BTV-14 Infection in Sheep Elicits Viraemia with Mild Clinical Symptoms
Abstract
1. Introduction
2. Materials and Methods
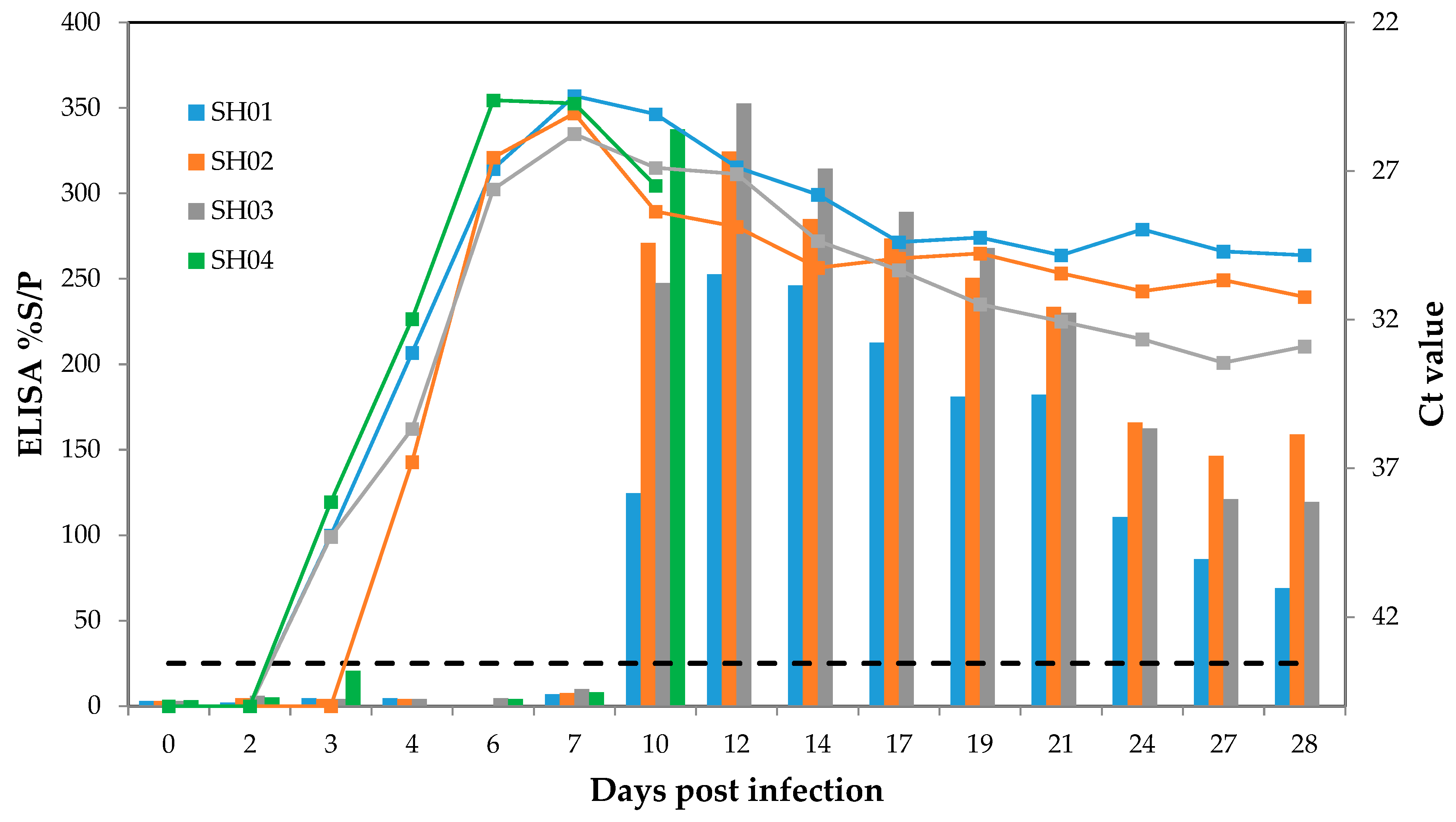
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Carpenter, S.; Groschup, M.H.; Garros, C.; Felippe-Bauer, M.L.; Purse, B.V. Culicoides biting midges, arboviruses and public health in Europe. Antivir. Res. 2013, 100, 102–113. [Google Scholar] [CrossRef]
- Schwartz-Cornil, I.; Mertens, P.P.C.; Contreras, V.; Hemati, B.; Pascale, F.; Breard, E.; Mellor, P.S.; MacLachlan, N.J.; Zientara, S. Bluetongue virus: Virology, pathogenesis and immunity. Vet. Res. 2008, 39, 46. [Google Scholar] [CrossRef]
- Darpel, K.E.; Monaghan, P.; Anthony, S.J.; Takamatsu, H.-H.; Mertens, P.P.C. Bluetongue virus in the mammalian host and the induced immune response. In Bluetongue, 1st ed.; Mellor, P.S., Baylis, M., Mertens, P.P.C., Eds.; Academic Press: London, UK, 2009; pp. 265–284. [Google Scholar] [CrossRef]
- Rajko-Nenow, P.; Golender, N.; Bumbarov, V.; Brown, H.; Frost, L.; Darpel, K.; Tennakoon, C.; Flannery, J.; Batten, C. Complete genome sequence of a novel Bluetongue virus isolated from a commercial sheeppox vaccine. Microbiol. Resour. Announc. 2020, 9, e01539-19. [Google Scholar] [CrossRef]
- Hofmann, M.A.; Renzullo, S.; Mader, M.; Chaignat, V.; Worwa, G.; Thuer, B. Genetic characterization of toggenburg Orbivirus, a new Bluetongue virus, from goats, Switzerland. Emerg. Infect. Dis. 2008, 14, 1855–1861. [Google Scholar] [CrossRef]
- Savini, G.; Puggioni, G.; Meloni, G.; Marcacci, M.; Di Domenico, M.; Rocchigiani, A.M.; Spedicato, M.; Oggiano, A.; Manunta, D.; Teodori, L.; et al. Novel putative Bluetongue virus in healthy goats from Sardinia, Italy. Infect. Genet. Evol. 2017, 51, 108–117. [Google Scholar] [CrossRef]
- Belbis, G.; Zientara, S.; Breard, E.; Sailleau, C.; Caignard, G.; Vitour, D.; Attoui, H. Bluetongue virus: From BTV-1 to BTV-27. Adv. Virus Res. 2017, 99, 161–197. [Google Scholar] [CrossRef]
- Sailleau, C.; Breard, E.; Viarouge, C.; Vitour, D.; Romey, A.; Garnier, A.; Fablet, A.; Lowenski, S.; Gorna, K.; Caignard, G.; et al. Re-Emergence of Bluetongue virus Serotype 8 in France, 2015. Transbound Emerg. Dis. 2017, 64, 998–1000. [Google Scholar] [CrossRef]
- Flannery, J.; Sanz-Bernardo, B.; Ashby, M.; Brown, H.; Carpenter, S.; Cooke, L.; Corla, A.; Frost, L.; Gubbins, S.; Hicks, H.; et al. Evidence of reduced viremia, pathogenicity and vector competence in a re-emerging European strain of Bluetongue virus serotype 8 in sheep. Transbound. Emerg. Dis. 2019, 66, 1177–1185. [Google Scholar] [CrossRef]
