Yeast-Free Doughs by Zymomonas mobilis: Evaluation of Technological and Fermentation Performances by Using a Metabolomic Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Z. mobilis Strains Maintenance and Biomass Production
2.2. Flour Characterization
2.3. Dough Production, Evaluation of Volume Increase, and Total CO2 Production
2.4. Dough Chemical Characterization, pH Monitoring and Microbial Counts
2.5. Volatile Compounds Determination by Solid-Phase Microextraction-Gas Chromatography-Mass Spectrometry (SPME-GC-MS)
2.6. Statistical Analysis
3. Results and Discussion
3.1. Microbial and Chemical Characterizations of Non-Inoculated Dough
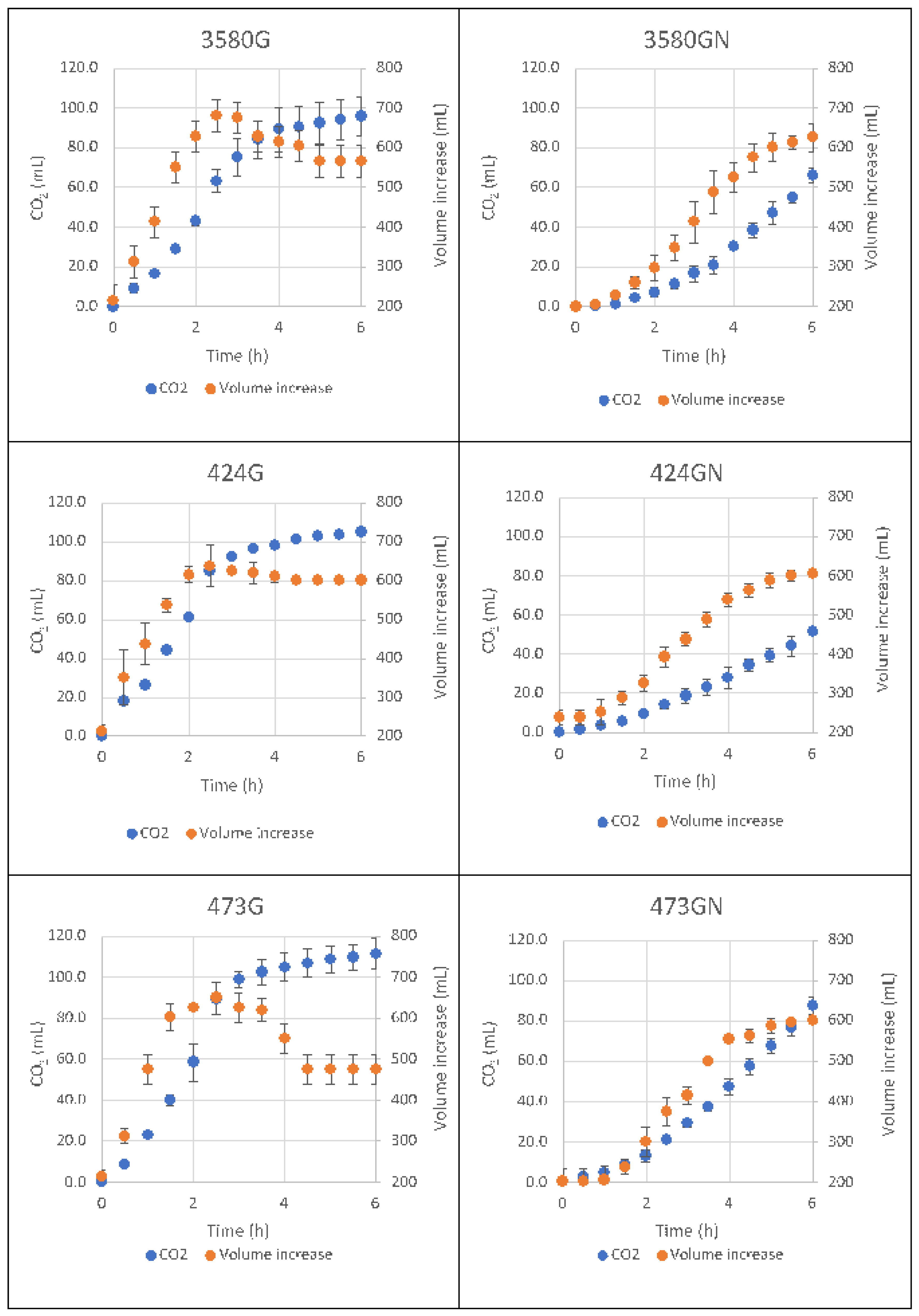
3.2. Microbial, Chemical and Technological Characterizations of Z. mobilis Inoculated Doughs
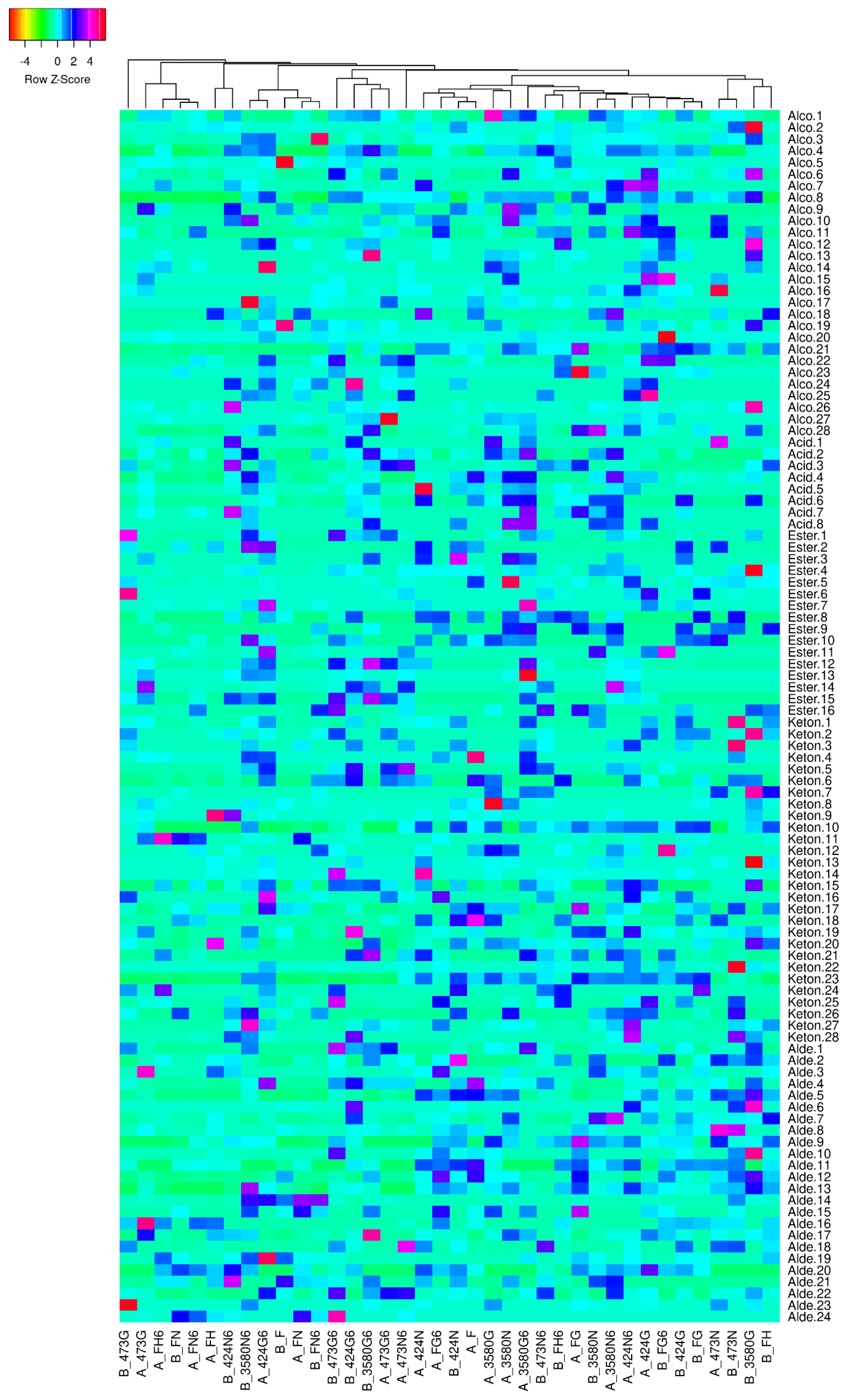
3.3. Volatilome Anlysis
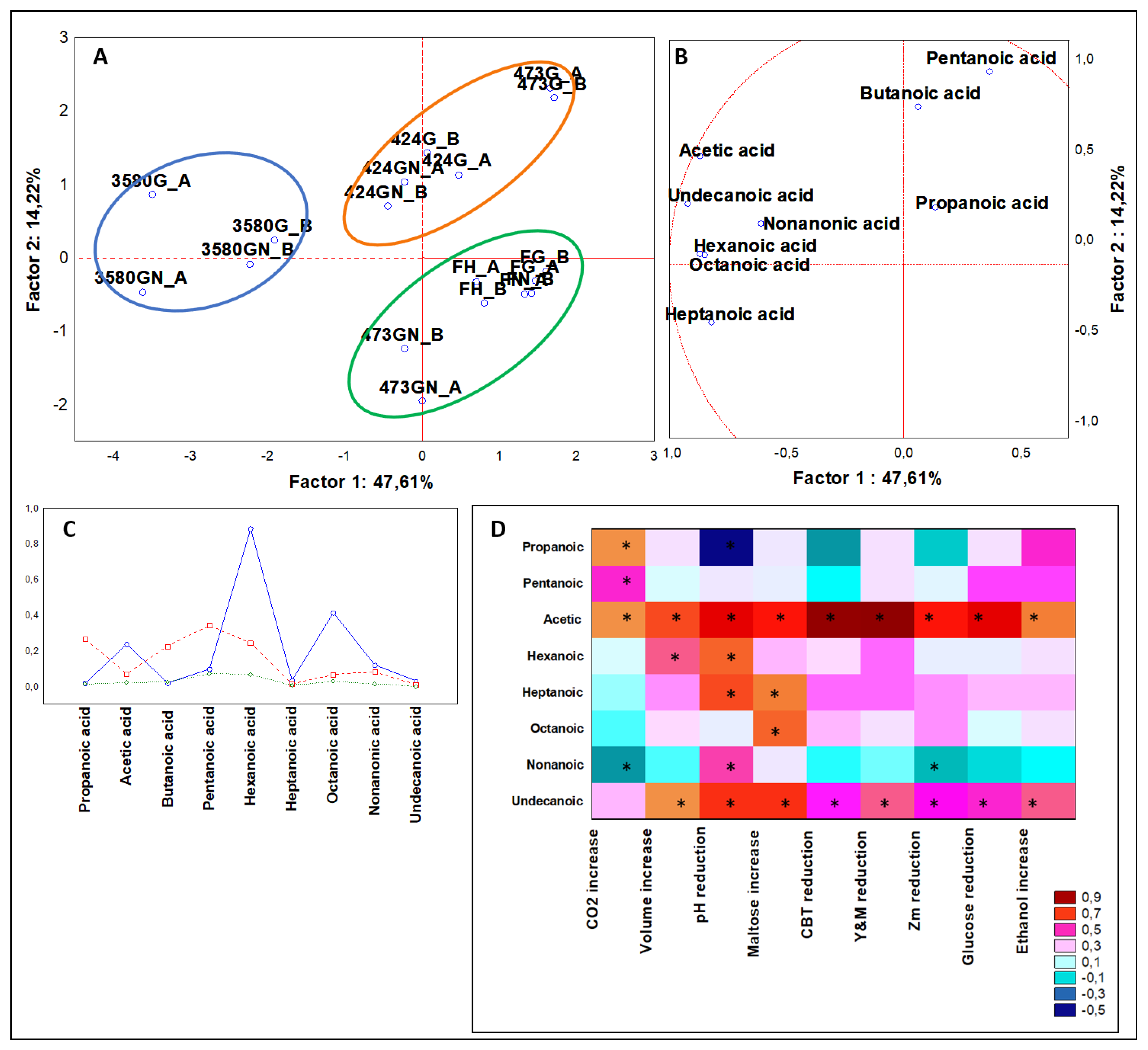
3.3.1. Multivariate Analysis of Organic Acids
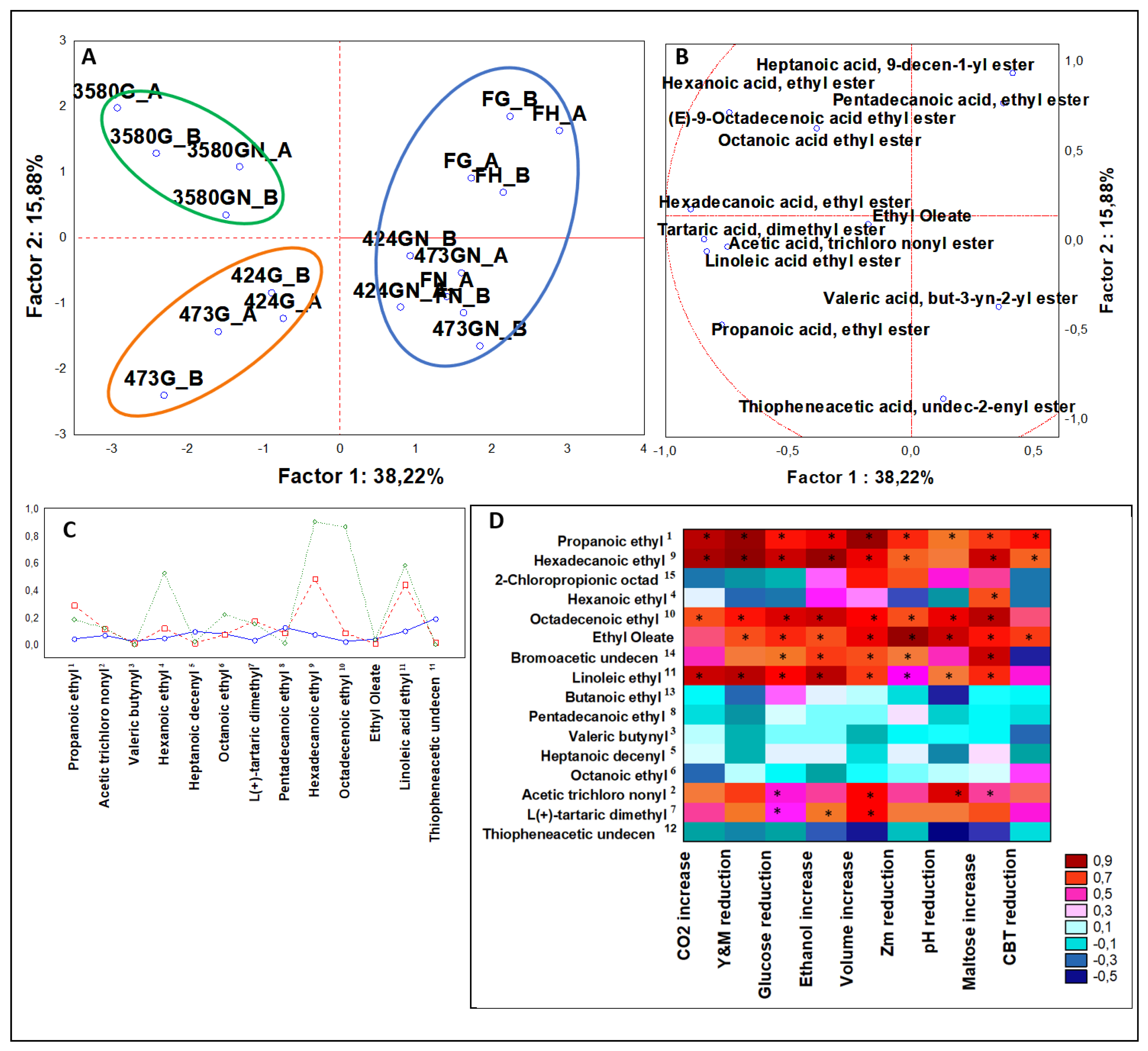
3.3.2. Multivariate Analysis of Organic Acid Esters
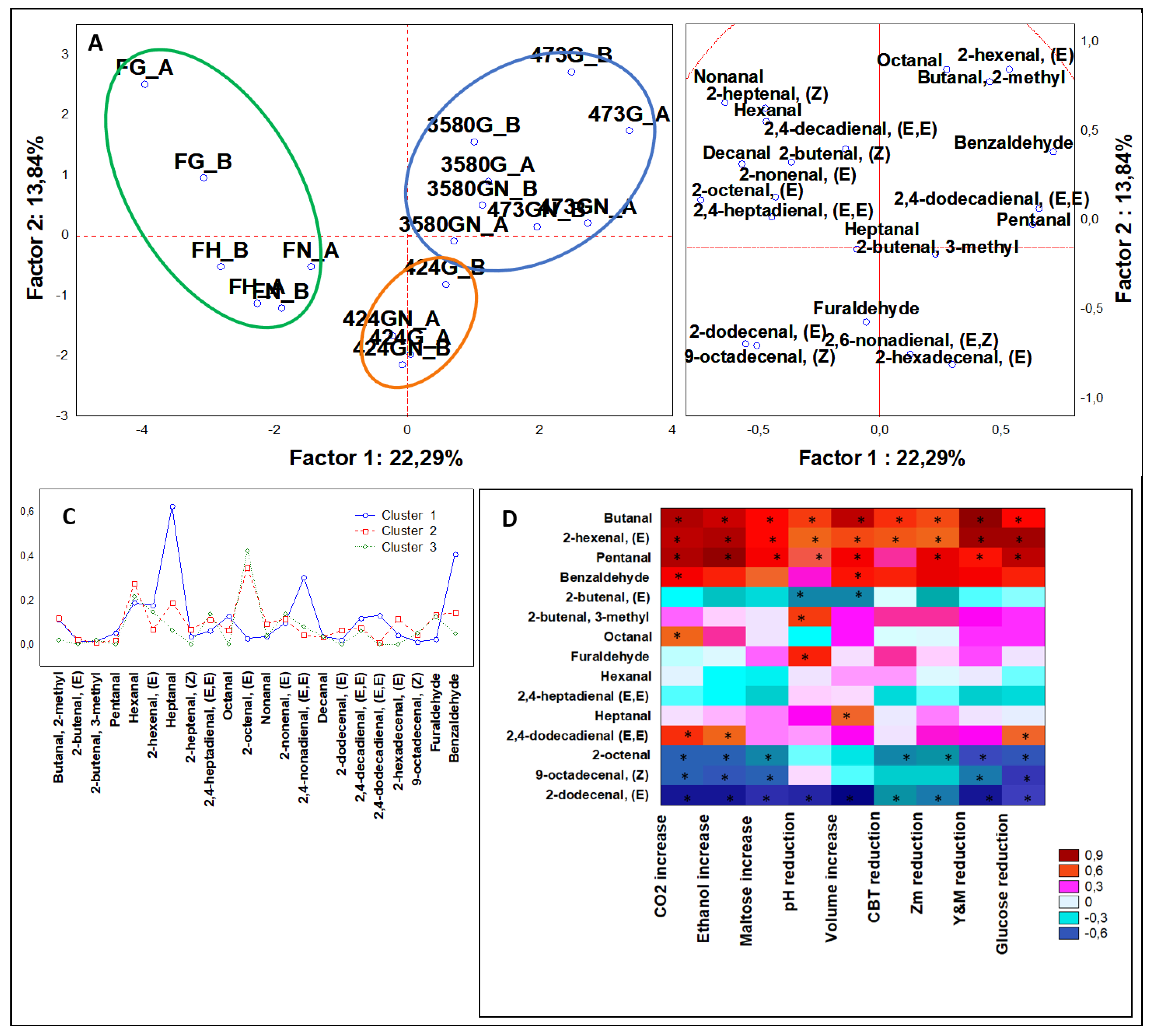
3.3.3. Multivariate Analysis of Aldehydes
3.3.4. Multivariate Analysis of Ketones
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Properties | Content | Method |
|---|---|---|
| Humidity (%) | <15.5 | Buhler method |
| Ash (%) | <0.55 | ISS1967 |
| Protein (%) | >11 | NIR |
| Gluten (%) | >19 | ICC 106 |
| Hagberg Index (s) | >250 | ISO 3093 |
| W 10−4 J | 240/250 | ISO 5530/4 |
| P/L 10−4 J | 0.45/0.65 | ISO 5530/4 |


References
- Brunner, B.; Scheurer, U.; Seibold, F. Differences in yeast intolerance between patients with Crohn’s disease and ulcerative colitis. Dis. Colon Rectum 2007, 50, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, M.; Perricone, R.; Blank, M.; Perricone, C.; Shoenfeld, Y. Anti-Saccharomyces cerevisiae autoantibodies in autoimmune diseases: From bread baking to autoimmunity. Clin. Rev. Allergy Immunol. 