Antimicrobial Activity of Six International Artisanal Kefirs against Bacillus cereus, Listeria monocytogenes, Salmonella enterica Serovar Enteritidis, and Staphylococcus aureus
Abstract
1. Introduction
2. Materials and Methods
2.1. Artisanal Kefir Preparation for Determining Kefir Antimicrobial Activity
2.2. Protein Concentration Measurements
2.3. Bacterial Strains, Microbiological Media, and Growth Conditions
2.4. Detection of Antimicrobial Activity in Artisanal Kefirs
2.5. Ruling-Out Any Antimicrobial Activity Due to Organic Acids, Hydrogen Peroxide, and Free Fatty Acids Produced in Artisanal Kefir
2.5.1. Artisanal Kefir Preparation
2.5.2. Bacterial Strains, Microbiological Media, and Growth Conditions
2.5.3. Detection of Antimicrobial Activity Due to Bacteriocin Production in Artisanal Kefir
2.6. Statistical Analysis
3. Results
3.1. Artisanal Kefir Products and Kefir Grains Description
3.2. The Antimicrobial Activity Spectra of Filter-Sterilized Artisanal Kefirs
3.2.1. Protein Concentration
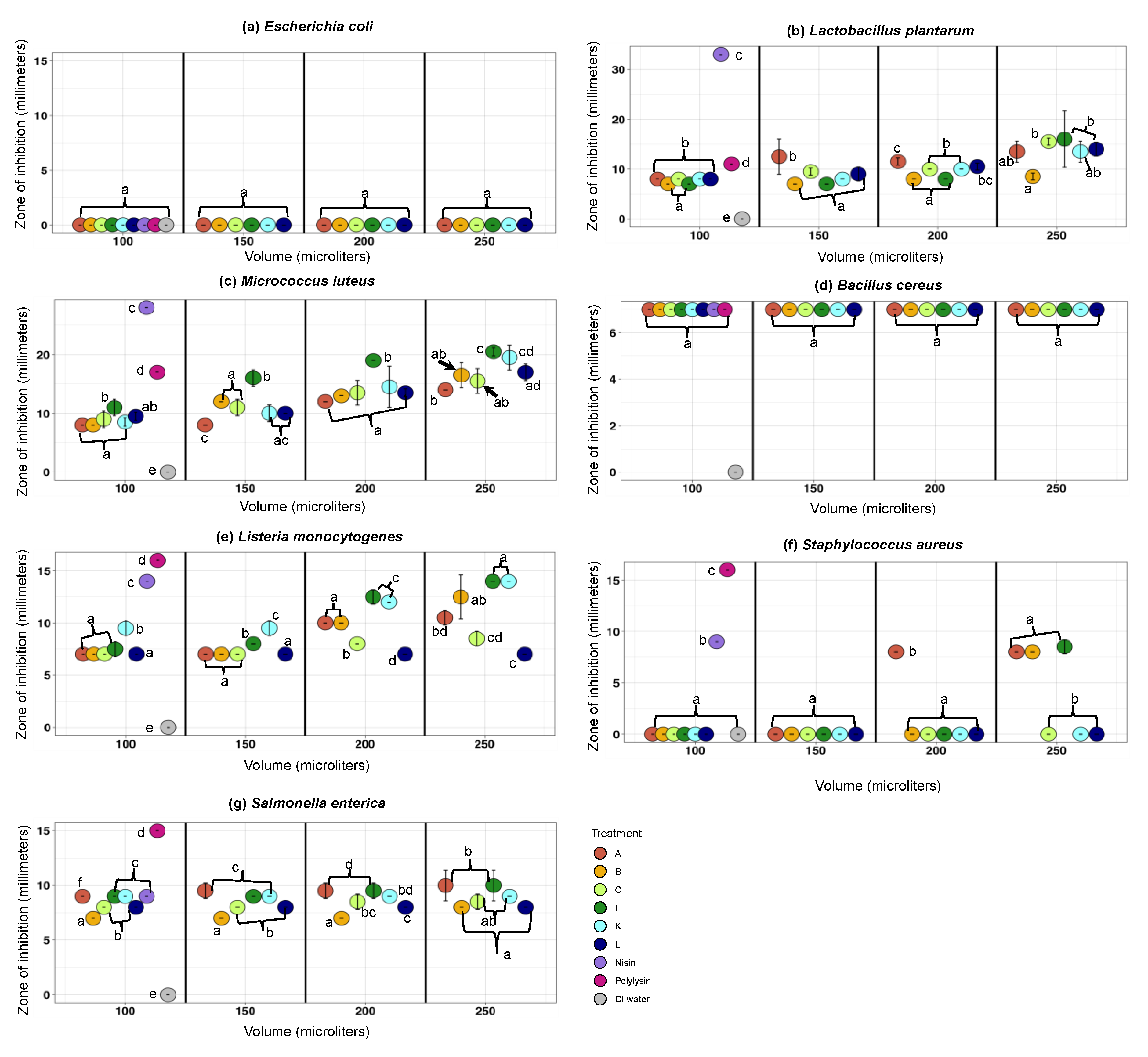