- Feenstra, F.; van Rijn, P.A. Current and next-generation bluetongue vaccines: Requirements, strategies, and prospects for different field situations. Crit. Rev. Microbiol. 2017, 43, 142–155. [Google Scholar] [CrossRef]
- More, S.; Bicout, D.; Botner, A.; Butterworth, A.; Depner, K.; Edwards, S.; Garin-Bastuji, B.; Good, M.; Schmidt, C.G.; Michel, V.; et al. Assessment of listing and categorisation of animal diseases within the framework of the Animal Health Law (Regulation(EU) No2016/429): Bluetongue. EFSA J. 2017, 15, 4957. [Google Scholar] [CrossRef]
- De Clercq, K.; Mertens, P.; De Leeuw, I.; Oura, C.; Houdart, P.; Potgieter, A.C.; Maan, S.; Hooyberghs, J.; Batten, C.; Vandemeulebroucke, E.; et al. Emergence of Bluetongue Serotypes in Europe, Part 2: The Occurrence of a BTV-11 Strain in Belgium. Transbound. Emerg. Dis. 2009, 56, 355–361. [Google Scholar] [CrossRef]
- Savini, G.; Lorusso, A.; Paladini, C.; Migliaccio, P.; Di Gennaro, A.; Di Provvido, A.; Scacchia, M.; Monaco, F. Bluetongue Serotype 2 and 9 Modified Live Vaccine Viruses as Causative Agents of Abortion in Livestock: A Retrospective Analysis in Italy. Transbound. Emerg. Dis. 2014, 61, 69–74. [Google Scholar] [CrossRef]
- Veronesi, E.; Darpel, K.E.; Hamblin, C.; Carpenter, S.; Takamatsu, H.-H.; Anthony, S.J.; Elliott, H.; Mertens, P.P.C.; Mellor, P.S. Viraemia and clinical disease in Dorset Poll sheep following vaccination with live attenuated Bluetongue virus vaccines serotypes 16 and 4. Vaccine 2010, 28, 1397–1403. [Google Scholar] [CrossRef]
- van Rijn, P.A. Prospects of next-generation vaccines for bluetongue. Front. Vet. Sci. 2019, 6, 15. [Google Scholar] [CrossRef]
- Koltsov, A.; Tsybanov, S.; Gogin, A.; Kolbasov, D.; Koltsova, G. Identification and characterization of Bluetongue virus serotype 14 in Russia. Front. Vet. Sci. 2020, 7, 8. [Google Scholar] [CrossRef]
- Orlowska, A.; Zmudzinski, J.F.; Smreczak, M.; Trebas, P.; Marzec, A. Diagnostic reliability of different RT-PCR protocols for the detection of Bluetongue virus serotype 14 (BTV-14). J. Vet. Res. 2017, 61, 391–395. [Google Scholar] [CrossRef][Green Version]
- Orlowska, A.; Trebas, P.; Smreczak, M.; Marzec, A.; Zmudzinski, J.F. First detection of Bluetongue virus serotype 14 in Poland. Arch. Virol. 2016, 161, 1969–1972. [Google Scholar] [CrossRef]
- Nomikou, K.; Hughes, J.; Wash, R.; Kellam, P.; Breard, E.; Zientara, S.; Palmarini, M.; Biek, R.; Mertens, P. Widespread Reassortment Shapes the Evolution and Epidemiology of Bluetongue virus following European Invasion. PLoS Pathog. 2015, 11, e1005056. [Google Scholar] [CrossRef]
- OIE. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals (mammals, birds and bees). In Manual of Diagnostic Tests and Vaccines for Terrestrial Animals; OIE: Paris, France, 2012; Volume 2, p. 1403. [Google Scholar]
