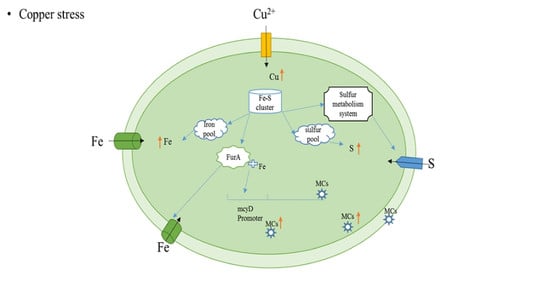
FurA-Dependent Microcystin Synthesis under Copper Stress in Microcystis aeruginosa
Abstract
1. Introduction
2. Materials and Methods
2.1. Strain and Culture Conditions
2.2. Copper Exposures
2.3. RNA Extraction and Reverse Transcription
2.4. M. aeruginosa Transcriptome Data Analyses
2.5. Quantitative Real-Time PCR
2.6. Intracellular Copper and Iron Concentrations of M. aeruginosa
2.7. Gel Mobility Shift Assays
2.7.1. Extraction of DNA
2.7.2. Overexpression and Purification of Fur Protein
2.7.3. Detect Protein–DNA Interactions
2.8. Determination of MCs
2.9. Statistical Analyses
3. Results
3.1. Copper Toxicity
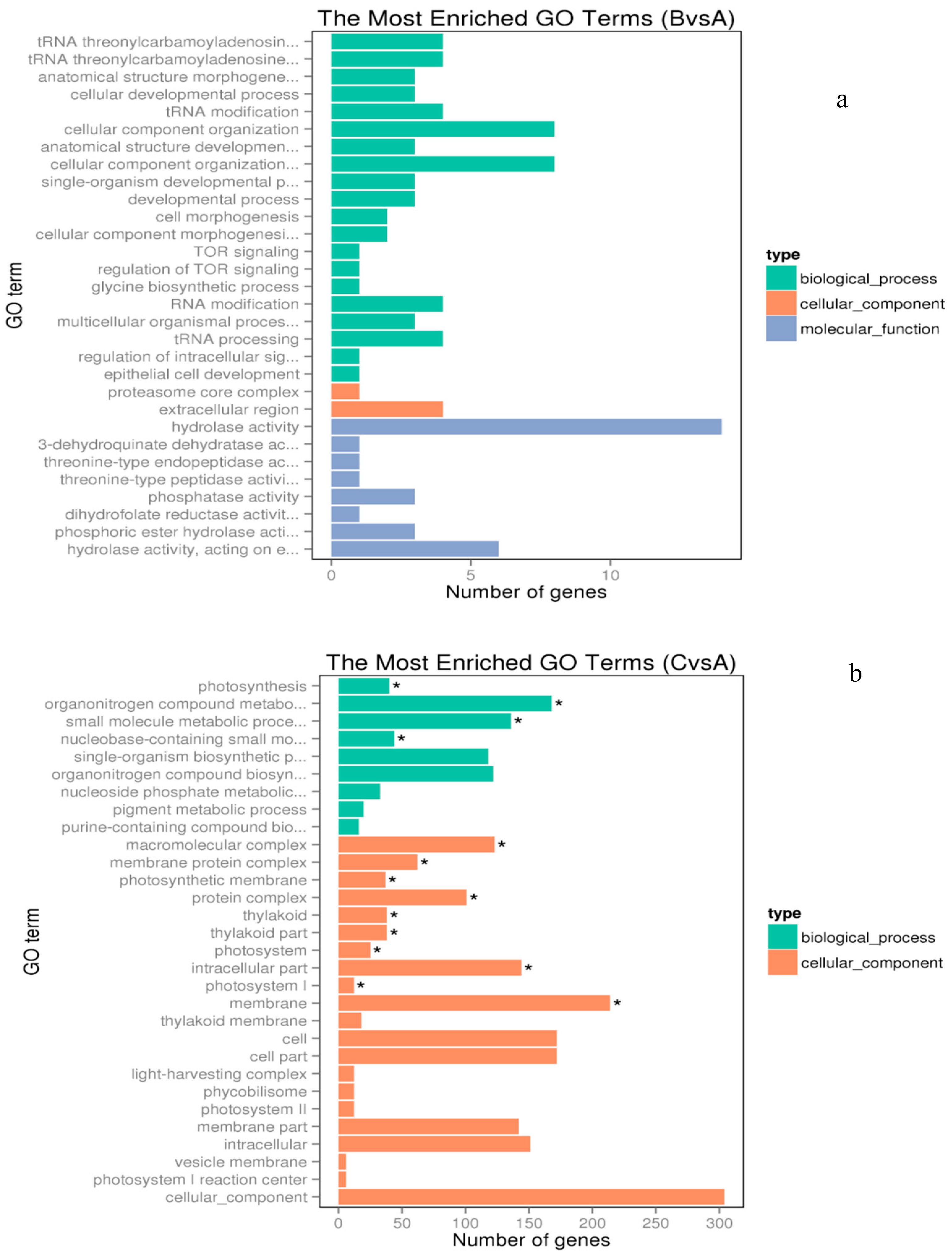
3.2. Transcriptomic Responses to Low and High Copper
3.3. Copper Stress Induces both Iron–Sulfur Cluster Biogenesis and Cluster Target Genes
3.4. Quantitative Real-Time PCR
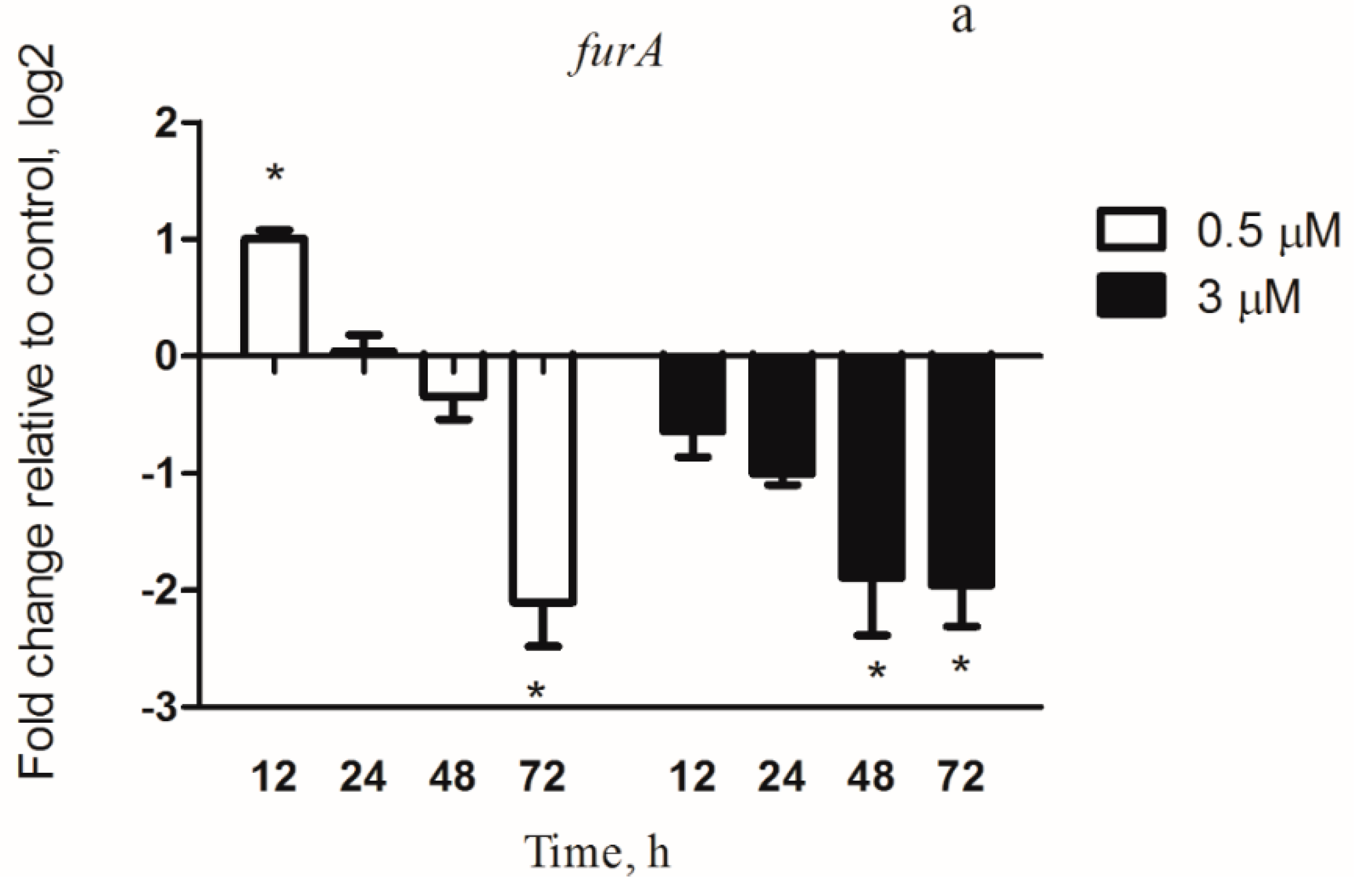
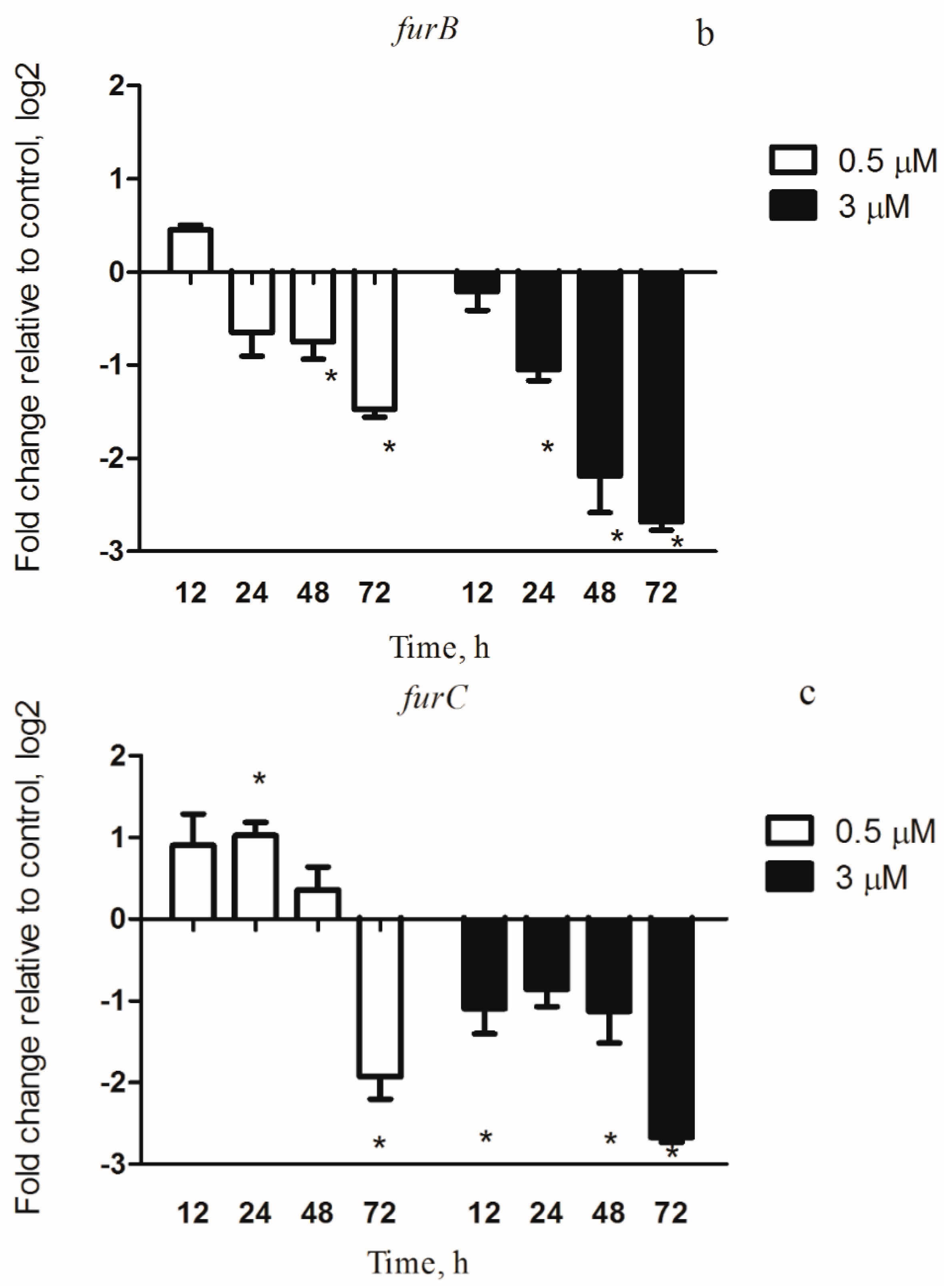
3.4.1. Iron Metabolism Genes under Copper Stress Conditions
3.4.2. Non-Ribosomal Peptide Synthesis Genes under Copper Stress Conditions
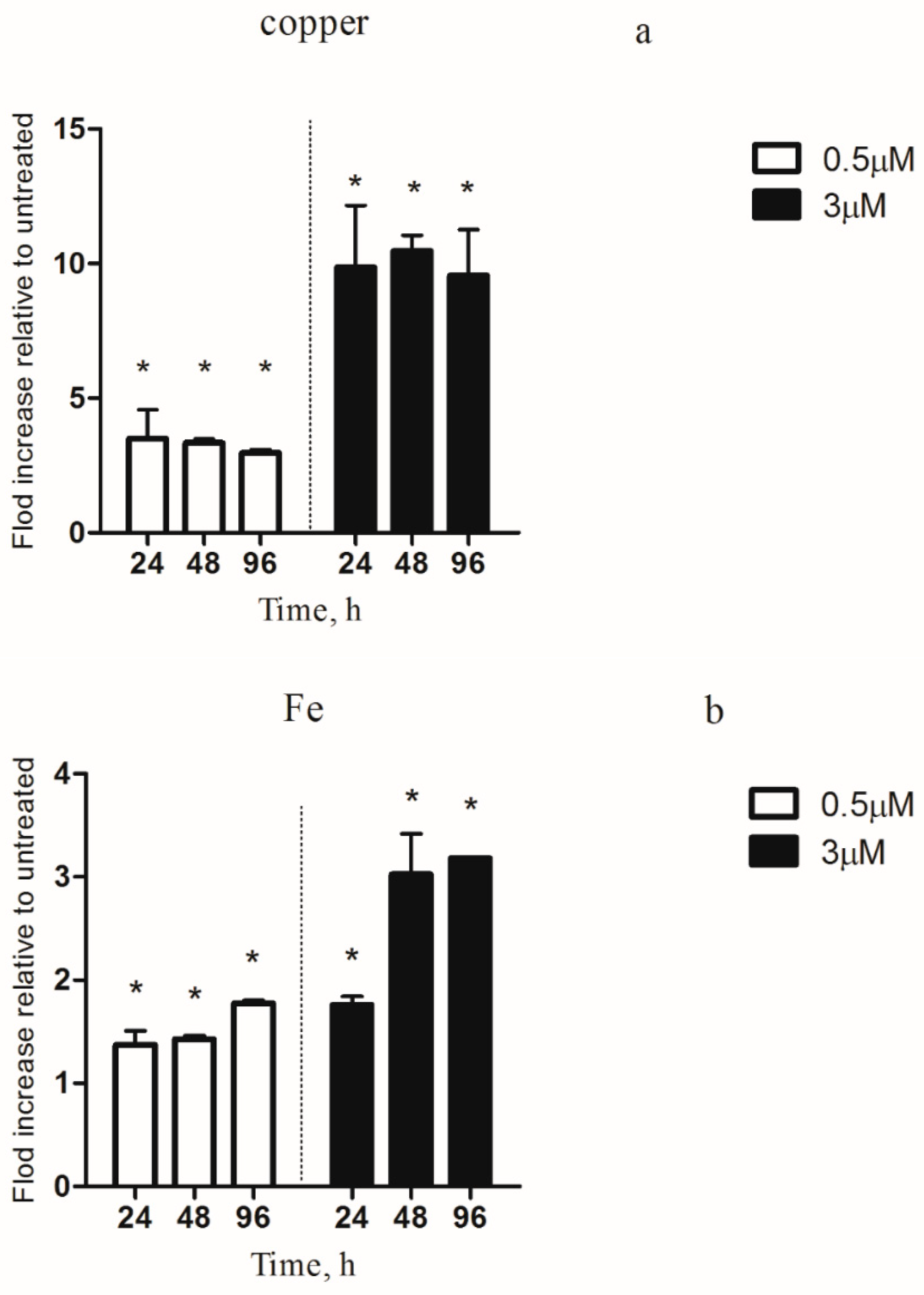
3.5. Intracellular Copper and Iron Content
3.6. FurA Controls the Expression of McyD Gene
3.7. MC Synthesis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Data Availability Statement
Appendix A
| GO_accession | Description | Term_Type |
|---|---|---|
| GO:0051539 | 4 iron, 4 sulfur cluster binding | molecular_function |
| GO:0051536 | iron–sulfur cluster binding | molecular_function |
| GO:0005381 | iron ion transmembrane transporter activity | molecular_function |
| GO:0006826 | iron ion transport | biological_process |
| GO:0044272 | sulfur compound biosynthetic process | biological_process |
| GO:0015093 | ferrous iron transmembrane transporter activity | molecular_function |
| GO:0015684 | ferrous iron transport | biological_process |
| GO:0016730 | oxidoreductase activity, acting on iron–sulfur proteins as donors | molecular_function |
| GO:0006790 | sulfur compound metabolic process | biological_process |
| GO:1901681 | sulfur compound binding | molecular_function |
| GO:0016667 | oxidoreductase activity, acting on a sulfur group of donors | molecular_function |
| GO:0005506 | iron ion binding | molecular_function |
| GO:0016226 | iron–sulfur cluster assembly | biological_process |