2013, 45, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Oda, Y.; Tonomura, K. Dough-leavening ability by Zymomonas mobilis and its application to breadmaking. J. Food Sci. 1994, 59, 171–174. [Google Scholar] [CrossRef]
- Musatti, A.; Rollini, M.; Sambusiti, C.; Manzoni, M. Zymomonas mobilis: Biomass production and use as a dough leavening agent. Ann. Microbiol. 2015, 65, 1583–1589. [Google Scholar] [CrossRef]
- Musatti, A.; Mapelli, C.; Foschino, R.; Picozzi, C.; Rollini, M. Unconventional bacterial association for dough leavening. Int. J. Food Microbiol 2016, 237, 28–34. [Google Scholar] [CrossRef]
- Musatti, A.; Mapelli, C.; Rollini, M.; Foschino, R.; Picozzi, C. Can Zymomonas mobilis substitute Saccharomyces cerevisiae in cereal dough leavening? Foods 2018, 7, 61. [Google Scholar] [CrossRef]
- Musatti, A.; Cappa, C.; Mapelli, C.; Alamprese, C.; Rollini, M. Use of sucrose to improve fermentative performances of Zymomonas mobilis in bread dough. Foods 2020, 9, 89. [Google Scholar] [CrossRef]
- Taneyo-Saa, D.L.; Di Silvestro, R.; Nissen, L.; Dinelli, G.; Gianotti, A. Effect of sourdough fermentation and baking process severity on bioactive fiber compounds in immature and ripe wheat flour bread. LWT-Food Sci. Technol. 2018, 89, 322–328. [Google Scholar] [CrossRef]
- Taneyo-Saa, D.L.; Nissen, L.; Gianotti, A. Metabolomic approach to study the impact of flour type and fermentation process on volatile profile of bakery products. Food Res. Int 2019, 119, 510–516. [Google Scholar] [CrossRef]
- Nissen, L.; Bordoni, A.; Gianotti, A. Shift of Volatile Organic Compounds (VOCs) in Gluten-Free Hemp-Enriched Sourdough Bread: A Metabolomic Approach. Nutrients 2020, 12, 1050. [Google Scholar] [CrossRef]
