1. Introduction
The burden of human foodborne diseases is substantial. The most common illnesses resulting from unsafe food include diarrheal diseases, affecting 550 million people per year [
1].
Salmonella is one of the four key global causes of diarrheal diseases. Indeed, the food-borne pathogen
Salmonella enterica is associated with a number of diseases in a wide range of animal hosts. Therefore,
Salmonella-related infections still represent an important health concern worldwide. Whether bacteria remain restricted to the gastrointestinal tract and local lymphatic system, or whether they spread to organs like the spleen or liver, is highly dependent on the individual host and the particular
Salmonella strain. Severe cases of infection may lead to serious clinical problems, including death [
2].
The flagellum is an important virulence factor, often involved in the initial phase of the infection. Flagella are long, helical appendages that allow bacteria to direct their movement towards nutrient sources or away from harmful substances [
3,
4].
Salmonella enterica serovar Typhimurium (
S. Typhimurium) has approximately 6 to 10 peritrichous flagella distributed around the cell [
5]. The flagellum is a complex structure made of approximately 30 different proteins that extends up to 20 µm beyond the cell surface [
6]. Typically, it consists of three main segments: the basal body, the hook, and the filament. The basal body anchors the flagellum into the bacterial inner and outer membranes and includes rotor and stator protein complexes, necessary for force generation and flagellar rotation [
7]. The flexible extracellular hook functions as a universal joint that connects the basal body with the third segment, the filament [
8,
9]. The filament forms a helical propeller and consists of approximately 20,000 subunits of flagellin. Many
Salmonella serovars alternate flagellin expression between two antigenically different proteins, FljB and FliC, in a process known as flagellar phase variation. Usually only one of the flagellins is produced at a time in a given cell [
5]. These flagellin variants display structural variability resulting in differences in swimming towards host cell surfaces. For example, bacteria expressing FliC-flagella are more efficient in the identification of target sites on host cell surfaces and therefore have an advantage when invading epithelial cells. They outcompete FljB-flagellated bacteria during intestinal colonization in both gastroenteritis and typhoid murine infection models even though their intracellular survival and the responses triggered on the host immune system are similar [
10].
Besides being involved in bacterial motility and chemotaxis, flagella are also required for many other processes that contribute to a successful colonization of the host. In mammalian systems, physical forces between flagella and the host cell surface allow
Salmonella to scan the host’s surface topology and to determine the optimal infection site [
11]. Moreover, the flagellar filament seems to be important for intestinal cell adhesion, which enables triggering of membrane ruffling and initiate invasion [
12].
While flagella actively contribute to the infection process during pathogenic interactions, flagellin may cause a disadvantage. Flagellin is one of the best-studied Pathogen-Associated Molecular Patterns (PAMPs) and is recognized in mammals and plants by the Toll-like receptor 5 (TLR5) and FLAGELLIN SENSING2 (FLS2) immune receptors, respectively [
13,
14,
15]. Although PAMPs are considered to be conserved, several reports revealed that some bacteria have evolved divergent flagellin sequences, leading to reduced Pattern-Triggered Immunity (PTI) activation [
16,
17,
18,
19].
In plant systems, reports regarding the importance of flagella for the survival and colonization of
Salmonella are conflicting. Shaw et al., reported that flagella do not have a role in attachment to tomato plants [
20]. In contrast, other studies showed that the absence of a flagellar filament has an impact on adhesion to various plants. Berger et al., reported reduced adhesion to basil leaves for the
S. Senftenberg
ΔfliC mutant [
21], whereas in
Arabidopsis thaliana the
ΔfliC mutant exhibited enhanced colonization of roots [
22]. Differences in the role of flagella in colonization of rhizosphere versus phyllosphere have been recently suggested [
23]. These authors reported decreased adhesion levels to corn salad (
Valerianella locusta) leaves for strains lacking flagella filaments. Hence, flagella were proposed to play an important role during both adhesion and motility, while in contact with corn salad. This discrepancy in results might be attributed to the different plant models used in each study. Additionally, variations in the physiological conditions of the plants used in the different studies, as well as analogous variations in the corresponding
Salmonella strains, could have added to those discrepancies. Noteworthy, a recent study reported differences in the structures and motility functions of FliC and FljB under high-viscosity conditions. The authors stated that
Salmonella strain expressing FljB showed a higher motility than the one expressing FliC under high viscosity. They attributed these differences to the density of D3 domain, which was much lower in FljB than FliC, offering more flexibility and mobility than to that of FliC [
24].
In this work, we hypothesize that flagella might play a versatile role during colonization of plants. We used tomato (
Solanum lycopersicum cultivar Moneymaker) as a representative crop plant, which fruits are widely consumed and have been associated to several salmonellosis outbreaks [
25]. We assessed the ability of
S. Typhimurium wild type strain 14028s and a double flagellin mutant
S. Typhimurium
ΔfljBΔfliC to persist inside the plant, as well as to reach distal, non-inoculated tissues of the plant. We compared the respective abilities of these strains using competitive index assays. The defense response of tomato plants to the presence of wild type
Salmonella strains and strains lacking flagella was evaluated through gene expression analysis of marker genes. We also analyzed the expression of motility and adhesion-related bacterial genes in response to plant media. Finally, we evaluated the expression of
fliC at the single-cell level following expression of a chromosome-located transcriptional fusion to Green Fluorescent Protein (GFP) by confocal microscopy and flow cytometry. We analyzed
fliC expression in bacteria growing in medium supplemented with plant extract, and also in bacteria directly extracted from the plant apoplast. Our results show that the majority of cells within the
S. Typhimurium apoplastic population down regulate the expression of flagella. However, a small subpopulation maintains flagellar expression
in planta at a very high level. Heterogeneous expression of flagella might be an adaptive strategy of
Salmonella to efficiently colonize the plant environment: non-motile cells not expressing flagellin could reduce recognition by the plant immune system or display increased fitness, while those retaining motility might be ready to colonize new ecological niches such as, for example, the herbivore. Taken together, our results provide new insights on how
Salmonella adapts to the plant environment and on which strategies
Salmonella uses in order to persist in this environment.
2. Materials and Methods
2.1. Bacterial Strains, Culture Conditions and Media Preparation
Salmonella enterica serovar Typhimurium strain 14028s (
S. Typhimurium 14028s), resistant to rifampicin, was used in this study as the wild type reference strain. In addition, a flagellin double mutant
Salmonella enterica serovar Typhimurium 14028s
∆fljB∆fliC, (
S. Typhimurium
∆fljB∆fliC) kindly provided by Michael Hensel (University of Osnabrück, Osnabrück, Germany) was used. Bacteria were grown in Luria-Bertani (LB) broth (Carl Roth GmbH & Co., KG, Karlsruhe, Germany) or on LB agar plates. Plant-based media, namely lettuce medium (LM) and tomato medium (TM), were prepared as described previously by [
26,
27], respectively. Minimal medium (MM) was used as a control medium and was prepared as described by [
27]. Xylose-lysine-desoxycholate (XLD) agar (Carl Roth GmbH & Co., KG) was used as a selective medium for
Salmonella. Antibiotics were used at the following concentrations: rifampicin 50 mg/L for
S. Typhimurium 14028s, rifampicin 50 mg/L plus kanamycin 50 mg/L for
∆fljB∆fliC, and chloramphenicol 50 mg/L for
fliC-gfp strain. Bacteria were incubated overnight on LB and XLD agar plates at 28–37 °C, or at 25–28 °C on plant-based media. All strains used in this study are listed in
Supplementary Table S1.