| GO:0031163 | metallo–sulfur cluster assembly | biological_process |
| GO:1901682 | sulfur compound transmembrane transporter activity | molecular_function |
| GO:1901678 | iron coordination entity transport | biological_process |
| GO:0016668 | oxidoreductase activity, acting on a sulfur group of donors, NAD(P) as acceptor | molecular_function |
| GO:0016699 | oxidoreductase activity, acting on hydrogen as donor, iron–sulfur protein as acceptor | molecular_function |
| GO:0006827 | high-affinity iron ion transmembrane transport | biological_process |
| GO:0033573 | high-affinity iron permease complex | cellular_component |
| GO:0034755 | iron ion transmembrane transport | biological_process |
| GO:0006879 | cellular iron ion homeostasis | biological_process |
| GO:0008199 | ferric iron binding | molecular_function |
| GO:0055072 | iron ion homeostasis | biological_process |
| GO:0008198 | ferrous iron binding | molecular_function |
| GO:0072348 | sulfur compound transport | biological_process |
| GO:0016671 | oxidoreductase activity, acting on a sulfur group of donors, disulfide as acceptor | molecular_function |
| GO:0000097 | sulfur amino acid biosynthetic process | biological_process |
| GO:0000096 | sulfur amino acid metabolic process | biological_process |
| GO:0051537 | 2 iron, 2 sulfur cluster binding | molecular_function |
| GO:0010309 | acireductone dioxygenase (iron(II)-requiring) activity | molecular_function |
| GO:0004089 | carbonate dehydratase activity | molecular_function |
| GO:0008124 | 4-alpha-hydroxytetrahydrobiopterin dehydratase activity | molecular_function |
| GO:0003855 | 3-dehydroquinate dehydratase activity | molecular_function |
| GO:0004424 | imidazoleglycerol-phosphate dehydratase activity | molecular_function |
| GO:0004664 | prephenate dehydratase activity | molecular_function |
| Locus Gene | Functional Description | 3 µM | |
|---|---|---|---|
| Fe–S cluster response | MAE_RS10070 | transcriptional regulator sufR | 7.06 × 10−31 |
| MAE_RS16045 | cysteine desufuration protein SufE | 0.00048425 | |
| MAE_RS10065 | Fe–S cluster assembly protein SufB | 0.00015271 | |
| MAE_RS00015 | (2Fe–2S)-binding protein | 0.00012458 | |
| MAE_RS23065 | (Fe–S)-binding protein | 9.8206 × 10−26 | |
| MAE_RS25860 | photosystem I iron–sulfur center | 0.001504 | |