3.2.2. The Antimicrobial Activity Spectra of Filter-Sterilized Artisanal Kefirs Determined by the Agar Well Diffusion Method
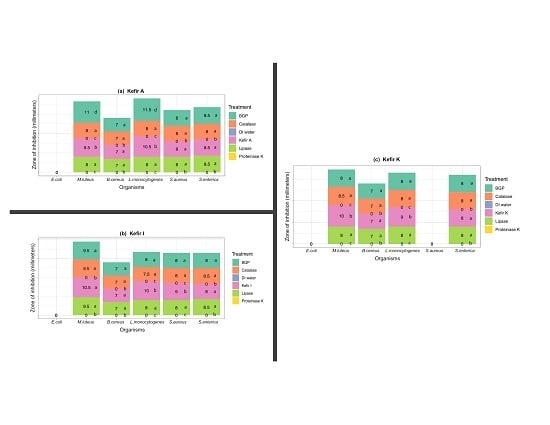
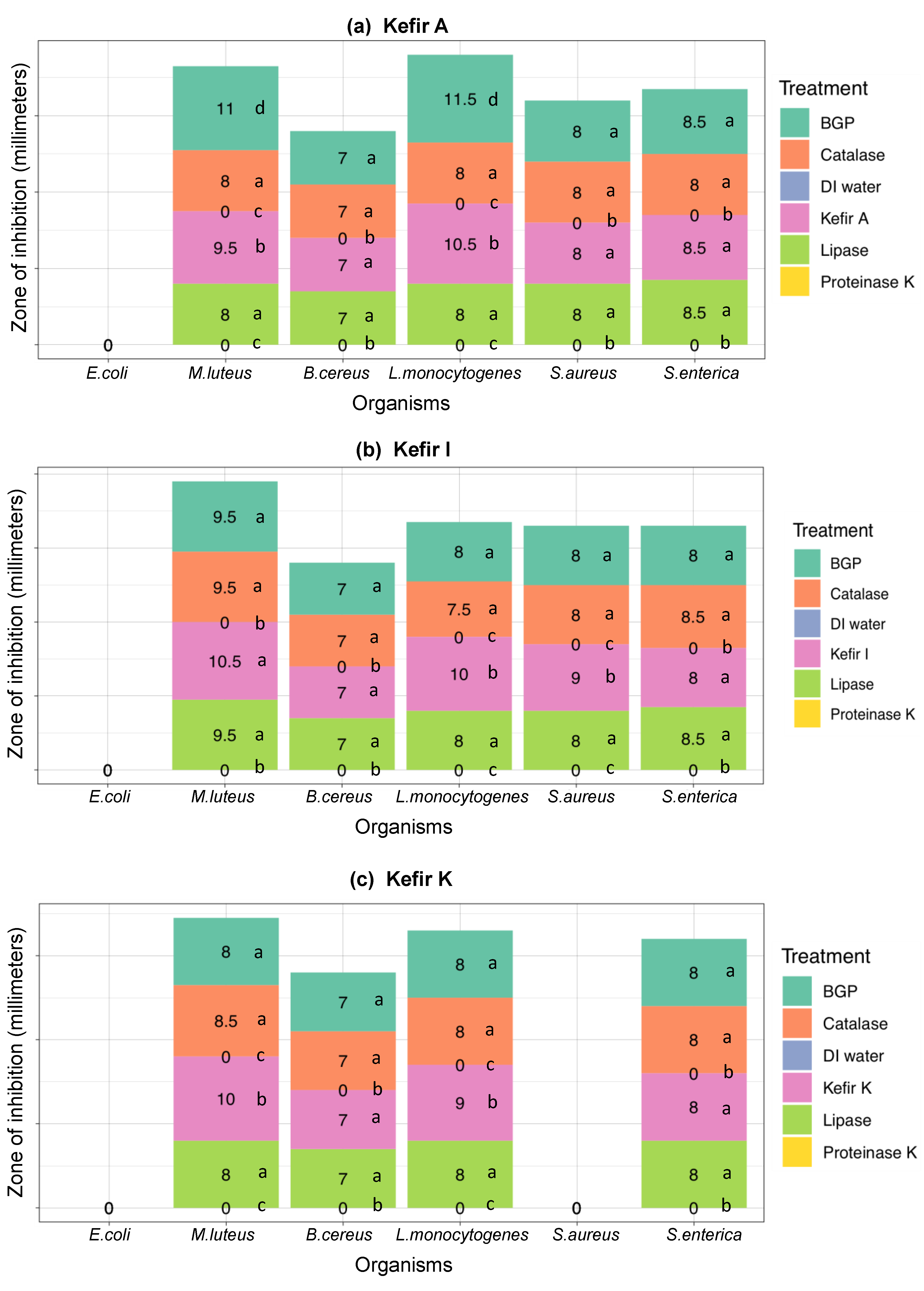
3.3. Ruling-Out Any Antimicrobial Activity Due to Organic Acids, Hydrogen Peroxide, and Free Fatty Acids Produced in Artisanal Kefir
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kirk, M.D.; Pires, S.M.; Black, R.E.; Caipo, M.; Crump, J.A.; Devleesschauwer, B.; Dopfer, D.; Fazil, A.; Fischer-Walker, C.L.; Hald, T.; et al. Correction: World Health Organization Estimates of the Global and Regional Disease Burden of 22 Foodborne Bacterial, Protozoal, and Viral Diseases, 2010: A Data Synthesis. PLoS Med. 2015, 12, e1001940. [Google Scholar] [CrossRef]
- The World Bank. Food-Borne Illnesses Cost US$ 110 Billion Per Year in Low- and Middle-Income Countries. 2018. Available online: https://www.worldbank.org/en/news/press-release/2018/10/23/food-borne-illnesses-cost-us-110-billion-per-year-in-low-and-middle-income-countries (accessed on 3 March 2020).