2.2. Fluorescent Labeling of Salmonella Strains
In order to visualize colonization patterns,
S. Typhimurium 14028s and
S. Typhimurium
∆fljB∆fliC were GFP-labeled with the pSM1890 GFP plasmid [
28]. The preparation was performed according to [
29]. Briefly,
Salmonella strains were allowed to mate with
E. coli carrying the IncQ plasmid pSM1890 GFP (derived from the IncQ plasmid pIE723 [
30] and
E. coli carrying the helper plasmid R751 [
31]. Cells were re-suspended from LB plates into 1 mL of a 10 mM MgCl
2 solution. The cell suspensions were combined, mixed and centrifuged and the supernatant discarded. The pellets were re-suspended in the remaining supernatant. The solution was placed on a filter disc (0.22 µm, Durapore membrane filters, Merck, Darmstadt, Germany) on LB agar and incubated overnight at 28 °C. Cells were re-suspended by vortexing the filter in 5 mL of 10 mM MgCl
2 solution and transconjugants were selected by plating serial dilutions on LB. After overnight incubation at 28 °C, green fluorescent colonies were picked and plated on XLD agar for further detection on the following day.
In addition, a transcriptional fusion to the
gfp gene was generated in
S. Typhimurium 14028s downstream of the stop codon of the
fliC ORF. The source of the promoterless
gfp ORF including its ribosomal-binding site, and the chloramphenicol resistance cassette, used for selecting the integration by allelic exchange of the
gfp-containing DNA fragment, was pZEP07 [
32]. The construct was integrated into the chromosome of
S. enterica using the Lambda Red recombination system [
33].
2.3. Plant Cultivation
Tomato (Solanum lycopersicum cultivar Moneymaker) seeds were surface sterilized with 70% ethanol for 1 min followed by incubation for 3 min in 3% sodium hypochlorite (NaClO) solution. The seeds were later vigorously washed with sterile distilled water. Seeds were germinated for 7–10 days in Petri dishes on sterile ¼-strength Murashige and Skoog (MS) agar medium (Sigma-Aldrich Chemie GmbH, München, Germany), pH 5.4 including vitamins and 5 g/ L sucrose. Seedlings were grown under sterile conditions with a light intensity of 150 µmol m2/s (16 h photoperiod) at 22 °C for either two additional weeks in sterile glass pots (for spray inoculation), or for 2 days in 50 mL conical tubes, containing 20 mL ¼ MS liquid medium for microscopic analysis.
In order to evaluate the survival of the bacteria in leaves, plants were grown under greenhouse conditions in standard bedding substrate (substrate 1, Klasmann-Deilmann GmbH, Geeste, Germany) at 22 °C and 16 h photoperiod for 4–5 weeks. The plants were watered as needed from the bottom to avoid contamination of the non-inoculated leaf parts.
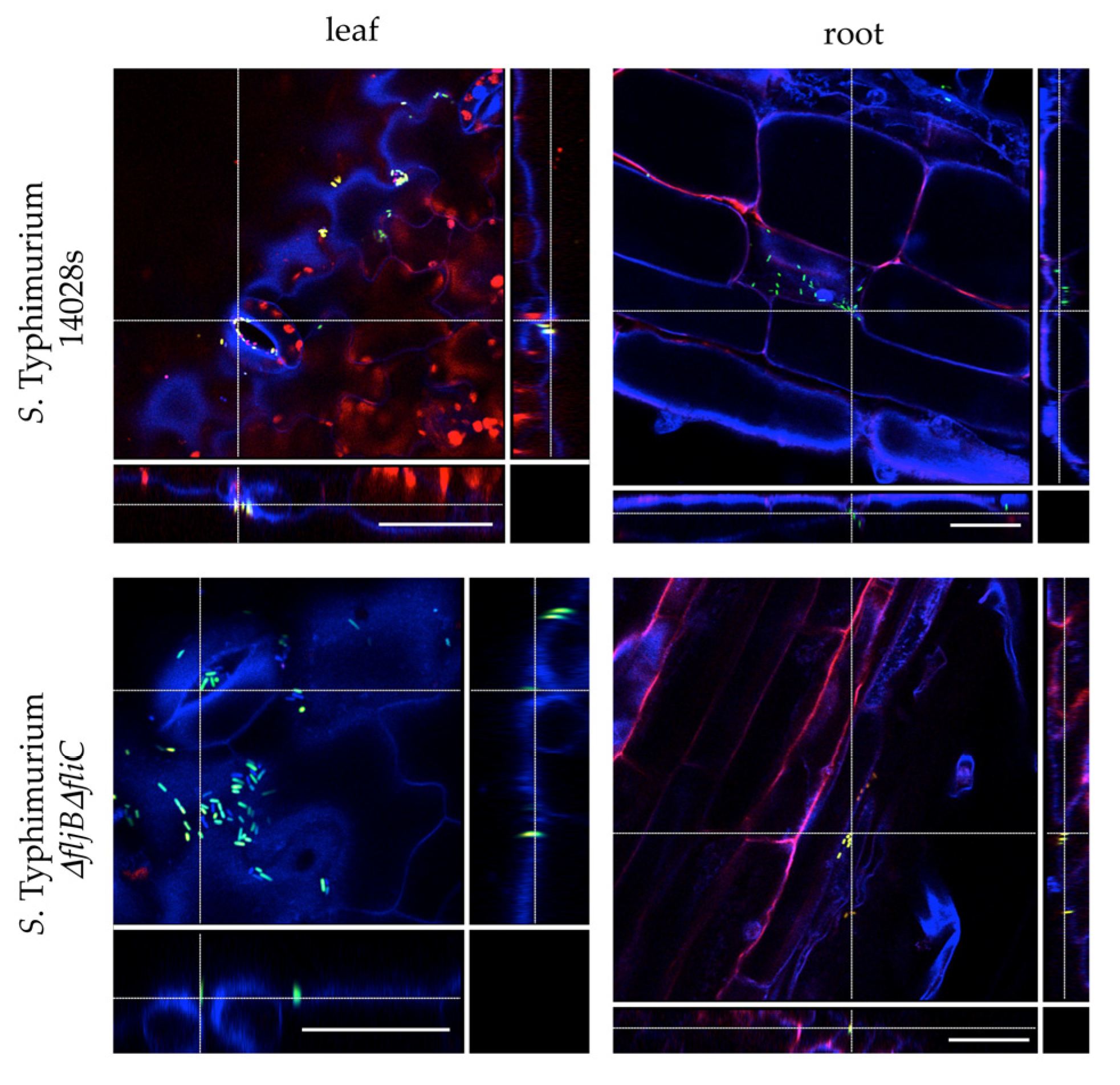
2.4. Confocal Laser Scanning Microscopy (CLSM)
Tomato plants were grown under sterile conditions in order to visualize the colonization patterns of Salmonella. Seven to ten-day old plants were transferred to conical tubes containing ¼-strength MS liquid medium. Plants were allowed to adapt to the medium for 24 h before the medium was inoculated with a final OD600 nm = 0.1 corresponding to 108 colony forming units/mL (CFU/mL) of either S. Typhimurium 14028s-GFP or S. Typhimurium ∆fljB∆fliC-GFP strain. Plants were sampled (leaves and roots) 24 h post inoculation (hpi), stained with propidium iodide (PI) solution (1 µg/ mL) for 5 min and mounted on a microscope slide in 4′, 6-diamidine-2′-phenylindole dihydrochloride (DAPI) solution (10 µg/ mL). Confocal laser scanning microscopy was performed using SP8 microscope (Leica Microsystems, Wetzlar, Germany) with excitation 405 nm, emission 430–480 nm (blue), excitation 488 nm, emission 500–550 nm (green), excitation 561 nm, emission 600–680 nm (red) including autofluorescence of chloroplasts.