- Mozzi, F.; Ortiz, M.E.; Bleckwedel, J.; De Vuyst, L.; Pescuma, M. Metabolomics as a tool for the comprehensive understanding of fermented and functional foods with lactic acid bacteria. Food Res. Int. 2013, 54, 1152–1161. [Google Scholar] [CrossRef]
- Nissen, L.; Demircan, B.; Taneyo-Saa, D.L.; Gianotti, A. Shift of Aromatic Profile in Probiotic Hemp Drink Formulations: A Metabolomic Approach. Microorganisms 2019, 7, 509. [Google Scholar] [CrossRef] [PubMed]
- Nissen, L.; di Carlo, E.; Gianotti, A. Prebiotic potential of hemp blended drinks fermented by probiotics. Food Res. Int. 2020, 131, 109029. [Google Scholar] [CrossRef] [PubMed]
- Rizo, J.; Guillén, D.; Farrés, A.; Díaz-Ruiz, G.; Sánchez, S.; Wacher, C.; Rodríguez-Sanoja, R. Omics in traditional vegetable fermented foods and beverages. Crit. Rev. Food Sci. 2018, 22, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Granato, D.; de Araujo Calado, M.V.; Jarvis, B. Observations on the use of statistical methods in Food Science and Technology. Food Res. Int. 2014, 55, 137–149. [Google Scholar] [CrossRef]
- McCann, T.H.; Day, L. Effect of sodium chloride on gluten network formation, dough microstructure and rheology in relation to breadmaking. J. Cereal Sci. 2013, 57, 444–452. [Google Scholar] [CrossRef]
- Baranyi, J.; Roberts, T.A. A dynamic approach to predicting bacterial growth in food. Int. J. Food Microbiol. 1994, 23, 277–294. [Google Scholar] [CrossRef]
- Homayouni, A.R.; Kasaie, Z. A comparative study on different methods for the evaluation of baker’s yeast bioactivity. Int. J. Food Prop. 2017, 20, 100–106. [Google Scholar] [CrossRef]
