Changes in the Intestinal Microbiota of Patients with Inflammatory Bowel Disease with Clinical Remission during an 8-Week Infliximab Infusion Cycle
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
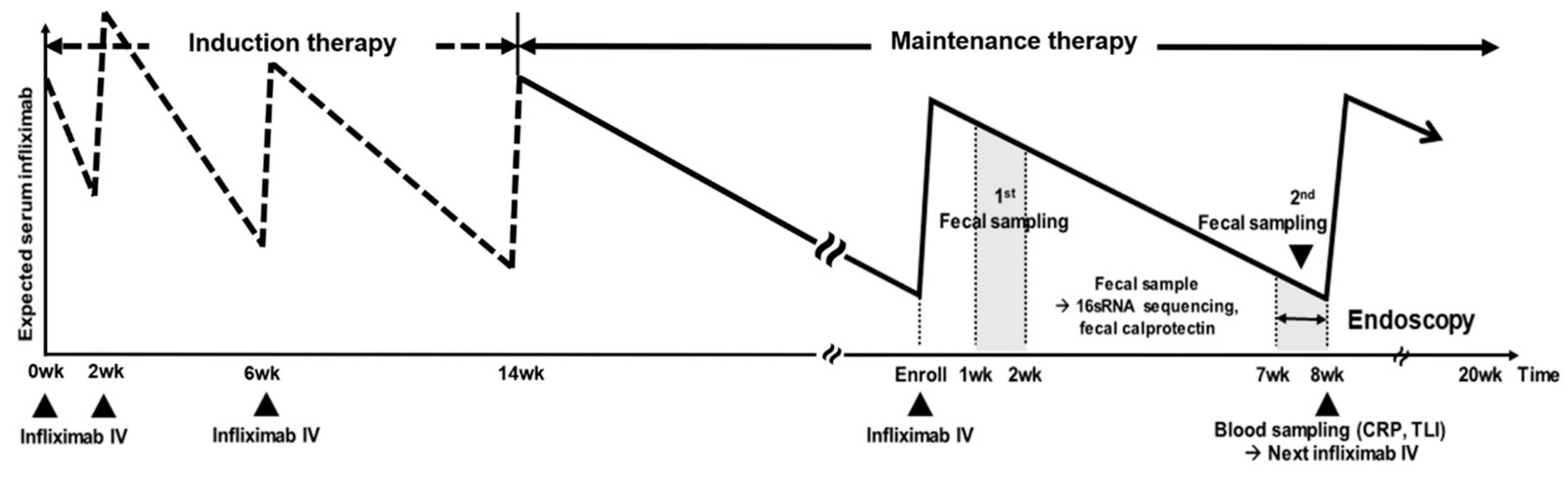
2.2. Study Design
2.3. 16.S rRNA Gene Sequencing
2.4. Taxonomic Comparison and Diversity Indices
2.5. Measurement of Fecal Calprotectin, CRP, and TLI
3. Results
3.1. Study Participants and Samples
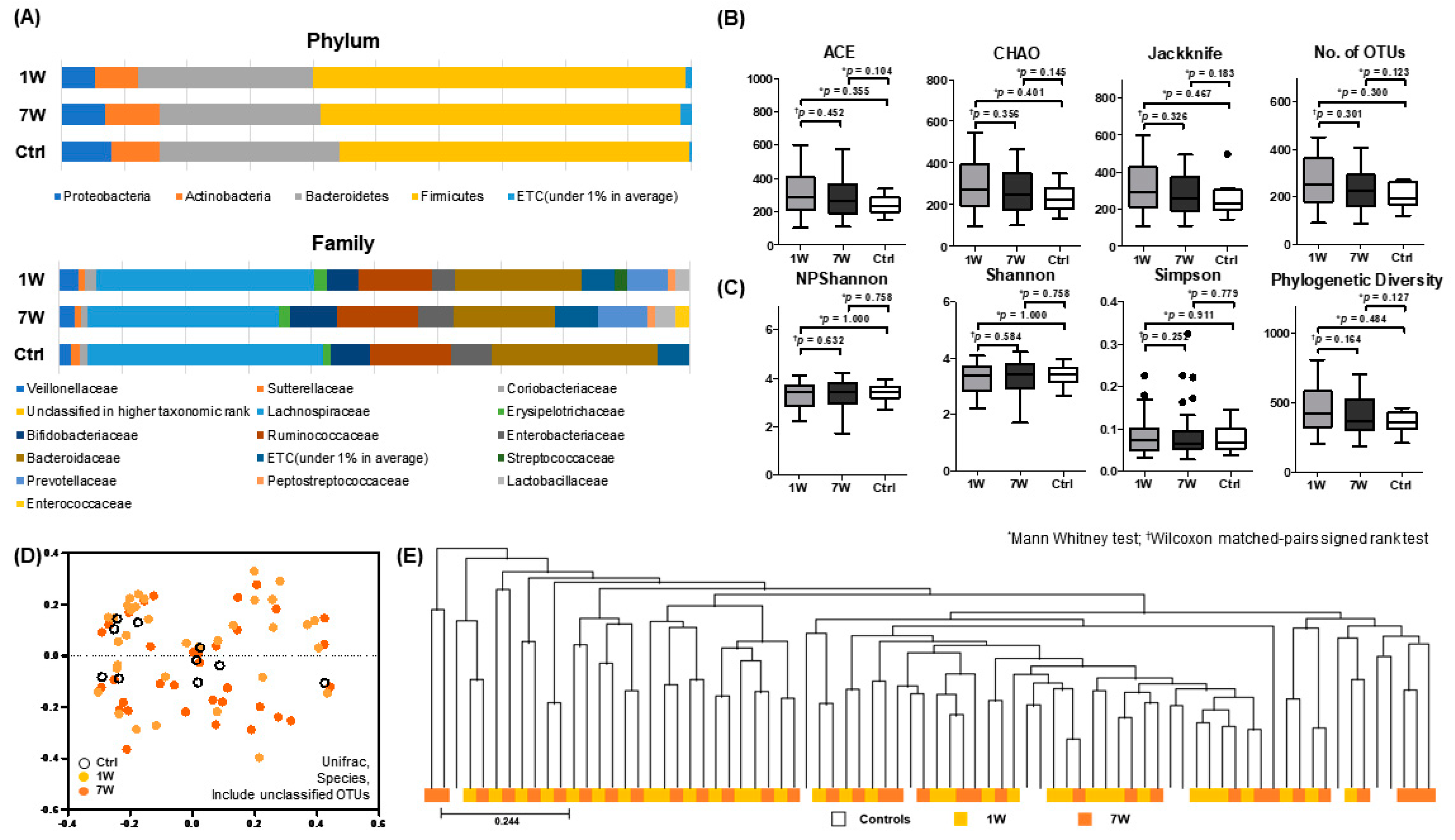
3.2. Changes in Microbiota Composition during 8-Week Infliximab Infusion Cycle
3.3. Comparison of Microbiota Composition of 7W Samples with TLI < 5 μg/mL and ≥5 μg/mL
3.4. Comparison of Microbial Profile in IBD Patients with Mucosal Healing (MH) and Patients with No Mucosal Healing (Non-MH)
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wallace, K.L.; Zheng, L.B.; Kanazawa, Y.; Shih, D.Q. Immunopathology of inflammatory bowel disease. World J. Gastroenterol. 2014, 20, 6–21. [Google Scholar] [CrossRef] [PubMed]
- Busquets, D.; Mas-de-Xaxars, T.; Lopez-Siles, M.; Martinez-Medina, M.; Bahi, A.; Sabat, M.; Louvriex, R.; Miquel-Cusachs, J.O.; Garcia-Gil, J.L.; Aldeguer, X. Anti-tumour Necrosis Factor Treatment with Adalimumab Induces Changes in the Microbiota of Crohn’s Disease. J. Crohns Colitis 2015, 9, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Bazin, T.; Hooks, K.B.; Barnetche, T.; Truchetet, M.E.; Enaud, R.; Richez, C.; Dougados, M.; Hubert, C.; Barre, A.; Nikolski, M.; et al. Microbiota Composition May Predict Anti-Tnf Alpha Response in Spondyloarthritis Patients: An Exploratory Study. Sci. Rep. 2018, 8, 5446. [Google Scholar] [CrossRef] [PubMed]
- Rajca, S.; Grondin, V.; Louis, E.; Vernier-Massouille, G.; Grimaud, J.C.; Bouhnik, Y.; Laharie, D.; Dupas, J.L.; Pillant, H.; Picon, L.; et al. Alterations in the intestinal microbiome (dysbiosis) as a predictor of relapse after infliximab withdrawal in Crohn’s disease. Inflamm. Bowel Dis. 2014, 20, 978–986. [Google Scholar] [PubMed]