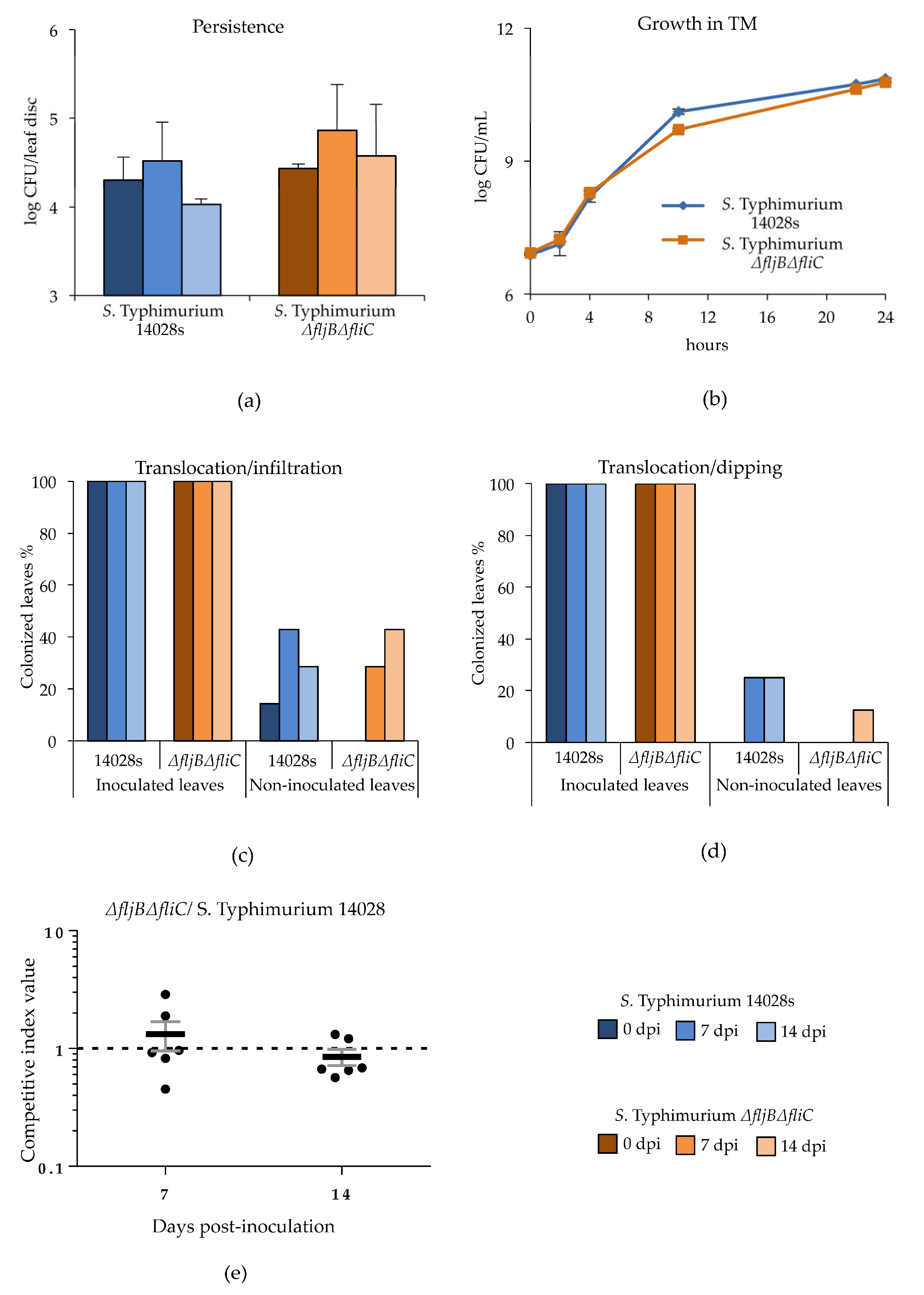
2.5. Persistence of Salmonella in Plants
To assess the survival of
Salmonella inside tomato leaves, plants were grown in substrate under greenhouse conditions as described above. Leaves were inoculated with
S. Typhimurium 14028s and
S. Typhimurium
∆fljB∆fliC strains using either syringe infiltration or dip-inoculation. To avoid contamination of the entire plant, the non-inoculated parts were covered with plastic bags until the inoculated parts dried (
Supplementary Figure S1). Bacterial inocula were prepared using fresh bacterial colonies grown on LB plates. Biomass thus obtained was adjusted to 10
7 CFU/mL (infiltration) or 10
8 CFU/mL (dipping) using 10 mM MgCl
2. Additionally, some plants were either inoculated with 10 mM MgCl
2 or non-treated (N.T.) and used as controls. Leaves were sampled three hours (0 day), 7 and 14 days post inoculation (dpi). Five mm diameter leaf discs were obtained using a sterile biopsy punch (GlaxoSmithKline PLC, Brentford, UK) and homogenized in 1 mL 10 mM MgCl
2 using a tissue homogenizer (Xenox, Götze, Berlin, Germany). Serial dilutions were prepared in duplicates and 10 µl of each dilution was dropped in duplicates onto XLD agar plates. CFUs were counted after an overnight incubation at 37 °C. The experiment was performed in three (infiltration) or four (dipping) replicates (one leaf per plant and replicate). Student’s
t-test was applied and
p ≤ 0.05 was considered significant. No colonies were found in negative control (N.T.) or MgCl
2-treated plants.
2.6. Translocation of Salmonella within Tomato Plants
To test the requirement of flagella in systemic colonization of tomato, plants were grown under greenhouse conditions as described previously. Leaves were inoculated with S. Typhimurium 14028s or S. Typhimurium ∆fljB∆fliC strains following the methods described above (infiltration or dipping). Leaves (inoculated and non-inoculated) were sampled 5 h (0 dpi), 7 and 14 dpi, cut with sterile scissors and placed in 50 mL conical tubes containing 10 mL Buffered Peptone Water (BPW) (Carl Roth GmbH & Co. KG). Tubes were incubated at 37 °C overnight under shaking conditions (140 rpm) and 10 µL of the suspension was transferred to 190 µL Rappaport Vassiliadis Broth (RVS) (Carl Roth GmbH & Co. KG) and incubated overnight at 42 °C. Ten µL of both enrichments (BPW and RVS) was dropped on XLD agar plates to confirm the presence of Salmonella after overnight incubation at 37 °C. The experiment was conducted in seven (infiltration) or eight (dipping) replicates. One leaf per plant per treatment and replicate was used.
2.7. Competitive Bacterial Colonization Assays
Competitive index (CI) assays were carried out as previously described in [
34]. Briefly, leaves from four to five-week old tomato plants, growing under greenhouse conditions, were infiltrated with a 5 × 10
5 CFU/mL of a mixed bacterial suspension, containing equal CFU of wild type (
S. Typhimurium 14028s) and mutant derivative strain (
S. Typhimurium
∆fljB∆fliC), using a blunt syringe. Serial dilutions of the inoculum were plated onto LB agar and LB agar with kanamycin to confirm dose and relative proportion between the strains, which should be close to one. Seven- or 14-days post-inoculation (dpi), five 10 mm-diameter leaf discs were taken from the infiltrated area of each leaf and homogenized together by mechanical disruption into 1 mL of 10 mM MgCl
2. Then, bacteria were enumerated by plating serial dilutions onto LB agar supplemented with cycloheximide, and the colonies obtained were replica-plated onto LB agar and LB agar with kanamycin, to differentiate the strains within the mixed infection. Bacterial enumeration was carried out in the dilution displaying between 50 and 500 colonies per plate. The CI is defined as the mutant-to-wild type ratio within the output sample divided by the mutant-to-wild type ratio within the input (inoculum), which should be close to one [
35,
36]. CIs presented were obtained from six plant replicates. Mean CI values are shown. Error bars represent standard error. Each CI was analyzed using a homoscedastic and 2-tailed Student’s
t-test and the null hypothesis that mean index is not significantly different from 1 (
p < 0.05).