- Birch, A.N.; Petersen, M.A.; Hansen, Å.S. The aroma profile of wheat bread crumb influenced by yeast concentration and fermentation temperature. LWT-Food Sci. Technol. 2013, 50, 480–488. [Google Scholar] [CrossRef]
- Petel, C.; Prost, C.; Onno, B. Sourdough volatile compounds and their contribution to bread: A review. Trends Food Sci. Technol. 2017, 59, 105–123. [Google Scholar] [CrossRef]
- Liu, T.; Li, Y.; Sadiq, F.A.; Yang, H.; Gu, J.; Yuan, L.; Lee, Y.K.; He, G. Predominant yeasts in Chinese traditional sourdough and their influence on aroma formation in Chinese steamed bread. Food Chem. 2018, 242, 404–411. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly-Y, M.; Glickman, J.N.; Garrett, W.S. The microbial metabolites, Short Chain Fatty Acids, regulate colonic Treg cell homeostatis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Augustin, K.; Khabbush, A.; Williams, S.; Eaton, S.; Orford, M.; Cross, J.H.; Heales, S.J.R.; Walker, M.C.; Williams, R.S.B. Mechanisms of action for the medium-chain triglyceride ketogenic diet in neurological and metabolic disorders. Lancet Neurol. 2018, 17, 84–93. [Google Scholar] [CrossRef]
- Dragone, G.; Mussatto, S.I.; Oliveira, J.M.; Teixeira, J.A. Characterisation of volatile compounds in an alcoholic beverage produced by whey fermentation. Food Chem. 2009, 112, 929–935. [Google Scholar] [CrossRef]
- Kenne, K.T.; Lognay, G.; Fauconnier, M.L. Probiotic as a sources of aroma in functional food: Selected examples and analytical methodology. In Trends in Probiotic Applications; Razafindralambo, H., Ed.; Studium Press LLC: Chicago, Il, USA; pp. 85–111.
- Maire, M.; Rega, B.; Cuvelier, M.E.; Soto, P.; Giampaoli, P. Lipid oxidation in baked products: Impact of formula and process on the generation of volatile compounds. Food Chem. 2013, 141, 510–3518. [Google Scholar] [CrossRef] [PubMed]