| MAE_RS06030 | (2Fe–2S)-binding protein | 0.0010832 | |
| MAE_RS07140 | ferredoxin:protochlorophyllide reductase (ATP-dependent) iron–sulfur ATP-binding protein | 1.3806 × 10−13 | |
| MAE_RS21970 | 4Fe-4S ferredoxin | 1.6106 × 10−8 | |
| MAE_RS24290 | zinc/iron-chelating domain-containing protein | 0.0035634 | |
| MAE_RS10060 | ABC transporter ATP-binding protein | 0.0020487 | |
| MAE_RS15100 | glycine cleavage system protein T | 0.0011042 | |
| Iron | MAE_RS00530 | ferredoxin | 0.0002315 |
| MAE_RS00890 | ferredoxin | 0.00014744 | |
| MAE_RS06385 | ferredoxin | 1.1706 × 10−8 | |
| MAE_RS04240 | ferrochelatase | 0.00043992 | |
| MAE_RS05555 | ferredoxin--NADP(+) reductase | 6.7706 × 10−10 | |
| MAE_RS10410 | iron ABC transporter substrate-binding protein | 7.2506 × 10−5 | |
| MAE_RS13725 | ferrous iron transporter B | 3.7706 × 10−34 | |
| MAE_RS13730 | iron transporter FeoA | 3.8906 × 10−54 | |
| MAE_RS14865 | ferrochelatase | 9.9806 × 10−7 | |
| MAE_RS18510 | ferredoxin | 0.0026747 | |
| MAE_RS19620 | iron ABC transporter substrate-binding protein | 4.8806 × 10−6 | |
| MAE_RS24680 | Fe(3+) ABC transporter substrate-binding protein | 1.1306 × 10−9 | |
| Sulfur | MAE_RS25765 | sulfurtransferase | 0.03116 |
| MAE_RS26150 | sulfurtransferase DndC | 5.5706 × 10−5 | |
| MAE_RS22440 | sulfurtransferase | 0.0012032 | |
| MAE_RS25470 | IscS subfamily cysteine desulfurase | 2.0906 × 10−10 | |
| MAE_RS05935 | cysteine desulfurase | 0.019161 | |
| MAE_RS06525 | cysteine desulfurase | 4.6006 × 10−6 | |
| MAE_RS07485 | molybdopterin synthase sulfur carrier subunit | 1.6906 × 10−6 | |
| Dehydratase | MAE_RS07940 | NAD-dependent dehydratase | 0.0031625 |
| MAE_RS13050 | GDP-mannose 4%2C6-dehydratase | 9.9906 × 10−6 | |
| MAE_RS13315 | 3-dehydroquinate dehydratase | 3.0206 × 10−8 | |
| MAE_RS17290 | 4a-hydroxytetrahydrobiopterin dehydratase | 0.00011614 | |
| MAE_RS20985 | methylthioribulose 1-phosphate dehydratase | 0.00015347 | |
| MAE_RS21370 | NAD-dependent dehydratase | 7.8206 × 10−6 | |
| MAE_RS25085 | dTDP-glucose 4%2C6-dehydratase | 0.0035724 | |
| MAE_RS25915 | imidazoleglycerol-phosphate dehydratase | 2.6606 × 10−7 | |
| Glutaredoxin 3 | MAE_RS01145 | arsenate reductase%2C glutathione/glutaredoxin type | 8.9406 × 10−18 |
| MAE_RS25430 | glutaredoxin 3 | 0.0021661 | |
| Microcystin | MAE_RS16655 | McyC protein | 0.0000487 |