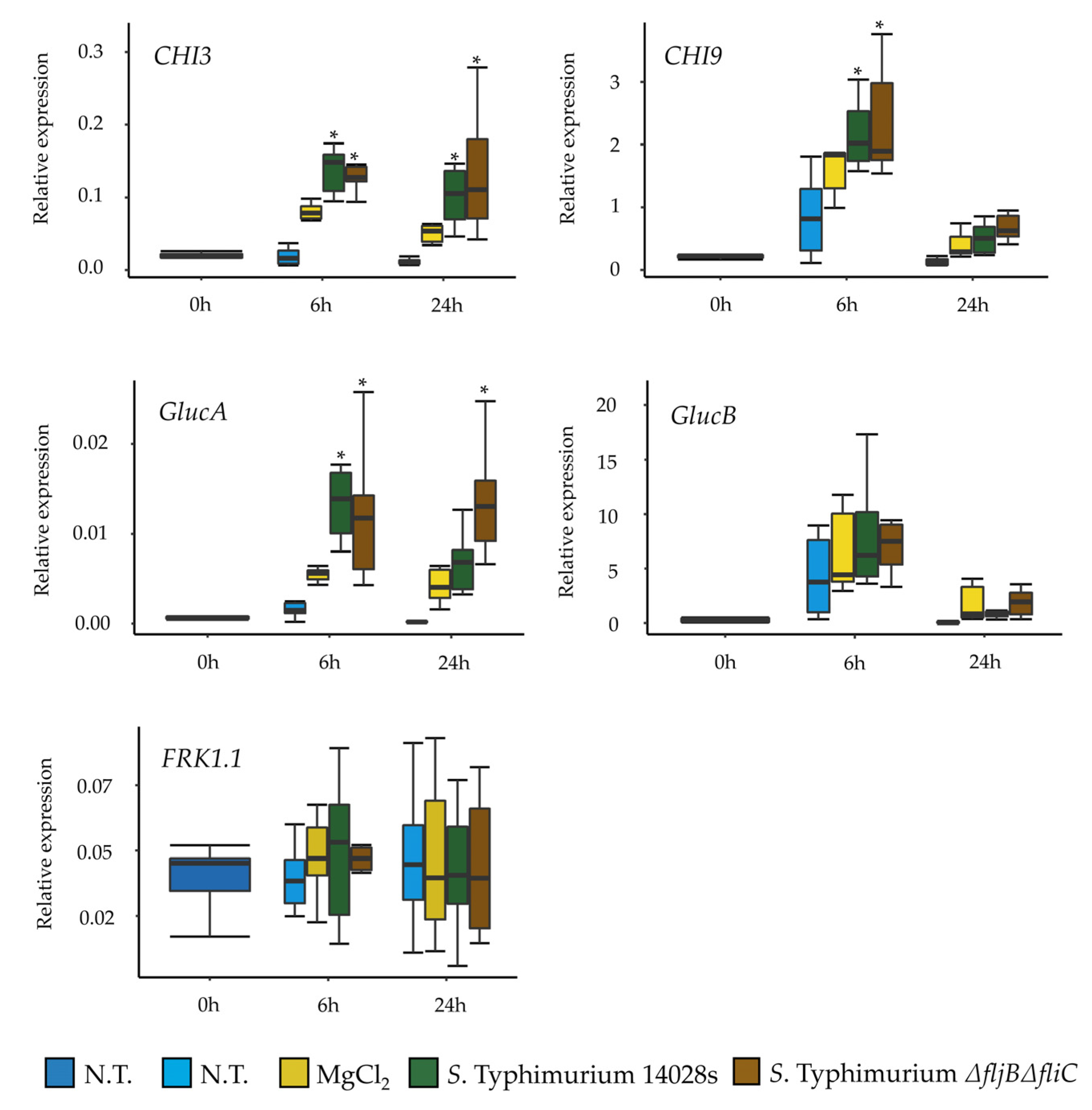
2.8. Quantitative Real Time PCR Analysis
To assess the response of tomato to the presence of
Salmonella and to evaluate the role of flagella in this response,
S. Typhimurium 14028s and
S. Typhimurium
∆fljB∆fliC were sprayed onto 3-week old sterile plants. Plants were grown on ¼-strength MS agar medium. In order to allow the bacteria to adapt to the plant environment, bacteria were pre-grown overnight on tomato-based medium (TM) at 25–28 °C. Plants were spray-inoculated with OD
600 nm = 0.1 (10
8 CFU/mL). Leaves were sampled at 0, 6 and 24 hpi and samples were pooled from three plants per treatment. About 100 mg of leaf tissue was homogenized in a TissueLyser (Qiagen, Hilden, Germany) and the total RNA was extracted using peqGOLD TriFast Reagent (Peqlab, Darmstadt, Germany) according to the manufacturer protocol. DNase treatments, using PerfeCTa DNase I, and cDNA synthesis were performed using 1 µg of total RNA and qScript cDNA Synthesis kit (Quanta BioSciences, Gaithersburg, MD, USA). Target cDNA was then amplified in a 20 µL reaction mixture containing 5 µL of sample DNA and LUNA Master Mix (New England Biolabs, Frankfurt, Germany) according to the manufacturer procedure. Reactions were run for 5 min at 95 °C, followed by 40 cycles of 30 s at 95 °C, 30 s at 58 °C and 60 s at 72 °C in the CFX connect System (Bio-Rad, München, Germany). Primers used for the qPCR are listed in
Supplementary Table S2. Relative gene expression was normalized to the expression of the
Actin gene. The experiment was performed in 6 replicates. Tukey HSD Test was applied with 95% family-wise confidence level on R version 3.6.1 and
p ≤ 0.05 was considered significant (
Supplementary Table S3). Additionally, the experiment was repeated using 100 nM flg22 peptide (
QRLSTGSRINSAKDDAAGLQIA) (AnaSpec. Inc., Fremont, CA, USA), as a control. The samples were obtained at 0 and 6 hpi and the experiment performed in 3 replicates.
2.9. Salmonella Response to Plant Media
Two experimental approaches were designed in order to test how well Salmonella adapts to the plant host and uses potential nutrients available in the plant environment. The first approach was intended to test the ability of the plant media to support growth of Salmonella, hence S. Typhimurium 14028s and S. Typhimurium ∆fljB∆fliC strains were grown in liquid tomato-based medium (TM) overnight at 25–28 °C. The initial bacterial concentration was set to OD600 nm = 0.01 and samples were taken at different time points. Serial dilutions were prepared in duplicates and 10 µl of each dilution was dropped onto XLD agar plates, and CFUs were quantified after overnight incubation at 37 °C. The experiment was performed in three replicates.