- Magnusson, M.K.; Strid, H.; Sapnara, M.; Lasson, A.; Bajor, A.; Ung, K.A.; Ohman, L. Anti-TNF Therapy Response in Patients with Ulcerative Colitis Is Associated with Colonic Antimicrobial Peptide Expression and Microbiota Composition. J. Crohns Colitis 2016, 10, 943–952. [Google Scholar] [CrossRef]
- Eun, C.S.; Kwak, M.J.; Han, D.S.; Lee, A.R.; Park, D.I.; Yang, S.K.; Kim, Y.S.; Kim, J.F. Does the intestinal microbial community of Korean Crohn’s disease patients differ from that of western patients? BMC Gastroenterol. 2016, 16, 28. [Google Scholar] [CrossRef][Green Version]
- Wang, Y.; Gao, X.; Ghozlane, A.; Hu, H.; Li, X.; Xiao, Y.; Li, D.; Yu, G.; Zhang, T. Characteristics of Faecal Microbiota in Paediatric Crohn’s Disease and Their Dynamic Changes During Infliximab Therapy. J. Crohns Colitis 2018, 12, 337–346. [Google Scholar] [CrossRef]
- Zhou, Y.; Xu, Z.Z.; He, Y.; Yang, Y.; Liu, L.; Lin, Q.; Nie, Y.; Li, M.; Zhi, F.; Liu, S.; et al. Gut Microbiota Offers Universal Biomarkers across Ethnicity in Inflammatory Bowel Disease Diagnosis and Infliximab Response Prediction. mSystems 2018, 3. [Google Scholar] [CrossRef]
- Koga, A.; Matsui, T.; Takatsu, N.; Takada, Y.; Kishi, M.; Yano, Y.; Beppu, T.; Ono, Y.; Ninomiya, K.; Hirai, F.; et al. Trough level of infliximab is useful for assessing mucosal healing in Crohn’s disease: A prospective cohort study. Intest. Res. 2018, 16, 223–232. [Google Scholar] [CrossRef]
- Adedokun, O.J.; Sandborn, W.J.; Feagan, B.G.; Rutgeerts, P.; Xu, Z.; Marano, C.W.; Johanns, J.; Zhou, H.; Davis, H.M.; Cornillie, F.; et al. Association between serum concentration of infliximab and efficacy in adult patients with ulcerative colitis. Gastroenterology 2014, 147, 1296–1307.e1295. [Google Scholar] [CrossRef]
- Bortlik, M.; Duricova, D.; Malickova, K.; Machkova, N.; Bouzkova, E.; Hrdlicka, L.; Komarek, A.; Lukas, M. Infliximab trough levels may predict sustained response to infliximab in patients with Crohn’s disease. J. Crohns Colitis 2013, 7, 736–743. [Google Scholar] [CrossRef]
- Seow, C.H.; Newman, A.; Irwin, S.P.; Steinhart, A.H.; Silverberg, M.S.; Greenberg, G.R. Trough serum infliximab: A predictive factor of clinical outcome for infliximab treatment in acute ulcerative colitis. Gut 2010, 59, 49–54. [Google Scholar] [CrossRef]