| MAE_RS16660 | McyB protein | 0.000000238 | |
| MAE_RS16665 | McyA protein | 0.00011205 | |
| MAE_RS16670 | type I polyketide synthase(McyD) | 5.5506 × 10−21 | |
| MAE_RS16675 | hybrid non-ribosomal peptide synthetase/type I polyketide synthase | 3.0306 × 10−13 | |
| MAE_RS16685 | type I polyketide synthase | 1.2406 × 10−12 | |
| MAE_RS24610 | non-ribosomal peptide synthetase | 1.6306 × 10−16 | |
| MAE_RS24620 | non-ribosomal peptide synthetase | 1.1206 × 10−15 | |
| MAE_RS24650 | non-ribosomal peptide synthetase | 2.8506 × 10−15 | |
| MAE_RS26180 | non-ribosomal peptide synthetase | 0.0000209 | |
| MAE_RS26185 | McnC protein | 0.000000339 | |
| MAE_RS26190 | McnB protein | 0.0000135 | |
| Cysteine | MAE_RS05935 | cysteine desulfurase | 0.019161 |
| MAE_RS06525 | cysteine desulfurase | 0.0000046 | |
| MAE_RS12370 | cysteine–tRNA ligase | 2.1306 × 10−8 | |
| MAE_RS16045 | cysteine desufuration protein SufE | 0.00048425 | |
| MAE_RS18355 | 5-histidylcysteine sulfoxide synthase | 0.000000907 | |
| MAE_RS22460 | cysteine synthase A | 0.0038637 | |
| MAE_RS25470 | IscS subfamily cysteine desulfurase | 2.0906 × 10−10 |
References
- Lee, W.; Westerhoff, P.; Croue, J.-P. Dissolved organic nitrogen as a precursor for chloroform, dichloroacetonitrile, N-Nitrosodimethylamine, and trichloronitromethane. Environ. Sci. Technol. 2007, 41, 5485–5490. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Yang, X.; Ma, J.; Shang, C.; Zhao, Q. Characterization of algal organic matter and formation of DBPs from chlor(am)ination. Water Res. 2010, 44, 5897–5906. [Google Scholar] [CrossRef] [PubMed]
- Carmichael, W.W. Health effects of toxin-producing cyanobacteria: “the CyanoHABs”. Hum. Ecol. Risk Assess. 2001, 7, 1393–1407. [Google Scholar] [CrossRef]
- Falconer, I.R. Effects on human health of some toxic cyanobacteria (blue green algae) in reservoirs, lakes and rivers. Environ. Toxicol. 1991, 4, 175–184. [Google Scholar] [CrossRef]
- Li, G.; Cai, F.; Yan, W.; Li, C.; Wang, J. A Proteomic Analysis of MCLR-induced Neurotoxicity: Implications for Alzheimer’s Disease. Toxicol. Sci. 2012, 127, 485–495. [Google Scholar] [CrossRef]
- Garcia-Villada, L.; Rico, M.; Altamirano, M.; Sanchez-Martin, L.; Lopez-Rodas, V.; Costas, E. Occurrence of copper resistant mutants in the toxic cyanobacteria Microcystis aeruginosa: Characterisation and future implications in the use of copper sulphate as algaecide. Water Res. 2004, 38, 2207–2213. [Google Scholar] [CrossRef]