- Scallan, E.; Griffin, P.M.; Angulo, F.J.; Tauxe, R.V.; Hoekstra, R.M. Foodborne illness acquired in the United States--unspecified agents. Emerg. Infect. Dis. 2011, 17, 16–22. [Google Scholar] [CrossRef]
- Scallan, E.; Hoekstra, R.M.; Angulo, F.J.; Tauxe, R.V.; Widdowson, M.A.; Roy, S.L.; Jones, J.L.; Griffin, P.M. Foodborne illness acquired in the United States–Major pathogens. Emerg. Infect. Dis. 2011, 17, 7–15. [Google Scholar] [CrossRef]
- Scallan, E.; Hoekstra, R.M.; Mahon, B.E.; Jones, T.F.; Griffin, P.M. An assessment of the human health impact of seven leading foodborne pathogens in the United States using disability adjusted life years. Epidemiol. Infect. 2015, 143, 2795–2804. [Google Scholar] [CrossRef]
- National Center for Emerging and Zoonotic Infectious Diseases. Estimated Annual Number of Episodes of Illnesses Caused by 31 Pathogens Transmitted Commonly by Food, United States. Available online: https://www.cdc.gov/foodborneburden/pdfs/scallan-estimated-illnesses-foodborne-pathogens.pdf (accessed on 3 March 2020).