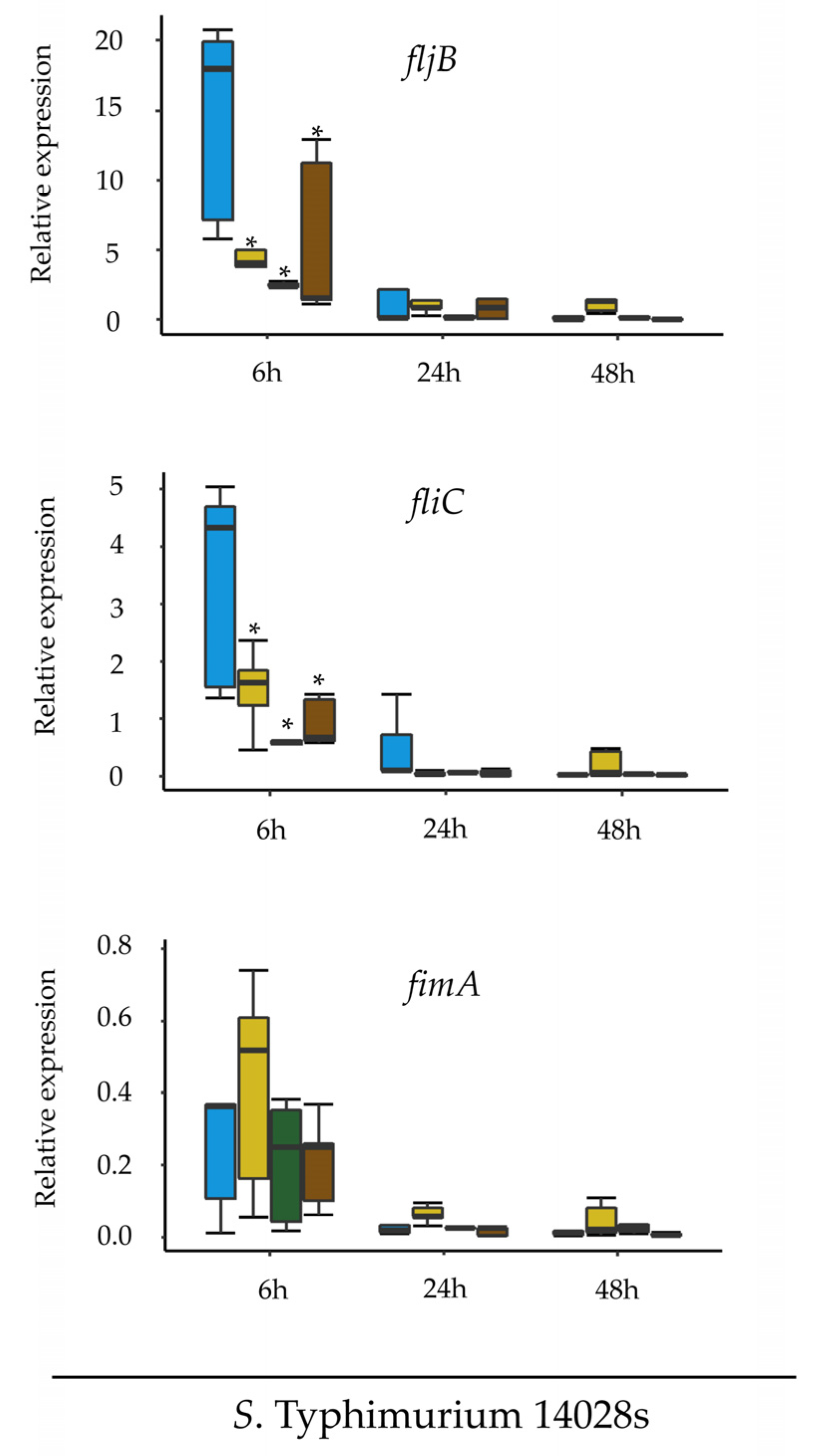
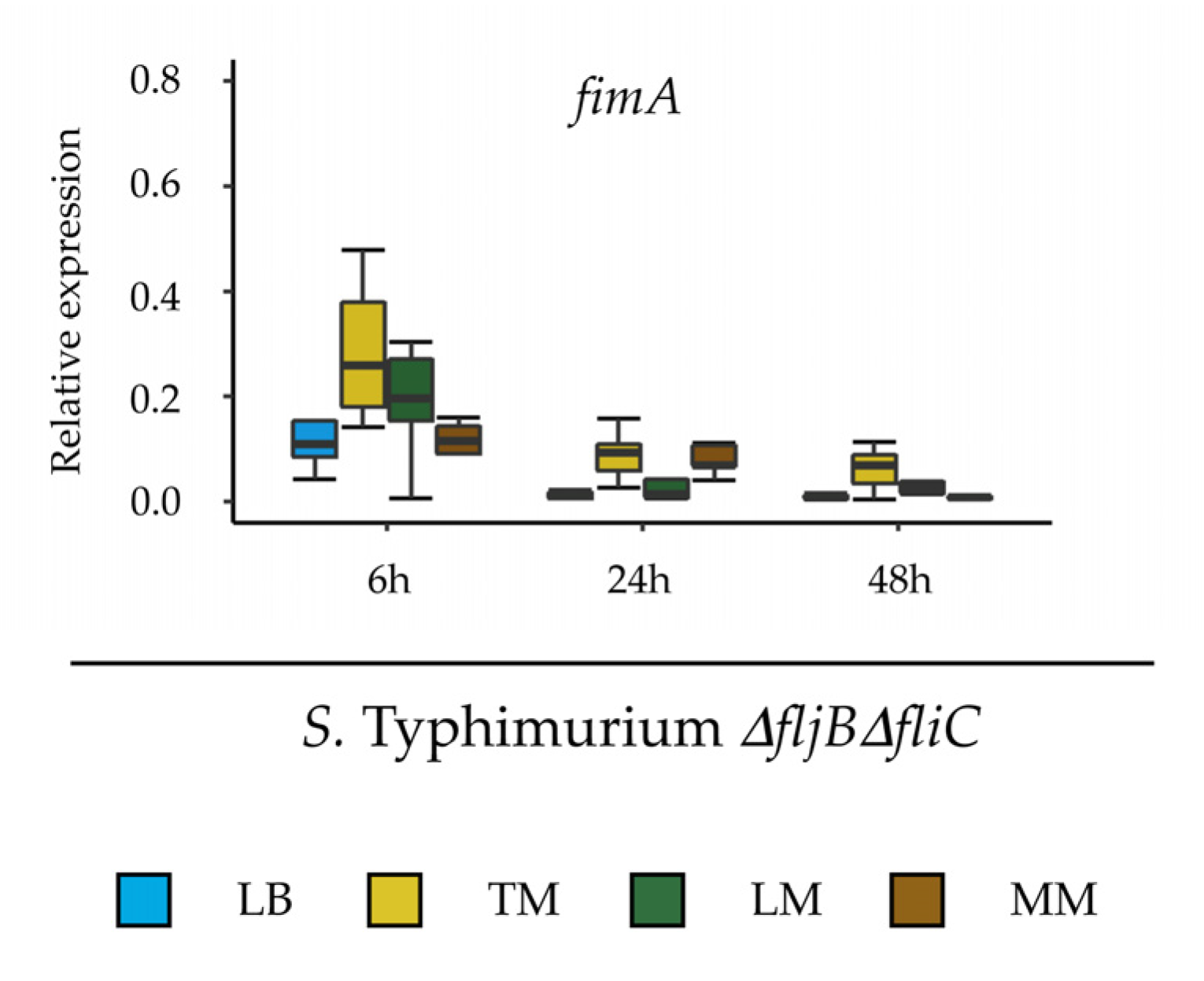
The second experimental approach was designed to assess the expression of
fljB, fliC and
fimA in
Salmonella in response to different media.
S. Typhimurium 14028s and
S. Typhimurium
∆fljB∆fliC were cultured in LB liquid medium overnight at 37 °C with aeration (150 rpm). Cells were pelleted at low speed (1500×
g, 10 min), washed and re-suspended in 1 mL of 10 mM MgCl
2. Two mL of the suspension (10
8 CFU/mL) was pipetted into cellulose ester dialysis membrane tubes with a pore size of 100 kD (Spectrum Europe, Breda, The Netherlands), which were knotted at both ends using dental floss. The dialysis membranes were placed into 50 mL conical tubes containing 30 mL of TM, LM, LB (positive control), or MM (negative control) media. The conical tubes were incubated at 25–28 °C for 24 h while shaking at 130 rpm. Subsequently, 2 × 0.5 mL from each dialysis membrane were mixed with RNAprotect (Qiagen), incubated for 5 min at room temperature and centrifuged at high speed (4000×
g, 10 min). Total RNA was extracted from the bacterial samples using the RNeasy Mini Kit (Qiagen) according to the manufacturer instructions. DNase digestion and cDNA synthesis were performed using Maxima H Minus First Strand cDNA Synthesis Kit (ThermoFisher Scientific, Braunschweig, Germany). The qPCR analysis was performed as described above. Primers used for the qPCR are listed in
Supplementary Table S2. Relative gene expression was normalized to the expression of the
rfaH gene from
Salmonella. All treatments were performed in five replicates. Tukey HSD Test was applied with 95% family-wise confidence level on R version 3.6.1 and
p ≤ 0.05 was considered significant (
Supplementary Table S3).