- Jancula, D.; Marsalek, B. Critical review of actually available chemical compounds for the prevention and management of cyanobacterial blooms. Chemosphere 2011, 85, 1415–1422. [Google Scholar] [CrossRef]
- Zhou, S.; Shao, Y.; Gao, N.; Deng, Y.; Qiao, J.; Ou, H.; Deng, J. Effects of different algaecides on the photosynthetic capacity, cell integrity and microcystin-LR release of Microcystis aeruginosa. Sci. Total Environ. 2013, 463, 111–119. [Google Scholar] [CrossRef]
- Boopathi, T.; Ki, J.-S. Impact of Environmental Factors on the Regulation of Cyanotoxin Production. Toxins 2014, 6, 1951–1978. [Google Scholar] [CrossRef]
- Hochmuth, J.D.; Asselman, J.; De Schamphelaere, K.A.C. Are interactive effects of harmful algal blooms and copper pollution a concern for water quality management? Water Res. 2014, 60, 41–53. [Google Scholar] [CrossRef]
- Kenefick, S.L.; Hrudey, S.E.; Peterson, H.G.; Prepas, E.E. Toxin release from Microcystis aeruginosa after chemical treatment. Water Sci. Technol. 1993, 27, 433–440. [Google Scholar] [CrossRef]
- Touchette, B.W.; Edwards, C.T.; Alexander, J. A comparison of cyanotoxin release following bloom treatments with copper sulfate or sodium carbonate peroxyhdrate. In Cyanobacterial Harmful Algal Blooms: State of the Science and Research Needs; Springer: Beilin, Germany, 2008; Volume 619, pp. 314–315. [Google Scholar]
- Tsai, K.-P. Effects of two copper compounds on Microcystis aeruginosa cell density, membrane integrity, and microcystin release. Ecotoxicol. Environ. Saf. 2015, 120, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Ho, L.; Hobson, P.; Brookes, J. Evaluating the effectiveness of copper sulphate, chlorine, potassium permanganate, hydrogen peroxide and ozone on cyanobacterial cell integrity. Water Res. 2013, 47, 5153–5164. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Hobson, P.; Ho, L.; Daly, R.; Brookes, J. The effects of various control and water treatment processes on the membrane integrity and toxin fate of cyanobacteria. J. Hazard. Mater. 2014, 264, 313–322. [Google Scholar] [CrossRef]
- Beasley, V.R.; Cook, W.O.; Dahlem, A.M.; Hooser, S.B.; Lovell, R.A.; Valentine, W.M. Algae intoxication in livestock and waterfowl. Vet. Clin. N. Am-Food A 1989, 5, 345–361. [Google Scholar] [CrossRef]