- Matthews, K.R.; Kniel, K.E.; Montville, T.J. Food Microbiology: An Introduction, 4th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Montville, T.J.; Matthews, K.R.; Kniel, K.E. Food Microbiology an Introduction, 3rd ed.; ASM Press: Washington, DC, USA, 2012; p. 419. [Google Scholar]
- Mills, S.; Serrano, L.M.; Griffin, C.; O’Connor, P.M.; Schaad, G.; Bruining, C.; Hill, C.; Ross, R.P.; Meijer, W.C. Inhibitory activity of Lactobacillus plantarum LMG P-26358 against Listeria innocua when used as an adjunct starter in the manufacture of cheese. Microb. Cell Fact. 2011, 10 (Suppl. 1), S7. [Google Scholar] [CrossRef]
- Gharsallaoui, A.; Joly, C.; Oulahal, N.; Degraeve, P. Nisin as a Food Preservative: Part 2: Antimicrobial Polymer Materials Containing Nisin. Crit. Rev. Food Sci. Nutr. 2016, 56, 1275–1289. [Google Scholar] [CrossRef]
- Nivedita, S.; Riti, K.; Neha, G.; Ranjana, K. Purification and Characterization of Bacteriocin Produced by Bacillus subtilis R75 Isolated from Fermented Chunks of Mung Bean (Phaseolus radiatus). Food Technol. Biotechnol. 2011, 49, 169–176. [Google Scholar]
- Badaoui Najjar, M.; Kashtanov, D.; Chikindas, M.L. Epsilon-poly-L-lysine and nisin A act synergistically against Gram-positive food-borne pathogens Bacillus cereus and Listeria monocytogenes. Lett. Appl. Microbiol. 2007, 45, 13–18. [Google Scholar] [CrossRef]
- Jeevaratnam, K.; Jamuna, M.; Bawa, A.S. Biological preservation of foods- Bacteriocins of lactic acid bacteria. Indian J. Biotechnol. 2005, 4, 446–454. [Google Scholar]
- Kolakowski, P. Ozimkiewicz, M. Restoration of kefir grains subjected to different treatments. Int. J. Dairy Technol. 2012, 65, 140–145. [Google Scholar] [CrossRef]
- Nejati, F.; Junne, S.; Neubauer, P. A Big World in Small Grain: A Review of Natural Milk Kefir Starters. Microorganisms 2020, 8, 192. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, B.; Gürakan, G.C.; Ünlü, G. Kefir: A multifaceted fermented dairy product. Probiotics Antimicrob. Proteins 2014, 6, 123–135. [Google Scholar] [CrossRef]
- Joao, S.; Tatiane, A.; Célia, F.; Simone, G. Evaluation of antagonistic activity of milk fermented with kefir grains of different origins. Braz. Arch. Biol. Technol. 2013, 56, 823–827. [Google Scholar]
- Chifiriuc, M.C.; Cioaca, A.B.; Lazar, V. In vitro assay of the antimicrobial activity of kephir against bacterial and fungal strains. Anaerobe 2011, 17, 433–435. [Google Scholar] [CrossRef]
- Kim, D.H.; Jeong, D.; Kim, H.; Kang, I.B.; Chon, J.W.; Song, K.Y.; Seo, K.H. Antimicrobial Activity of Kefir against Various Food Pathogens and Spoilage Bacteria. Korean J. Food Sci. Anim. Resour. 2016, 36, 787–790. [Google Scholar] [CrossRef] [PubMed]
- Ünlü, G.; Nielsen, B.; Ionita, C. Production of Antilisterial Bacteriocins from Lactic Acid Bacteria in Dairy-Based Media: A Comparative Study. Probiotics Antimicrob. Proteins 2015, 7, 259–274. [Google Scholar] [CrossRef] [PubMed]
- Dimitrieva-Moats, G.Y.; Ünlü, G. Development of Freeze-Dried Bacteriocin-Containing Preparations from Lactic Acid Bacteria to Inhibit Listeria monocytogenes and Staphylococcus aureus. Probiotics Antimicrob. Proteins 2012, 4, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Ünlü, G.; Nielsen, B.; Ionita, C. Inhibition of Listeria monocytogenes in Hot Dogs by Surface Application of Freeze-Dried Bacteriocin-Containing Powders from Lactic Acid Bacteria. Probiotics Antimicrob. Proteins 2016, 8, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.A.; Jomaa, S.A.; El-Wahed, A.A.; El-Seedi, A.H.R. The Many Faces of Kefir Fermented Dairy Products: Quality Characteristics, Flavour Chemistry, Nutritional Value, Health Benefits, and Safety. Nutrients 2020, 12, 346. [Google Scholar] [CrossRef] [PubMed]