- Ye, B.D.; Jang, B.I.; Jeen, Y.T.; Lee, K.M.; Kim, J.S.; Yang, S.K. Diagnostic guideline of Crohn’s disease. Korean J. Gastroenterol. 2009, 53, 161–176. [Google Scholar]
- Choi, C.H.; Kim, Y.H.; Kim, Y.S.; Ye, B.D.; Lee, K.M.; Lee, B.I.; Jung, S.A.; Kim, W.H.; Lee, H. Guidelines for the management of ulcerative colitis. Korean J. Gastroenterol. 2012, 59, 118–140. [Google Scholar] [CrossRef] [PubMed]
- Levin, A.D.; Wildenberg, M.E.; van den Brink, G.R. Mechanism of Action of Anti-TNF Therapy in Inflammatory Bowel Disease. J. Crohns Colitis 2016, 10, 989–997. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.H.; Ha, S.M.; Kwon, S.; Lim, J.; Kim, Y.; Seo, H.; Chun, J. Introducing EzBioCloud: A taxonomically united database of 16S rRNA gene sequences and whole-genome assemblies. Int. J. Syst. Evol. Microbiol. 2017, 67, 1613–1617. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Langille, M.G.; Zaneveld, J.; Caporaso, J.G.; McDonald, D.; Knights, D.; Reyes, J.A.; Clemente, J.C.; Burkepile, D.E.; Vega Thurber, R.L.; Knight, R.; et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotechnol. 2013, 31, 814–821. [Google Scholar] [CrossRef]
- Colombel, J.F.; Panaccione, R.; Bossuyt, P.; Lukas, M.; Baert, F.; Vanasek, T.; Danalioglu, A.; Novacek, G.; Armuzzi, A.; Hebuterne, X.; et al. Effect of tight control management on Crohn’s disease (CALM): A multicentre, randomised, controlled phase 3 trial. Lancet 2018, 390, 2779–2789. [Google Scholar] [CrossRef]
- Feuerstein, J.D.; Nguyen, G.C.; Kupfer, S.S.; Falck-Ytter, Y.; Singh, S.; American Gastroenterological Association Institute Clinical Guidelines Committee. American Gastroenterological Association Institute Guideline on Therapeutic Drug Monitoring in Inflammatory Bowel Disease. Gastroenterology 2017, 153, 827–834. [Google Scholar] [CrossRef]
- Pascal, V.; Pozuelo, M.; Borruel, N.; Casellas, F.; Campos, D.; Santiago, A.; Martinez, X.; Varela, E.; Sarrabayrouse, G.; Machiels, K.; et al. A microbial signature for Crohn’s disease. Gut 2017, 66, 813–822. [Google Scholar] [CrossRef] [PubMed]
