during ripening under refrigeration conditions. J. Food Eng. 2018, 219, 62–70. [Google Scholar] [CrossRef]
- González-Fernández, J.J.; Galea, Z.; Álvarez, J.M.; Hormaza, J.; López, R.; López, R. Evaluation of composition and performance of composts derived from guacamole production residues. J. Environ. Manag. 2015, 147, 132–139. [Google Scholar] [CrossRef]
- Kendall, M.E.; Mody, R.K.; Mahon, B.E.; Doyle, M.P.; Herman, K.M.; Tauxe, R.V. Emergence of Salsa and Guacamole as Frequent Vehicles of Foodborne Disease Outbreaks in the United States, 1973–2008. Foodborne Pathog. Dis. 2013, 10, 316–322. [Google Scholar] [CrossRef]
- López-Malo, A.; Palou, E.; Barbosa-Cánovas, G.; Welti-Chanes, J.; Swanson, B. Polyphenoloxidase activity and color changes during storage of high hydrostatic pressure treated avocado puree. Food Res. Int. 1998, 31, 549–556. [Google Scholar] [CrossRef]
- Palou, E.; Hernández-Salgado, C.; López-Malo, A.; Barbosa-Cánovas, G.; Swanson, B.; Welti-Chanes, J. High pressure-processed guacamole. Innov. Food Sci. Emerg. Technol. 2000, 1, 69–75. [Google Scholar] [CrossRef]
- Jacobo-Velázquez, D.A.; Hernández-Brenes, C. Biochemical Changes during the Storage of High Hydrostatic Pressure Processed Avocado Paste. J. Food Sci. 2010, 75, S264–S270. [Google Scholar] [CrossRef]
- Jacobo-Velázquez, D.A.; Hernández-Brenes, C. Sensory Shelf-Life Limiting Factor of High Hydrostatic Pressure Processed Avocado Paste. J. Food Sci. 2011, 76, S388–S395. [Google Scholar] [CrossRef] [PubMed]
- Patterson, M.F.; Kilpatrick, D.J. The Combined Effect of High Hydrostatic Pressure and Mild Heat on Inactivation of Pathogens in Milk and Poultry. J. Food Prot. 1998, 61, 432–436. [Google Scholar] [CrossRef] [PubMed]
- Krebbers, B.; Matser, A.M.; Hoogerwerf, S.W.; Moezelaar, R.; Tomassen, M.M.; Berg, R.W.V.D. Combined high-pressure and thermal treatments for processing of tomato puree: Evaluation of microbial inactivation and quality parameters. Innov. Food Sci. Emerg. Technol. 2003, 4, 377–385. [Google Scholar] [CrossRef]
- Moerman, F.; Mertens, B.; Demey, L.; Huyghebaert, A. Reduction of Bacillus subtilis, Bacillus stearothermophilus and Streptococcus faecalis in meat batters by temperature-high hydrostatic pressure pasteurization. Meat Sci. 2001, 59, 115–125. [Google Scholar] [CrossRef]
- Murano, E.A.; Murano, P.S.; Brennan, R.E.; Shenoy, K.; Moreira, R.G. Application of High Hydrostatic Pressure to Eliminate Listeria monocytogenes from Fresh Pork Sausage. J. Food Prot. 1999, 62, 480–483. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.V.M. Heat assisted HPP for the inactivation of bacteria, moulds and yeasts spores in foods: Log reductions and mathematical models. Trends Food Sci. Technol. 2019, 88, 143–156. [Google Scholar] [CrossRef]
- Kim, M.; Weigand, M.R.; Oh, S.; Hatt, J.K.; Krishnan, R.; Tezel, U.; Pavlostathis, S.G.; Konstantinidis, K.T. Widely Used Benzalkonium Chloride Disinfectants Can Promote Antibiotic Resistance. Appl. Environ. Microbiol. 2018, 84, e01201-18. [Google Scholar] [CrossRef]