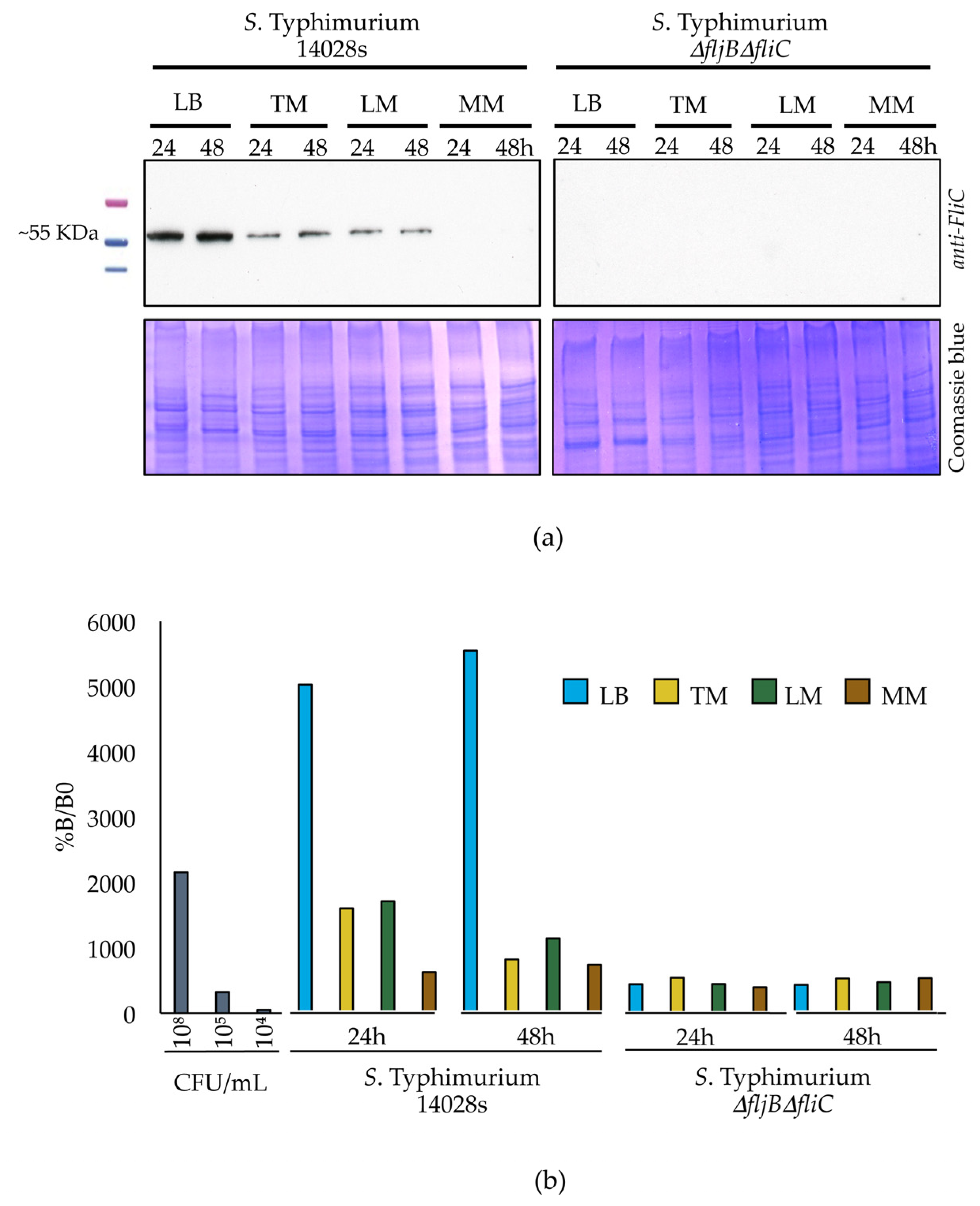
2.10. Western Blot Analysis
S. Typhimurium 14028s and S. Typhimurium ∆fljB∆fliC were cultured in dialysis membranes merged into conical tubes containing different media, as described earlier. Half mL of the bacterial culture was sampled from the dialysis membranes and centrifuged at low speed (1500× g, 10 min, 4 °C) in order to reduce shearing and loss of flagella. Pellets were re-suspended in 100 µL of 10 mM MgCl2. Proteins were extracted using methanol/chloroform. Protein concentration was measured at OD595 nm using Bradford assay Roti® Quant 5 x concentrated (Carl Roth GmbH & Co. KG). Five µg proteins were separated on SDS-PAGE using 12% polyacrylamide gels and electrophoretically transferred to a PVDF membrane (Immun-Blot® PVDF, Bio-Rad) for the following western blot analysis. Anti-flagellin FliC monoclonal antibody (1:5000; InvivoGen, San Diego, CA, USA) was used as a primary antibody and goat anti-mouse polyclonal-HRP (1:5000; Carl Roth GmbH & Co. KG) was used as a secondary antibody. Bands were detected after adding substrate mixture (SERVA Electrophoresis GmbH, Heidelberg, Germany) using an Optimax X-ray film processor (PROTEC Medizintechnik GmbH & Co., Oberstenfeld, Germany). The experiment was performed in three replicates.
2.11. Quantification of Flagellin Protein in Different Media
Salmonella strains were grown and proteins were extracted as described above. We used 2 × 50 µL of each protein sample to coat clear bottom and high binding 96-well plates (Greiner bio-one GmbH, Frickenhausen, Germany). Coating was done for 3 h at 37 °C, the 96-well plates were subsequently washed and proteins were blocked overnight using 10% milk in PBS. Afterwards, samples were incubated with anti-flagellin FliC monoclonal antibody (1:1000; InvivoGen) for 1 h followed by another 1 h incubation with goat anti-mouse polyclonal-HRP (1:5000; Carl Roth GmbH & Co. KG). After each incubation step, 4 washing steps were performed. To measure absorbance, TMB substrate kit (ThermoFisher Scientific) was added and incubated in darkness for 30 min followed by addition of the stop solution (ThermoFisher Scientific). Measurements were carried out using TriStar2S multimode Reader (Berthold Technologies GmbH & Co., Wildbad, Germany) at 450 nm and 652 nm wavelengths applying a single endpoint protocol. To calculate the percentages of flagellin present, the values from the 652 nm measurement were subtracted from those obtained at 450 nm in order to correct the optical imperfections in the microplate. B0 (No antigen was added) and NSB (No primary antibody was added) were used as controls for non-specific binding between primary-secondary antibodies and antigen-secondary antibody, respectively. The experiment was performed in 3 replicates.
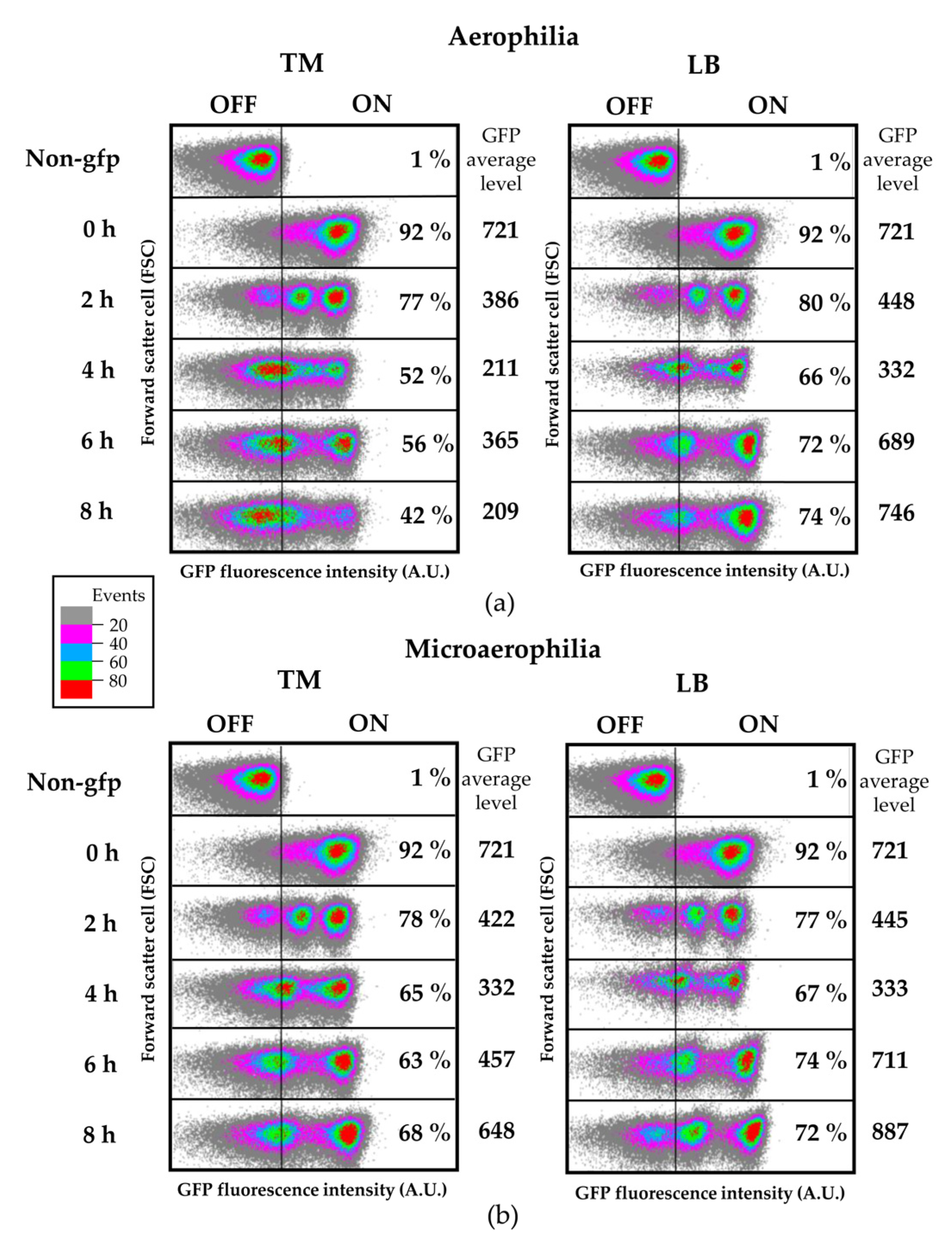
2.12. Flow Cytometry and Confocal Laser Scanning Microscopy (CLSM)
Salmonella cells from steady-state cultures in LB were washed with 10 mM MgCl
2 and diluted in TM or LB at OD
600 = 0.1, followed by incubation at 28 °C with shaking (aerophilia) or without shaking (microaerophilia) for time-course flow cytometry analyses. At different time points 300 µL of each culture were washed with 10 mM MgCl
2 and analyzed by flow cytometry. For flow cytometry analysis of apoplast-extracted bacteria, tomato leaves were syringe infiltrated at OD
600 = 0.1 and both inoculum and apoplast-extracted bacteria were used. To recover bacteria from tomato leaves an apoplastic fluid extraction was carried out at the indicated time point post inoculation as previously described by [
34]. Summarily, the apoplastic fluid was obtained by pressure infiltrating a whole leaf with 10 mL of 10 mM MgCl
2 solution inside a 20 mL syringe. Following five cycles of pressure application, the flow-through was removed and placed in a fresh 50 mL tube. Both tubes were centrifuged for 30 min at low speed (900×
g) at 4 °C. Pellets were re-suspended into 1 mL MgCl
2 and analyzed by flow cytometry. Cultures and apoplast-extracted bacterial suspensions were collected using a BD FACSVerse cytometer and data were analyzed with Kaluza Analysis Software (Beckman Coulter, Brea, CA, USA). To analyze cytometry data the following conventions were applied: all SSC, FSC, and fluorescence zero values were excluded. Data were excluded that fell within the forward scatter (FSC) and side scatter (SSC) region where significant counts appeared in “buffer only” controls. FSC and SSC medians were calculated and a series of circular gates expanding out from the FSC and SSC medians were applied. All data were collected for 100,000 events per sample and were compared with the data from the control strain (without GFP reporter) to establish the fraction of fliC
ON cells. Live/dead staining was carried out using propidium iodide at 20 mM (Sigma, St. Louis, MO, USA). Each independent experiment included two replicate samples, as indicated for each figure. Figures show typical results.