- Plessas, S.; Alexopoulos, A.; Mantzourani, I.; Koutinas, A.; Voidarou, C.; Stravropoulou, E.; Bezirtzoglou, E. Application of novel starter cultures for sourdough bread production. Anaerobe 2011, 17, 486–489. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Lim, H.J.; Chang, J.W.; Hurh, B.S.; Kim, Y.S. Investigation on the formations of volatile compounds, fatty acids, and γ-lactones in white and brown rice during fermentation. Food Chem. 2018, 269, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.F.; Chen, W.; Chen, H.; Chen, W.; Zhong, Q. Combination of Lactobacillus plantarum and Saccharomyces cerevisiae DV10 as Starter Culture to Produce Mango Slurry: Microbiological, Chemical Parameters and Antioxidant Activity. Molecules 2019, 24, 4349. [Google Scholar] [CrossRef]
- Arn, H.; Acree, T.E. Flavornet: A database of aroma compounds based on odor potency in natural products. Dev. Food Sci. 1998, 40, 27. [Google Scholar]
- Lee, Y.; Min, S.; Choe, E.O.; Min, D.B. Formation of Volatile Compounds in Soy Flour by Singlet Oxygen Oxidation During Storage Under Light. J. Food Sci. 2003, 68, 1933–1937. [Google Scholar] [CrossRef]
- Aslankoohi, E.; Herrera-Malaver, B.; Rezaei, M.N.; Steensels, J.; Courtin, C.M.; Verstrepen, K.J. Non-Conventional Yeast Strains Increase the Aroma Complexity of Bread. PLoS ONE 2016, 11, e0165126. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Li, Y.; Yang, Y.; Yi, H.; Zhang, L.; He, G. The influence of different lactic acid bacteria on sourdough flavor and a deep insight into sourdough fermentation through RNA sequencing. Food Chem. 2020, 307, 125529. [Google Scholar] [CrossRef] [PubMed]

| Dough | Flour (g/100 gflour) | Water Absorption (g/100 gflour) | Glucose (g/100 gflour) | NaCl (g/100 gflour) | Arrival Time (min) | Dough Stability (min) |