- Tezel, U.; Pavlostathis, S.G. Quaternary ammonium disinfectants: Microbial adaptation, degradation and ecology. Curr. Opin. Biotechnol. 2015, 33, 296–304. [Google Scholar] [CrossRef]
- Morrison, B.J.; Rubin, J.E. Carbapenemase Producing Bacteria in the Food Supply Escaping Detection. PLoS ONE 2015, 10, e0126717. [Google Scholar] [CrossRef]
- Bonardi, S.; Pitino, R. Carbapenemase-producing bacteria in food-producing animals, wildlife and environment: A challenge for human health. Ital. J. Food Saf. 2019, 8, 7956. [Google Scholar] [CrossRef]
- Iseppi, R.; De Niederhäusern, S.; Bondi, M.; Messi, P.; Sabia, C. Extended-Spectrum β-Lactamase, AmpC, and MBL-Producing Gram-Negative Bacteria on Fresh Vegetables and Ready-to-Eat Salads Sold in Local Markets. Microb. Drug Resist. 2018, 24, 1156–1164. [Google Scholar] [CrossRef] [PubMed]
- Zurfluh, K.; Poirel, L.; Nordmann, P.; Klumpp, J.; Stephan, R. First detection of Klebsiella variicola producing OXA-181 carbapenemase in fresh vegetable imported from Asia to Switzerland. Antimicrob. Resist. Infect. Control 2015, 4, 38. [Google Scholar] [CrossRef] [PubMed]
- Nocker, A.; Sossa-Fernandez, P.; Burr, M.D.; Camper, A.K. Use of Propidium Monoazide for Live/Dead Distinction in Microbial Ecology. Appl. Environ. Microbiol. 2007, 73, 5111–5117. [Google Scholar] [CrossRef] [PubMed]
- Elizaquível, P.; Sánchez, G.; Aznar, R. Quantitative detection of viable foodborne E. coli O157:H7, Listeria monocytogenes and Salmonella in fresh-cut vegetables combining propidium monoazide and real-time PCR. Food Control 2012, 25, 704–708. [Google Scholar] [CrossRef]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glöckner, F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2012, 41, e1. [Google Scholar] [CrossRef]
- Schmieder, R.; Edwards, R.A. Quality control and preprocessing of metagenomic datasets. Bioinformatics 2011, 27, 863–864. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, N.; Lozupone, C.A.; Turnbaugh, P.J.; Fierer, N.; Knight, R. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc. Natl. Acad. Sci. USA 2010, 108, 4516–4522. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2012, 41, D590–D596. [Google Scholar] [CrossRef]
- Waite, J.; Jones, J.; Turek, E.; Dunne, C.; Wright, A.; Yang, T.; Beckwitt, R.; Yousef, A.E. Production of Shelf-Stable Ranch Dressing Using High-Pressure Processing. J. Food Sci. 2009, 74, M83–M93. [Google Scholar] [CrossRef]
- Del Árbol, J.T.; Pulido, R.P.; La Storia, A.; Grande, M.J.; Lucas, R.; Ercolini, D.; Gálvez, A. Microbial diversity in pitted sweet cherries (Prunus avium L.) as affected by High-Hydrostatic Pressure treatment. Food Res. Int. 2016, 89, 790–796. [Google Scholar] [CrossRef] [PubMed]
- Smelt, J.P.P.M. Recent advances in the microbiology of high pressure processing. Trends Food Sci. Technol. 1998, 9, 152–158. [Google Scholar] [CrossRef]