- Zuo, T.; Ng, S.C. The Gut Microbiota in the Pathogenesis and Therapeutics of Inflammatory Bowel Disease. Front. Microbiol. 2018, 9, 2247. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Tedjo, D.I.; Smolinska, A.; Savelkoul, P.H.; Masclee, A.A.; van Schooten, F.J.; Pierik, M.J.; Penders, J.; Jonkers, D.M. The fecal microbiota as a biomarker for disease activity in Crohn’s disease. Sci. Rep. 2016, 6, 35216. [Google Scholar] [CrossRef]
- Walker, A.W.; Sanderson, J.D.; Churcher, C.; Parkes, G.C.; Hudspith, B.N.; Rayment, N.; Brostoff, J.; Parkhill, J.; Dougan, G.; Petrovska, L. High-throughput clone library analysis of the mucosa-associated microbiota reveals dysbiosis and differences between inflamed and non-inflamed regions of the intestine in inflammatory bowel disease. BMC Microbiol. 2011, 11, 7. [Google Scholar] [CrossRef]
- Knox, N.C.; Forbes, J.D.; Van Domselaar, G.; Bernstein, C.N. The Gut Microbiome as a Target for IBD Treatment: Are We There Yet? Curr. Treat. Options Gastroenterol. 2019, 17, 115–126. [Google Scholar] [CrossRef]
- Gevers, D.; Kugathasan, S.; Denson, L.A.; Vazquez-Baeza, Y.; Van Treuren, W.; Ren, B.; Schwager, E.; Knights, D.; Song, S.J.; Yassour, M.; et al. The treatment-naive microbiome in new-onset Crohn’s disease. Cell Host Microbe 2014, 15, 382–392. [Google Scholar] [CrossRef]
- Ferreira-Halder, C.V.; Faria, A.V.S.; Andrade, S.S. Action and function of Faecalibacterium prausnitzii in health and disease. Best Pract. Res. Clin. Gastroenterol. 2017, 31, 643–648. [Google Scholar] [CrossRef]
- Louis, P.; Flint, H.J. Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol. Lett. 2009, 294, 1–8. [Google Scholar] [CrossRef]
- Sokol, H.; Pigneur, B.; Watterlot, L.; Lakhdari, O.; Bermudez-Humaran, L.G.; Gratadoux, J.J.; Blugeon, S.; Bridonneau, C.; Furet, J.P.; Corthier, G.; et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc. Natl. Acad. Sci. USA 2008, 105, 16731–16736. [Google Scholar] [CrossRef]
- Schaffler, H.; Kaschitzki, A.; Alberts, C.; Bodammer, P.; Bannert, K.; Koller, T.; Warnke, P.; Kreikemeyer, B.; Lamprecht, G. Alterations in the mucosa-associated bacterial composition in Crohn’s disease: A pilot study. Int. J. Colorectal Dis. 2016, 31, 961–971. [Google Scholar] [CrossRef] [PubMed]