|---|---|---|---|---|---|---|
| F | 100 | 55.9 | - | - | 2.3 | 9.5 |
| FG | 100 | 53.3 | 3 | - | 1.6 | 9.1 |
| FGN | 100 | 51.4 | 3 | 1 | 1.7 | 16.6 |
| Parameter | Time (h) | Dough | DSM 3580 | DSM 424 | DSM 473 | Negative Control (Non-Inoculated) |
|---|---|---|---|---|---|---|
| Aerobic | 0 | FG | 3.75 ± 0.19 A | 2.30 ± 0.43 B | 4.31 ± 0.38 A | 3.49 ± 0.07 A |
| Mesophilic | 2 | 3.57 ± 0.22 | 3.18 ± 0.17 | 4.20 ± 0.18 | 3.21 ± 0.18 | |
| Counts AMC | 4 | 3.48 ± 0.16 A | 3.47 ± 0.13 A | 4.15 ± 0.47 B | 3.42 ± 0.04 A | |
| (log CFU/g) | 6 | 3.18 ± 0.31 A | 3.43 ± 0.52 A,B | 4.12 ± 0.34 B | 3.68 ± 0.03 B | |
| 0 | FGN | 3.32 ± 0.46 A | 2.66 ± 0.51 B | 3.96 ± 0.03 A | 3.50 ± 0.04 A | |
| 2 | 2.95 ± 0.43 | 3.36 ± 0.48 | 3.94 ± 0.02 | 3.73 ± 0.09 | ||
| 4 | 3.23 ± 0.12 A | 3.29 ± 0.13 A | 3.81 ± 0.01 B | 3.62 ± 0.16 A | ||
| 6 | 2.83 ± 0.07 A | 3.12 ± 0.59 A,B | 3.56 ± 0.01 B | 3.92 ± 0.33 B | ||
| Yeasts and | 0 | FG | 3.15 ± 0.64 | 2.82 ± 0.48 | 3.20 ± 0.08 | 3.44 ± 0.11 |
| moulds Y&M | 2 | 2.61 ± 0.29 A | 2.80 ± 0.21 A | 2.60 ± 0.43 A | 3.34 ± 0.06 B | |
| (log CFU/g) | 4 | 2.81 ± 0.38 A,B | 2.33 ± 0.89 A | 2.76 ± 0.40 A,B | 3.34 ± 0.29 B | |
| 6 | 2.52 ± 0.31 A | 2.53 ± 0.18 A | 2.66 ± 0.38 A | 3.21 ± 0.13 B | ||
| 0 | FGN | 2.76 ± 0.08 | 2.92 ± 0.63 | 2.60 ± 0.08 | 3.39 ± 0.07 | |
| 2 | 2.73 ± 0.11 A | 2.60 ± 0.60 A | 2.50 ± 0.71 A | 3.60 ± 0.28 B | ||
| 4 | 2.83 ± 0.02 A,B | 2.66 ± 0.51 A | 2.51 ± 0.05 A | 3.47 ± 0.14 B | ||
| 6 | 2.27 ± 0.38 A | 2.70 ± 0.00 A | 2.29 ± 0.16 A | 3.47 ± 0.11 B | ||
| Z. mobilis | 0 | FG | 8.23 ± 0.22 | 9.18 ± 0.05 | 8.67 ± 0.47 | n.d.* |
| counts | 2 | 8.20 ± 0.87 | 8.97 ± 0.55 | 8.72 ± 0.34 | n.d.* | |
| (log CFU/g) | 4 | 8.33 ± 0.15 A | 9.33 ± 0.16 B | 8.69 ± 0.41 A,B | n.d.* | |