- Plessas, S.; Nouska, C.; Mantzourani, I.; Kourkoutas, Y.; Alexopoulos, A.; Bezirtzoglou, E. Microbiological Exploration of Different Types of Kefir Grains. Fermentation 2017, 3, 1. [Google Scholar] [CrossRef]
- Lakshmi, T.S.; MaryPramela, A.; Iyer, P. Anti-microbial, anti-fungal and anti-carcinogenic properties of coconut milk kefir. Int. J. Home Sci. 2017, 3, 365–369. [Google Scholar]
- Iraporda, C.; Abatemarco Junior, M.; Neumann, E.; Nunes, A.C.; Nicoli, J.R.; Abraham, A.G.; Garrote, G.L. Biological activity of the non-microbial fraction of kefir: Antagonism against intestinal pathogens. J. Dairy Res. 2017, 84, 339–345. [Google Scholar] [CrossRef] [PubMed]
| Kefir Origin | Source | Grains’ Description | Products’ Description |
|---|---|---|---|
| Lithuania | Etsy Inc. | Cauliflower-like appearance, off-white to pale yellow, medium size (1–10 mm) and firm grains | Mild, smooth, and not sour (sweet) |
| Ireland | Etsy Inc. | Soft, small size (>1 mm) grains | Mild, sweet and pleasant taste, smooth, sweet aroma, fresh, and cheesy |
| The Caucuses region | Etsy Inc. | Cauliflower-like appearance, off-white to pale yellow, size 2–10 mm, firm, rubbery with smooth grains | Earthy, cheesy aroma, and sour taste |
| South Korea | [19] | Soft, curling, size 2–10 mm | Earthy, cheesy aroma, and sour taste |
| Britain | Etsy Inc. | Cauliflower-like appearance, small to large size (2.5–50 mm), rubbery, firm, smooth grains | Creamy, earthy, cheesy aroma, slightly sour |
| Fusion Tea | Amazon | Cauliflower-like appearance, off-white to pale yellow, mixed sizes (2–7 mm), firm, rubbery textured grains | Smooth, mild sour, creamy, pleasant, and fresh, sweet, yeasty aroma |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sindi, A.; Badsha, M.B.; Nielsen, B.; Ünlü, G. Antimicrobial Activity of Six International Artisanal Kefirs against Bacillus cereus, Listeria monocytogenes, Salmonella enterica Serovar Enteritidis, and Staphylococcus aureus. Microorganisms 2020, 8, 849. https://doi.org/10.3390/microorganisms8060849
Sindi A, Badsha MB, Nielsen B, Ünlü G. Antimicrobial Activity of Six International Artisanal Kefirs against Bacillus cereus, Listeria monocytogenes, Salmonella enterica Serovar Enteritidis, and Staphylococcus aureus. Microorganisms. 2020; 8(6):849. https://doi.org/10.3390/microorganisms8060849
Chicago/Turabian StyleSindi, Abrar, Md. Bahadur Badsha, Barbara Nielsen, and Gülhan Ünlü. 2020. "Antimicrobial Activity of Six International Artisanal Kefirs against Bacillus cereus, Listeria monocytogenes, Salmonella enterica Serovar Enteritidis, and Staphylococcus aureus" Microorganisms 8, no. 6: 849. https://doi.org/10.3390/microorganisms8060849
APA StyleSindi, A., Badsha, M. B., Nielsen, B., & Ünlü, G. (2020). Antimicrobial Activity of Six International Artisanal Kefirs against Bacillus cereus, Listeria monocytogenes, Salmonella enterica Serovar Enteritidis, and Staphylococcus aureus. Microorganisms, 8(6), 849. https://doi.org/10.3390/microorganisms8060849