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut microbiota functions: Metabolism of nutrients and other food components. Eur. J. Nutr. 2018, 57, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Son, D.O.; Satsu, H.; Shimizu, M. Histidine inhibits oxidative stress- and TNF-alpha-induced interleukin-8 secretion in intestinal epithelial cells. FEBS Lett. 2005, 579, 4671–4677. [Google Scholar] [CrossRef] [PubMed]
- Guerville, M.; Boudry, G. Gastrointestinal and hepatic mechanisms limiting entry and dissemination of lipopolysaccharide into the systemic circulation. Am. J. Physiol. Gastrointest. Liver Physiol. 2016, 311, G1–G15. [Google Scholar] [CrossRef] [PubMed]
- Neish, A.S. Microbes in gastrointestinal health and disease. Gastroenterology 2009, 136, 65–80. [Google Scholar] [CrossRef]
- Burrough, E.R.; Arruda, B.L.; Plummer, P.J. Comparison of the Luminal and Mucosa-Associated Microbiota in the Colon of Pigs with and without Swine Dysentery. Front. Vet. Sci. 2017, 4, 139. [Google Scholar] [CrossRef]



| Crohn’s Disease (n = 30) | Ulcerative Colitis (n = 10) | p * | IBD (n = 40) | Healthy Control (n = 10) | p† | |
|---|---|---|---|---|---|---|
| Age (years), median (IQR) | 33.0 (25.7–42.2) | 48.0 (36.0–51.5) | 0.016 | 37.5 (27.8–48.0) | 30.5 (27.00–33.5) | 0.074 |
| Male, n (%) | 25 (83.3) | 4 (40.0) | 0.008 | 29 (72.5) | 6 (60.0) | 0.440 |
| Current smoker, n (%) | 1 (4.2) | 0 (0) | 1.000 | 1 (2.9) | 0 (0) | 1.000 |
| Abdominal surgery, n (%) | 7 (29.2) | 0 (0–0) | 0.078 | 7 (20.6) | 0 (0–0) | 0.161 |
| Montreal classification | ||||||
| A1/A2/A3, n (%) | 2 (8.3)/22 (91.7)/0 (0) | - | ||||
| L1/L2/L3, n (%) | 4 (16.7)/5 (20.8)/15 (62.5) | - | ||||
| B1/B2/B3, n (%) | 10 (41.7)/11 (45.8)/3 (12.5) | - | ||||
| E1/E2/E3, n (%) | - | 2 (20.0)/2 (20.0)/6 (60.0) | ||||
| Azathiopurine use, n (%) | 11 (45.8) | 1 (10.0) | 12 (35.3) | |||
| BMI (kg/m2), median (IQR) | 21.6 (19.0–24.0) | 22.4 (20.5–24.7) | 0.322 | 21.8 (19.7–24.4) | ||