For microscopy images of apoplast-extracted bacteria, samples were stained with 20 µM of FM4-64 (N-3-triethylammoniumpropyl-4-6-4-diethylaminophenylhexatrienylpyridinium dibromide) (Thermo Fisher Scientific, Waltham, MA, USA) and observed under either a SP8 microscope (Leica Microsystems) or a confocal microscope LSM 800 (Zeiss, Wetzlar, Germany) with excitation 488 nm, emission 500–533 nm for GFP and excitation 488 nm, emission 604–674 nm for FM4-64.
4. Discussion
During the last decades, there have been many reports on salmonellosis outbreaks associated to fresh produce, leading to public health concerns on the safety of fresh produce and the efficiency of food protection measures during agricultural handling. The most recent outbreak occurred in 14 states in the USA, affected a total of 165 people, 73 of which were hospitalized. The source was associated to cut fruits, including honeydew melons, cantaloupes, pineapples and grapes infected with
S. Javiana [
43].
Several studies have already attempted to comprehend the interaction between
Salmonella and plant hosts. Flagella’s role in the
Salmonella-plant interaction has been extensively studied, mostly considering flagellin as a conserved PAMP. Typically, the recognition of PAMPs activates PTI in plants and leads to the onset of several responses, including activation of kinase cascades, production of reactive oxygen species (ROS), activation of the expression of defense genes, stomata closure, and accumulation of defense hormones [
14,
44,
45,
46,
47]. Nonetheless, several reports have indicated the possible evolvement of divergent flagellin sequences in some bacteria, modifications which would have impact on its recognition and therefore on the interaction between the plant and the bacterium [
16,
17,
18,
19]. The N-terminal and C-terminal regions of flagellin are conserved in different bacterial species, while variations take place in the central part. The elicitor activity of flagellin has been attributed to the most conserved domain within the N-terminal part [
16,
48], which is recognized in plants by the FLS2 receptor [
14]. Very intriguing is the fact that the animal ortholog of FLS2 (TLR5) recognizes a different domain [
13].
In our previous work [
27], results obtained from transcriptomic analysis of
S. Typhimurium 14028s in tomato-based medium showed that the majority of motility and adhesion related genes were not differentially expressed in this plant-mimicking medium. This led us to speculate that flagella might not be necessary for the survival of
Salmonella in plants.
Our current work shows that
S. Typhimurium wild type strain 14028s and the double flagellin mutant
S. Typhimurium
ΔfljBΔfliC display the same ability to persist within the inoculated tissue and to colonize non-inoculated areas of tomato plants. Consistent with our results, some studies have reported that
Salmonella lacking flagellin was able to survive better in alfalfa (
Medicago sativa) than the respective wild type, suggesting that down-regulation of flagellin synthesis may increase
Salmonella fitness in plants [
22,
49]. Likewise, using non-flagellated mutants, flagella were found to be non-essential for attachment of both
S. Typhimurium and
S. Senftenberg to tomatoes [
20]. Contrarily, in the attachment of
S. Senftenberg to salad leaves, flagella seemed to play a major role [
21]. Furthermore, a recent study showed the importance of flagellum-mediated motility in adhesion of
S. Typhimurium to
Valerianella locusta (corn salad) leaves [
23]. In addition,
Salmonella colonization or internalization in
Arabidopsis,
Medicago sativa and lettuce was reduced in mutants impaired in motility [
50,
51]. Noteworthy, it was previously shown that
Salmonella from infected
Arabidopsis retain virulence in human cells and mice [
52]. This may suggest an adaptive value for the loss of flagella while in contact with plants, thereby gaining more virulence while infecting mammals by not triggering defense mechanisms. Interestingly, previous reports have shown that flagella may play a minor role for endophytic bacteria since endophytes are usually non-motile upon entering plants [
49,
53,
54]. Our results indicate that
Salmonella inside a tomato plant could adopt a similar lifestyle.
Generally, bacteria express various adhesive structures such as capsule, fimbriae or pili. However, these structures are usually not expressed at the same time as flagellin [
55]. For instance, flagella are often involved in reversible attachment that permits individual cells to swim toward suitable biotic or abiotic surfaces. On the other hand, irreversible attachment often involves loss of flagella and synthesis of curli or type 1 fimbriae. Such changes are triggered by different environmental conditions, such as temperature, osmolarity, or pH, which regulate the expression of the flagellar master operon,
flhDC [
47,
56]. The assessment of the flagellin expression and synthesis, while in contact with plant-supplemented medium, revealed reduction in both transcript and protein levels of flagellin. This could indicate that
Salmonella down-regulates the expression of flagella in order to either improve its fitness or to evade plant defense mechanisms associated to the activation of PTI.