- Kalchayanand, N.; Sikes, A.; Dunne, C.P.; Ray, B. Interaction of Hydrostatic Pressure, Time and Temperature of Pressurization and Pediocin AcH on Inactivation of Foodborne Bacteria. J. Food Prot. 1998, 61, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Zabat, M.; Sano, W.; Wurster, J.; Cabral, D.; Belenky, P. Microbial Community Analysis of Sauerkraut Fermentation Reveals a Stable and Rapidly Established Community. Foods 2018, 7, 77. [Google Scholar] [CrossRef] [PubMed]
- Fusco, V.; Quero, G.M.; Cho, G.-S.; Kabisch, J.; Meske, D.; Neve, H.; Bockelmann, W.; Franz, C.M.A.P. The genus Weissella: Taxonomy, ecology and biotechnological potential. Front. Microbiol. 2015, 6, 155. [Google Scholar] [CrossRef] [PubMed]
| Total Aerobic Mesophiles | T0 | T5 | T10 | T20 | T30 | T40 | T50 |
|---|---|---|---|---|---|---|---|
| Controls | 4.89 ± 0.09 | 5.03 ± 0.11 | 5.98 ± 0.13 a | 5.79 ± 0.21 a | 6.70 ± 0.12 a | 6.55 ± 0.06 a | 6.25 ± 0.19 a |
| Treatment A | 2.57 ± 0.14 b | 3.29 ± 0.10 b | 3.24 ± 0.14 b | 3.30 ± 0.18 b | 3.27 ± 0.15 b | 3.21 ± 0.27 b | 3.24 ± 0.13 b |
| Treatment B | 2.07 ± 0.12 b,c | 2.70 ± 0.14 b,c | 2.85 ± 0.17 b | 2.51 ± 0.17 b,c | 2.59 ± 0.09 b,c | 3.39 ± 0.13 b | 2.43 ± 0.10 b,c |
| Treatment C | 2.60 ± 0.11 b | 3.34 ± 0.16 b | 3.32 ± 0.14 b | 3.40 ± 0.08 b | 3.42 ± 0.14 b | 3.94 ± 0.20 b | 3.14 ± 0.21 b |
| Treatment D | 1.55 ± 0.15 b,d | 2.08 ± 0.15 b,d | 2.50 ± 0.16 b,d | 2.12 ± 0.13 b,d | 2.42 ± 0.11 b,d | 1.82 ± 0.26 b,d | 1.75 ± 0.20 b,d |
| Enterobacteriaceae | T0 | T5 | T10 | T20 | T30 | T40 | T50 |
| Controls | 4.63 ± 0.14 | 4.48 ± 0.12 | 4.17 ± 0.14 e | 3.61 ± 0.13 e | 2.55 ± 0.12 e | <1.48 | 1.55 ± 0.15 e |
| Treatment A | <1.48 | <1.48 | <1.48 | <1.48 | <1.48 | <1.48 | <1.48 |
| Treatment B | <1.48 | <1.48 | <1.48 | <1.48 | <1.48 | <1.48 | <1.48 |
| Treatment C | <1.48 | <1.48 | <1.48 | <1.48 | <1.48 | <1.48 | <1.48 |
| Treatment D | <1.48 | <1.48 | <1.48 | <1.48 | <1.48 | <1.48 | <1.48 |
| Yeasts and molds | T0 | T5 | T10 | T20 | T30 | T40 | T50 |
| Controls | 2.55 ± 0.12 | 2.83 ± 0.27 | 4.79 ± 0.10 a | 5.87 ± 0.18 a | 6.46 ± 0.16 a | 5.60 ± 0.29 a | 5.33 ± 0.20 a |
| Treatment A | 1.82 ± 0.26 f | <1.48 | <1.48 | 2.17 ± 0.22 f | <1.48 | 3.24 ± 0.24 f | <1.48 |
| Treatment B | <1.48 | <1.48 | <1.48 | <1.48 | <1.48 | <1.48 | <1.48 |
| Treatment C | <1.48 | <1.48 | <1.48 | 1.60 ± 0.09 | <1.48 | <1.48 | <1.48 |
| Treatment D | <1.48 | <1.48 | <1.48 | <1.48 | <1.48 | <1.48 | <1.48 |
| pH | T0 | T5 | T10 | T20 | T30 | T40 | T50 |
| Controls | 4.53 ± 0.01 | 4.34 ± 0.02 g | 4.35 ± 0.03 g | 4.33 ± 0.01 g | 4.30 ± 0.01 g | 4.23 ± 0.01 g | 4.07 ± 0.08 g |
| Treatment A | 4.47 ± 0.01 | 4.41 ± 0.01 | 4.44 ± 0.03 | 4.41 ± 0.01 | 4.35 ± 0.08 | 4.25 ± 0.10 h | 4.35 ± 0.01 |
| Treatment B | 4.41 ± 0.01 | 4.43 ± 0.01 | 4.45 ± 0.01 | 4.43 ± 0.01 | 4.43 ± 0.03 | 4.34 ± 0.03 | 4.35 ± 0.03 |
| Treatment C | 4.46 ± 0.01 | 4.42 ± 0.03 | 4.44 ± 0.01 | 4.42 ± 0.06 | 4.42 ± 0.03 | 4.33 ± 0.04 | 4.34 ± 0.01 |
| Treatment D | 4.42 ± 0.04 | 4.45 ± 0.01 | 4.43 ± 0.01 | 4.45 ± 0.01 | 4.45 ± 0.03 | 4.36 ± 0.06 | 4.35 ± 0.04 |
| Antimicrobial | T0 | T5 | T10 | T20 | T30 | T40 | T50 |
|---|---|---|---|---|---|---|---|
| Cefotaxime | <1.48 | <1.48 | <1.48 | 2.85 ± 0.07 | 2.84 ± 0.16 | 1.50 ± 0.03 a | <1.48 |