| 6 | 8.05 ± 0.14 A | 8.91 ± 0.57 B | 8.62 ± 0.23 A,B | n.d.* | ||
| 0 | FGN | 8.75 ± 0.72 | 8.23 ± 0.04 | 9.01 ± 0.30 | n.d.* | |
| 2 | 8.08 ± 0.48 | 8.33 ± 0.03 | 8.75 ± 0.05 | n.d.* | ||
| 4 | 8.16 ± 0.33 A | 8.44 ± 0.06 A,B | 8.74 ± 0.36 B | n.d.* | ||
| 6 | 7.61 ± 0.75 A | 8.30 ± 0.09 A,B | 8.73 ± 0.57 B | n.d.* | ||
| pH | 0 | FG | 5.86 ± 0.01 | 5.88 ± 0.07 | 5.87 ± 0.03 | 6.00 ± 0.01 |
| (units) | 2 | 5.61 ± 0.23 A | 5.66 ± 0.08 A | 5.73 ± 0.04 A,B | 5.93 ± 0.01 B | |
| 4 | 5.44 ± 0.05 A | 5.52 ± 0.08 A,B | 5.63 ± 0.09 B | 5.91 ± 0.01 C | ||
| 6 | 5.39 ± 0.01 A | 5.50 ± 0.09 B | 5.53 ± 0.00 B | 5.92 ± 0.06 C | ||
| 0 | FGN | 5.89 ± 0.00 | 5.87 ± 0.14 | 5.87 ± 0.07 | 6.00 ± 0.00 | |
| 2 | 5.23 ± 0.29 A | 5.61 ± 0.01 A,B | 5.68 ± 0.08 A,B | 5.94 ± 0.03 B | ||
| 4 | 4.92 ± 0.26 A | 5.32 ± 0.19 A,B | 5.55 ± 0.07 B | 5.91 ± 0.01 C | ||
| 6 | 4.82 ± 0.14 A | 5.08 ± 0.20 B | 5.43 ± 0.04 B | 5.90 ± 0.01 C | ||
| Glucose | 0 | FG | 20.68 ± 0.53 | 19.05 ± 2.20 | 18.24 ± 0.95 | 22.47 ± 1.88 |
| (mg/g) | 2 | 6.44 ± 1.81 A,a | 7.32 ± 1.07 A,a | 3.04 ± 1.58 A,a | 22.32 ± 2.23 B | |
| 4 | 1.53 ± 0.81 A,a | 0.47 ± 0.29 A,a | 0.90 ± 0.51 A,a | 22.30 ± 1.91 B | ||
| 6 | 0.30 ± 0.42 A,a | 0.72 ± 0.16 A,a | 0.56 ± 0.15 A,a | 22.40 ± 1.04 B | ||
| 0 | FGN | 22.46 ± 0.02 | 21.21 ± 1.50 | 21.44 ± 0.45 | 21.15 ± 0.19 | |
| 2 | 17.09 ± 0.30 A,b | 18.22 ± 0.41 A,b | 17.55 ± 0.92 A,b | 21.54 ± 0.42 B | ||
| 4 | 14.15 ± 0.46 A,b | 14.50 ± 2.27 A,b | 10.23 ± 1.16 A,b | 21.59 ± 1.44 B | ||
| 6 | 5.73 ± 2.06 A,b | 10.23 ± 1.04 A,b | 2.77 ± 1.68 A,b | 21.65 ± 1.26 B | ||
| Maltose | 0 | FG | 10.33 ± 0.24 | 9.81 ± 1.12 | 10.78 ± 0.64 | 10.50 ± 1.43 |
| (mg/g) | 2 | 15.25 ± 1.24 a | 14.54 ± 0.91a | 15.92 ± 0.36 a | 13.53 ± 0.83 | |