Previous reports indicated that
S. enterica flagellin mutants triggered reduced defense responses in
Arabidopsis and tomato [
57]. This may implicate that tomato, at least partially recognizes
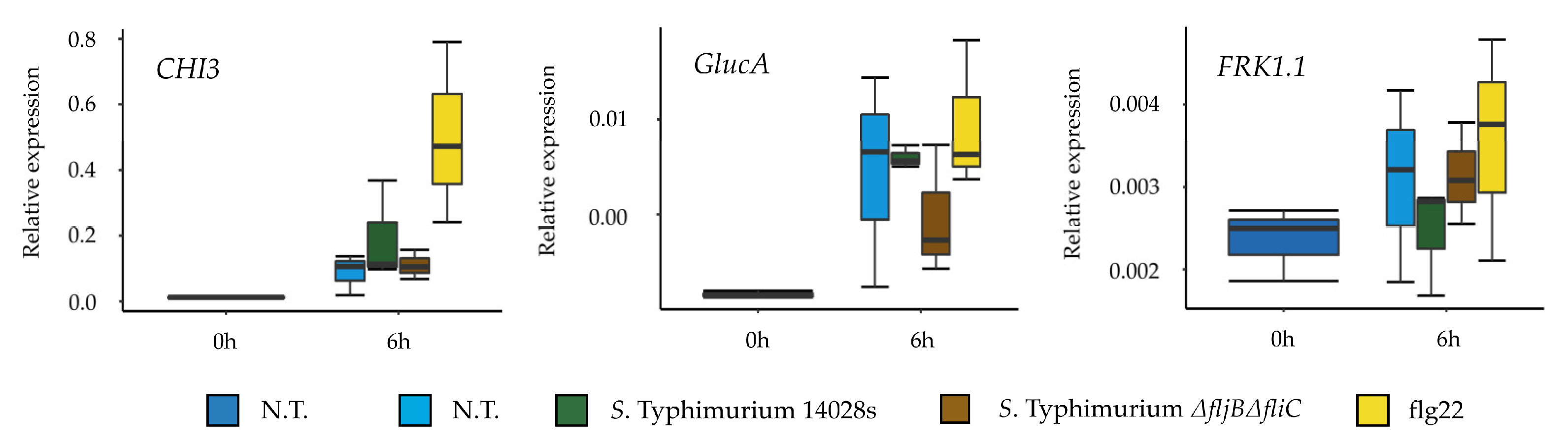
Salmonella’s flagella as a PAMP. Interestingly, our results showed no differences in the expression of several tomato defense-related genes in plants treated either with the wild type or with the mutant. This raises the question of whether
Salmonella is generally recognized by tomato Pattern Recognition Receptors (PRRs), and if it does, whether this recognition is due to flagella or to other PAMPs. Another explanation could be attributed to the highly efficient mechanisms of neutralizing ROS that
Salmonella possesses, which allows it to proliferate inside plants, even when triggering the plant immune response [
58,
59]. However, our results showed that the expression and synthesis of FliC was higher in LB than in the plant-related media at the population level. Hence, yet another possibility could be that
Salmonella switches off the production of flagella once in contact with plants in order to evade plant defense system as an evolutionary or adaptive strategy.
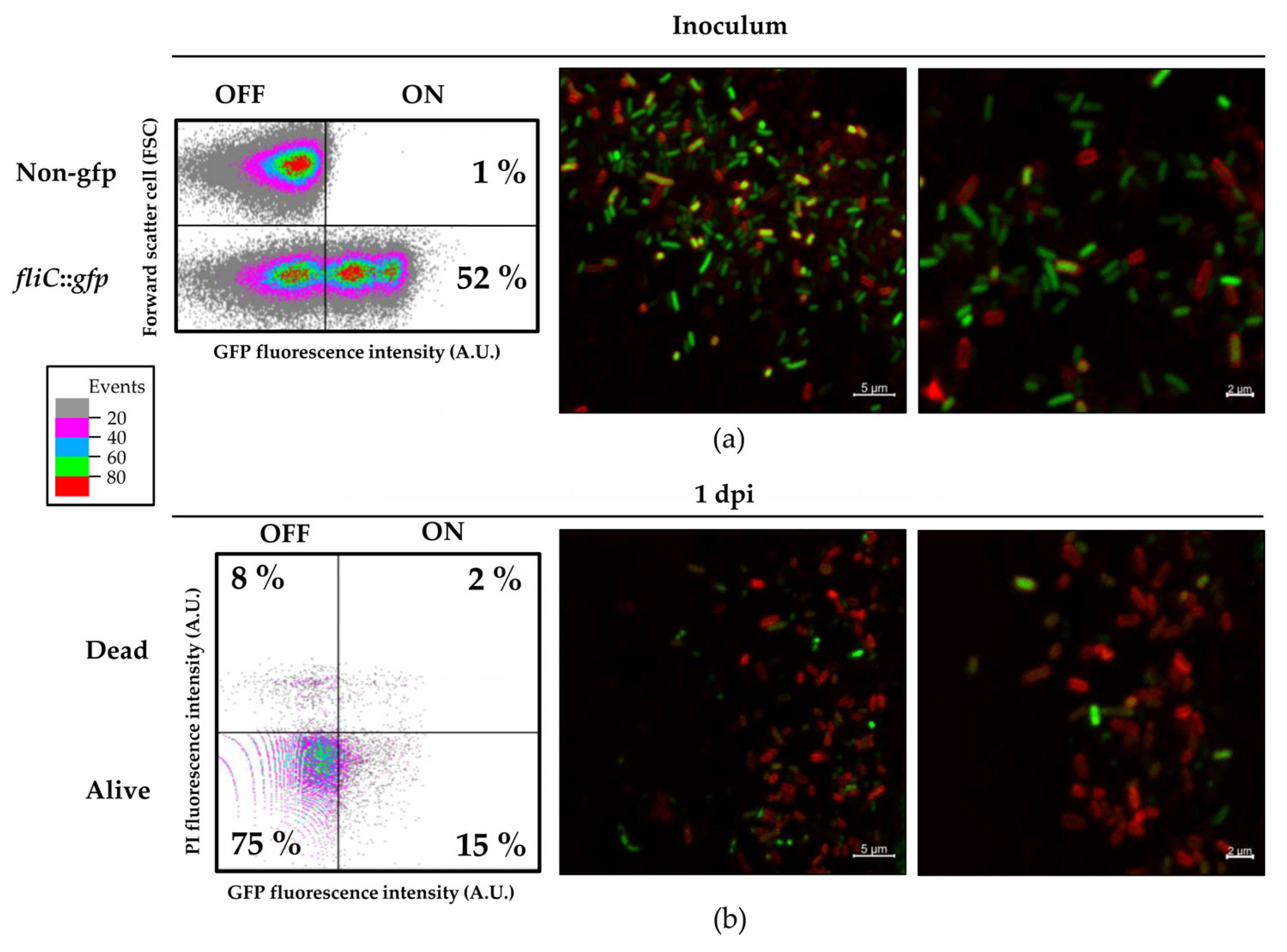
Here, we analyzed the S. Typhimurium strain expressing a fliC::gfp transcriptional fusion to follow fliC expression at a single-cell level using both flow cytometry and confocal microscopy. After infiltration of this strain into tomato leaves, we observed significant heterogeneity within the population in regards to the levels of fliC expression, detecting FliCOFF and FliCON bacterial subpopulations. This heterogeneity was also clearly observable using the confocal laser-scanning microscope.
Particularly striking were the big differences in the level of
fliC expression between individual cells. It was apparent that a small subpopulation of
Salmonella cells continued to express
fliC at a very high level. Such heterogeneous expression has been already observed, and even associated to higher virulence in animal hosts. The phenotypic heterogeneity in
Salmonella flagellar gene expression has been shown to provide a mechanism by which the pathogen maximizes fitness within the mammalian host [
60]. Whether such behavior is part of
Salmonella’s adaptation to colonize plant tissues or plays a role beyond the plant host as part of an adaptation to the eventual progress to an herbivorous host, is a very intriguing question. Further work will be necessary to explore these hypotheses.
Further characterization of additional virulence genes and their expression patterns within the population could define the specificity of interactions between Salmonella and crop plants and open doors to new insights of this interaction, leading thereby to the design of informed strategies for enhanced food safety.