- Christoffersen, K.; Kaas, H. Toxic cyanobacteria in water. A guide to their public health consequences, monitoring, and management. Limnol. Oceanog. 2000, 45, 1212. [Google Scholar] [CrossRef]
- Calomeni, A.J.; Rodgers, J.H., Jr. Evaluation of the utility of six measures for algal (Microcystis aeruginosa, Planktothrix agardhii and Pseudokirchneriella subcapitata) viability. Ecotoxicol. Environ. Saf. 2015, 111, 192–198. [Google Scholar] [CrossRef]
- Alexova, R.; Fujii, M.; Birch, D.; Cheng, J.; Waite, T.D.; Ferrari, B.C.; Neilan, B.A. Iron uptake and toxin synthesis in the bloom-forming Microcystis aeruginosa under iron limitation. Environ. Microbiol. 2011, 13, 1064–1077. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Li, N.; Zhang, L.; Li, F.; Wang, Y.; Zhu, Y.; Kang, H.; Wang, S.; Qin, S. Metagenome of microorganisms associated with the toxic Cyanobacteria Microcystis aeruginosa analyzed using the 454 sequencing platform. Chin. J. Oceanol. Limnol. 2011, 29, 505–513. [Google Scholar] [CrossRef]
- Martin-Luna, B.; Hernandez, J.A.; Bes, M.T.; Fillat, M.F.; Peleato, M.L. Identification of a Ferric uptake regulator from Microcystis aeruginosa PCC7806. Fems Microbiol. Lett. 2006, 254, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Martin-Luna, B.; Sevilla, E.; Hernandez, J.A.; Bes, M.T.; Fillat, M.F.; Peleato, M.L. Fur from Microcystis aeruginosa binds in vitro promoter regions of the microcystin biosynthesis gene cluster. Phytochemistry 2006, 67, 876–881. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, J.A.; Lopez-Gomollon, S.; Muro-Pastor, A.; Valladares, A.; Bes, M.T.; Peleato, M.L.; Fillat, M.F. Interaction of FurA from Anabaena sp PCC 7120 with DNA: A reducing environment and the presence of Mn2+ are positive effectors in the binding to isiB and furA promoters. Biometals 2006, 19, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.; Teresa Bes, M.; Barja, F.; Luisa Peleato, M.; Fillat, M.F. Overexpression of FurA in Anabaena sp. PCC 7120 Reveals New Targets for This Regulator Involved in Photosynthesis, Iron Uptake and Cellular Morphology. Plant Cell Physiol. 2010, 51, 1900–1914. [Google Scholar] [CrossRef] [PubMed]