| Imipenem | <1.48 | <1.48 | 1.87 ± 0.12 | 3.13 ± 0.25 b | 1.63 ± 0.21 | <1.48 | <1.48 |
| KPC aerobiosis | 3.26 ± 0.12 | 4.13 ± 0.07 c | 4.63 ± 0.23 c | 5.64 ± 0.12 c | 6.17 ± 0.13 c | 6.05 ± 0.18 c | 5.49 ± 0.13 c |
| KPC anaerobiosis | 2.52 ± 0.05 d | 1.79 ± 0.10 d | 2.06 ± 0.15 d | <1.48 | <1.48 | <1.48 | <1.48 |
| Benzalkonium chloride | 2.55 ± 0.05 | <1.48 | <1.48 | <1.48 | <1.48 | <1.48 | <1.48 |
| Sample | No Reads | Chao1 | Shannon | Simpson |
|---|---|---|---|---|
| U0 | 3478 | 35 | 0.67 | 0.36 |
| U5 | 3440 | 37 | 0.62 | 0.34 |
| U10 | 9275 | 48 | 0.81 | 0.35 |
| U20 | 7689 | 34 | 0.87 | 0.39 |
| U30 | 4641 | 33 | 0.43 | 0.17 |
| U40 | 69,978 | 19 | 0.87 | 0.47 |
| U50 | 162,425 | 23 | 0.51 | 0.26 |
| A0 | 2926 | 38 | 0.49 | 0.24 |
| A5 | 2987 | 44 | 0.38 | 0.16 |
| A10 | 2483 | 36 | 0.52 | 0.26 |
| A20 | 2823 | 40 | 0.4 | 0.17 |
| A30 | 2384 | 28 | 0.36 | 0.16 |
| A40 | 4474 | 42 | 0.5 | 0.2 |
| A50 | 3294 | 44 | 0.54 | 0.24 |
| B0 | 2113 | 36 | 0.43 | 0.21 |
| B5 | 4865 | 55 | 0.4 | 0.15 |
| B10 | 1876 | 31 | 0.42 | 0.2 |
| B20 | 2571 | 39 | 0.38 | 0.15 |
| B30 | 2312 | 30 | 0.38 | 0.17 |
| B40 | 3750 | 39 | 0.41 | 0.16 |
| B50 | 4096 | 45 | 0.4 | 0.15 |
| C0 | 2948 | 33 | 0.53 | 0.29 |
| C5 | 1689 | 41 | 0.41 | 0.19 |
| C10 | 6809 | 51 | 0.47 | 0.19 |
| C20 | 1565 | 36 | 0.38 | 0.17 |
| C30 | 1968 | 24 | 0.29 | 0.13 |
| C40 | 2074 | 36 | 0.44 | 0.21 |
| C50 | 8033 | 62 | 0.51 | 0.2 |
| D0 | 7764 | 52 | 0.61 | 0.24 |
| D5 | 4082 | 43 | 0.41 | 0.16 |
| D10 | 4209 | 43 | 0.49 | 0.2 |
| D20 | 3967 | 41 | 0.39 | 0.15 |
| D30 | 5623 | 41 | 0.43 | 0.17 |
| D40 | 4514 | 35 | 0.45 | 0.18 |
| D50 | 6665 | 39 | 0.48 | 0.19 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez López, J.; Grande, M.J.; Pérez-Pulido, R.; Galvez, A.; Lucas, R. Impact of High-Hydrostatic Pressure Treatments Applied Singly or in Combination with Moderate Heat on the Microbial Load, Antimicrobial Resistance, and Bacterial Diversity of Guacamole. Microorganisms 2020, 8, 909. https://doi.org/10.3390/microorganisms8060909
Rodríguez López J, Grande MJ, Pérez-Pulido R, Galvez A, Lucas R. Impact of High-Hydrostatic Pressure Treatments Applied Singly or in Combination with Moderate Heat on the Microbial Load, Antimicrobial Resistance, and Bacterial Diversity of Guacamole. Microorganisms. 2020; 8(6):909. https://doi.org/10.3390/microorganisms8060909
Chicago/Turabian StyleRodríguez López, Javier, Mª. José Grande, Rubén Pérez-Pulido, Antonio Galvez, and Rosario Lucas. 2020. "Impact of High-Hydrostatic Pressure Treatments Applied Singly or in Combination with Moderate Heat on the Microbial Load, Antimicrobial Resistance, and Bacterial Diversity of Guacamole" Microorganisms 8, no. 6: 909. https://doi.org/10.3390/microorganisms8060909
APA StyleRodríguez López, J., Grande, M. J., Pérez-Pulido, R., Galvez, A., & Lucas, R. (2020). Impact of High-Hydrostatic Pressure Treatments Applied Singly or in Combination with Moderate Heat on the Microbial Load, Antimicrobial Resistance, and Bacterial Diversity of Guacamole. Microorganisms, 8(6), 909. https://doi.org/10.3390/microorganisms8060909