- Hofmann, M.; Griot, C.; Chaignat, V.; Perler, L.; Thur, B. Blauzungenkrankheit erreicht die Schweiz. Schweiz. Arch. Tierheilkd. 2008, 150, 49–56. [Google Scholar] [CrossRef]
- Schulz, C.; Sailleau, C.; Breard, E.; Flannery, J.; Viarouge, C.; Zientara, S.; Beer, M.; Batten, C.; Hoffmann, B. Experimental infection of sheep, goats and cattle with a Bluetongue virus serotype 4 field strain from Bulgaria, 2014. Transbound. Emerg. Dis. 2018, 65, e243–e250. [Google Scholar] [CrossRef] [PubMed]
- van der Sluijs, M.T.W.; Schroer-Joosten, D.P.H.; Fid-Fourkour, A.; Smit, M.; Vrijenhoek, M.P.; Moulin, V.; de Smit, A.J.; Moormann, R.J.M. Transplacental transmission of BTV-8 in sheep: BTV viraemia, antibody responses and vaccine efficacy in lambs infected in utero. Vaccine 2013, 31, 3726–3731. [Google Scholar] [CrossRef] [PubMed]
- Flannery, J.; Rajko-Nenow, P.; Hicks, H.; Hill, H.; Gubbins, S.; Batten, C. Evaluating the most appropriate pooling ratio for EDTA blood samples to detect Bluetongue virus using real-time RT-PCR. Vet. Microbiol. 2018, 217, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Darpel, K.E.; Batten, C.A.; Veronesi, E.; Shaw, A.E.; Anthony, S.; Bachanek-Bankowska, K.; Kgosana, L.; Bin-Tarif, A.; Carpenter, S.; Muueller-Doblies, U.U.; et al. Clinical signs and pathology shown by British sheep and cattle infected with Bluetongue virus serotype 8 derived from the 2006 outbreak in northern Europe. Vet. Rec. 2007, 161, 253–261. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flannery, J.; Frost, L.; Fay, P.; Hicks, H.; Henstock, M.; Smreczak, M.; Orłowska, A.; Rajko-Nenow, P.; Darpel, K.; Batten, C. BTV-14 Infection in Sheep Elicits Viraemia with Mild Clinical Symptoms. Microorganisms 2020, 8, 892. https://doi.org/10.3390/microorganisms8060892
Flannery J, Frost L, Fay P, Hicks H, Henstock M, Smreczak M, Orłowska A, Rajko-Nenow P, Darpel K, Batten C. BTV-14 Infection in Sheep Elicits Viraemia with Mild Clinical Symptoms. Microorganisms. 2020; 8(6):892. https://doi.org/10.3390/microorganisms8060892
Chicago/Turabian StyleFlannery, John, Lorraine Frost, Petra Fay, Hayley Hicks, Mark Henstock, Marcin Smreczak, Anna Orłowska, Paulina Rajko-Nenow, Karin Darpel, and Carrie Batten. 2020. "BTV-14 Infection in Sheep Elicits Viraemia with Mild Clinical Symptoms" Microorganisms 8, no. 6: 892. https://doi.org/10.3390/microorganisms8060892
APA StyleFlannery, J., Frost, L., Fay, P., Hicks, H., Henstock, M., Smreczak, M., Orłowska, A., Rajko-Nenow, P., Darpel, K., & Batten, C. (2020). BTV-14 Infection in Sheep Elicits Viraemia with Mild Clinical Symptoms. Microorganisms, 8(6), 892. https://doi.org/10.3390/microorganisms8060892