- Sevilla, E.; Martin-Luna, B.; Vela, L.; Bes, M.T.; Fillat, M.F.; Peleato, M.L. Iron availability affects mcyD expression and microcystin-LR synthesis in Microcystis aeruginosa PCC7806. Environ. Microbiol. 2008, 10, 2476–2483. [Google Scholar] [CrossRef] [PubMed]
- Chillappagari, S.; Seubert, A.; Trip, H.; Kuipers, O.P.; Marahiel, M.A.; Miethke, M. Copper Stress Affects Iron Homeostasis by Destabilizing Iron-Sulfur Cluster Formation in Bacillus subtilis. J. Bacteriol. 2010, 192, 2512–2524. [Google Scholar] [CrossRef] [PubMed]
- Macomber, L.; Imlay, J.A. The iron-sulfur clusters of dehydratases are primary intracellular targets of copper toxicity. Proc. Natl. Acad. Sci. USA 2009, 106, 8344–8349. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.M.; Gaballa, A.; Hui, M.; Ye, R.W.; Helmann, J.D. Genetic and physiological responses of Bacillus subtilis to metal ion stress. Mol. Microbiol. 2005, 57, 27–40. [Google Scholar] [CrossRef]
- Outten, F.W.; Theil, E.C. Iron-Based Redox Switches in Biology. Antioxid. Redox Signal. 2009, 11, 1029–1046. [Google Scholar] [CrossRef]
- Wang, T.; Shen, G.Z.; Balasubramanian, R.; McIntosh, L.; Bryant, D.A.; Golbeck, J.H. The sufR gene (sll0088 in Synechocystis sp strain PCC 6803) functions as a repressor of the sufBCDS operon in iron-sulfur cluster biogenesis in cyanobacteria. J. Bacteriol. 2004, 186, 956–967. [Google Scholar] [CrossRef]
- Shen, G.; Balasubramanian, R.; Wang, T.; Wu, Y.; Hoffart, L.M.; Krebs, C.; Bryant, D.A.; Golbeck, J.H. SufR coordinates two 4Fe-4S (2+,1+) clusters and functions as a transcriptional repressor of the sufBCDS operon and an autoregulator of sufR in cyanobacteria. J. Biol. Chem. 2007, 282, 31909–31919. [Google Scholar] [CrossRef] [PubMed]
- Outten, F.W.; Wood, M.J.; Munoz, F.M.; Storz, G. The SufE protein and the SufBCD complex enhance SufS cysteine desulfurase activity as part of a sulfur transfer pathway for fe-s cluster assembly in Escherichia coli. Antioxid. Redox Signal. 2003, 278, 45713–45719. [Google Scholar] [CrossRef] [PubMed]
- Outten, F.W.; Djaman, O.; Storz, G. A suf operon requirement for Fe-S cluster assembly during iron starvation in Escherichia coli. Mol. Microbiol. 2004, 52, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Fillat, M.F. The FUR (ferric uptake regulator) superfamily: Diversity and versatility of key transcriptional regulators. Arch. Biochem. Biophys. 2014, 546, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Escolar, L.; Perez-Martin, J.; de Lorenzo, V. Opening the iron box: Transcriptional metalloregulation by the Fur protein. J. Bacteriol. 1999, 181, 6223–6229. [Google Scholar] [CrossRef]