| Hemoglobin (g/dL), median (IQR) | 13.0 (11.6–15.0) | 13.7 (12.9–14.9) | 0.589 | 13.3 (11.9–14.9) | ||
| Hematocrit (%), median (IQR) | 39.8 (37.2–44.4) | 42.3 (40.3–44.7) | 0.539 | 42.1 (37.6–44.6) | ||
| Albumin (g/dL), median (IQR) | 4.6 (4.3–4.7) | 4.6 (4.4–4.70) | 0.411 | 4.6 (4.3–4.7) | ||
| ESR (mm/hr), median (IQR) | 8.5 (5.0–19.5) | 14.0 (6.5–41.0) | 0.752 | 10.5 (5.3–20.5) | ||
| CRP (mg/L), median (IQR) | 0.5 (0.3–2.6) | 0.6 (0.3–1.2) | 0.752 | 0.6 (0.3–1.6) | ||
| Calprotectin (μg/mL), median (IQR) | 19.8 (3.0–41.0) | 2.2 (1.7–51.8) | 0.006 | 4.21 (1.9–38.2) | 1.60 (0.78–2.72) | 0.001 |
| Trough level of Infliximab (μg/mL), median (IQR) | 4.3 (3.2–5.8) | 4.8 (4.3–6.4) | 0.534 | 4.4 (3.4–5.9) | ||
| Mucosal healing (n, %) | 12 (50.0) | 7 (70.0) | 0.451 | 19 (55.9) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seong, G.; Kim, N.; Joung, J.-G.; Kim, E.R.; Chang, D.K.; Chun, J.; Hong, S.N.; Kim, Y.-H. Changes in the Intestinal Microbiota of Patients with Inflammatory Bowel Disease with Clinical Remission during an 8-Week Infliximab Infusion Cycle. Microorganisms 2020, 8, 874. https://doi.org/10.3390/microorganisms8060874
Seong G, Kim N, Joung J-G, Kim ER, Chang DK, Chun J, Hong SN, Kim Y-H. Changes in the Intestinal Microbiota of Patients with Inflammatory Bowel Disease with Clinical Remission during an 8-Week Infliximab Infusion Cycle. Microorganisms. 2020; 8(6):874. https://doi.org/10.3390/microorganisms8060874
Chicago/Turabian StyleSeong, Gyeol, Namil Kim, Je-Gun Joung, Eun Ran Kim, Dong Kyung Chang, Jongsik Chun, Sung Noh Hong, and Young-Ho Kim. 2020. "Changes in the Intestinal Microbiota of Patients with Inflammatory Bowel Disease with Clinical Remission during an 8-Week Infliximab Infusion Cycle" Microorganisms 8, no. 6: 874. https://doi.org/10.3390/microorganisms8060874
APA StyleSeong, G., Kim, N., Joung, J.-G., Kim, E. R., Chang, D. K., Chun, J., Hong, S. N., & Kim, Y.-H. (2020). Changes in the Intestinal Microbiota of Patients with Inflammatory Bowel Disease with Clinical Remission during an 8-Week Infliximab Infusion Cycle. Microorganisms, 8(6), 874. https://doi.org/10.3390/microorganisms8060874