- Mey, A.R.; Wyckoff, E.E.; Kanukurthy, V.; Fisher, C.R.; Payne, S.M. Iron and fur regulation in Vibrio cholerae and the role of Fur in virulence. Infect. Immun. 2005, 73, 8167–8178. [Google Scholar] [CrossRef]
- Pajuelo, D.; Hernandez-Cabanyero, C.; Sanjuan, E.; Lee, C.-T.; Xavier Silva-Hernandez, F.; Hor, L.-I.; Mackenzie, S.; Amaro, C. Iron and Fur in the life cycle of the zoonotic pathogen Vibrio vulnificus. Environ. Microbiol. 2016, 18, 4005–4022. [Google Scholar] [CrossRef]
- Lopez-Gomollon, S.; Sevilla, E.; Bes, M.T.; Peleato, M.L.; Fillat, M.F. New insights into the role of Fur proteins: FurB (AII2473) from Anabaena protects DNA and increases cell survival under oxidative stress. Biochem. J. 2009, 418, 201–207. [Google Scholar] [CrossRef]
- Singh, A.K.; McIntyre, L.M.; Sherman, L.A. Microarray analysis of the genome-wide response to iron deficiency and iron reconstitution in the cyanobacterium Synechocystis sp PCC 6803. Plant Physiol. 2003, 132, 1825–1839. [Google Scholar] [CrossRef]
- Daly, R.I.; Lionel, H.O.; Brookes, J.D. Effect of Chlorination on Microcystis aeruginosa Cell Integrity and Subsequent Microcystin Release and Degradation. Environ. Environ-Ence Technol. 2007, 41, 4447–4453. [Google Scholar]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Yin, J.; Wei, J.; Zhang, X. FurA-Dependent Microcystin Synthesis under Copper Stress in Microcystis aeruginosa. Microorganisms 2020, 8, 832. https://doi.org/10.3390/microorganisms8060832
Chen Y, Yin J, Wei J, Zhang X. FurA-Dependent Microcystin Synthesis under Copper Stress in Microcystis aeruginosa. Microorganisms. 2020; 8(6):832. https://doi.org/10.3390/microorganisms8060832
Chicago/Turabian StyleChen, Yuanyuan, Jiaojiao Yin, Jin Wei, and Xuezhen Zhang. 2020. "FurA-Dependent Microcystin Synthesis under Copper Stress in Microcystis aeruginosa" Microorganisms 8, no. 6: 832. https://doi.org/10.3390/microorganisms8060832
APA StyleChen, Y., Yin, J., Wei, J., & Zhang, X. (2020). FurA-Dependent Microcystin Synthesis under Copper Stress in Microcystis aeruginosa. Microorganisms, 8(6), 832. https://doi.org/10.3390/microorganisms8060832