| 4 | 17.97 ± 2.81 | 17.59 ± 0.83 | 18.59 ± 1.39 | 15.61 ± 1.01 | ||
| 6 | 20.74 ± 0.93 | 20.19 ± 1.93 | 20.37 ± 0.43 | 18.01 ± 0.70 | ||
| 0 | FGN | 9.82 ± 0.20 | 9.66 ± 0.18 | 7.95 ± 0.81 | 9.38 ± 0.15 | |
| 2 | 12.99 ± 0.26 b | 13.24 ± 0.27b | 12.90 ± 0.42 b | 13.48 ± 0.30 | ||
| 4 | 17.88 ± 1.61 | 15.49 ± 0.71 | 15.77 ± 0.06 | 15.67 ± 1.01 | ||
| 6 | 19.46 ± 1.22 | 18.57 ± 0.20 | 17.54 ± 1.48 | 18.29 ± 0.98 | ||
| Ethanol | 0 | FG | 1.03 ± 0.17 | 1.47 ± 0.78 | 2.09 ± 0.85 | n.d.§ |
| (mg/g) | 2 | 6.76 ± 0.46 a | 7.18 ± 1.53 a | 8.27 ± 2.31 a | n.d.§ | |
| 4 | 9.09 ± 1.39 a | 10.15 ± 0.05 a | 10.24 ± 0.57 a | n.d.§ | ||
| 6 | 10.40 ± 0.52 a | 10.85 ± 0.06 a | 11.15 ± 0.14 a | n.d.§ | ||
| 0 | FGN | 0.49 ± 0.01 | 0.44 ± 0.04 | 0.44 ± 0.11 | n.d.§ | |
| 2 | 1.49 ± 0.01 b | 1.72 ± 0.00 b | 2.38 ± 0.41 b | n.d.§ | ||
| 4 | 3.77 ± 0.66 b | 3.48 ± 0.32 b | 5.53 ± 0.30 b | n.d.§ | ||
| 6 | 7.15 ± 1.77 b | 5.88 ± 0.18 b | 9.10 ± 0.21 b | n.d.§ |
| Parameter | Dough | Z. mobilis Strain | ||
|---|---|---|---|---|
| DSM 3580 | DSM 424 | DSM 473 | ||
| Leavening Rate | FG | 34.88 ± 0.18 A | 38.37 ± 0.96 AB | 46.15 ± 3.70 B |
| (mL/h) | FGN | 16.69 ± 0.46 A | 10.89 ± 0.335 B | 19.53 ± 1.28 C |
| Lag leavening | FG | 0.96 ± 0.01 | 0.75 ± 0.01 | 0.80 ± 0.12 |
| time (h) | FGN | 2.20 ± 0.28 A | 1.50 ± 0.54 B | 1.70 ± 0.17 AB |
| Max. CO2 | FG | 97.59 ± 0.81 | 101.26 ± 0.89 | 107.38 ± 6.50 |
| production (mL) | FGN | - | - | - |
| R2 | FG | 0.995 ± 0.000 | 0.990 ± 0.001 | 0.990 ± 0.003 |
| FGN | 0.992 ± 0.002 | 0.994 ± 0.007 | 0.998 ± 0.001 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nissen, L.; Rollini, M.; Picozzi, C.; Musatti, A.; Foschino, R.; Gianotti, A. Yeast-Free Doughs by Zymomonas mobilis: Evaluation of Technological and Fermentation Performances by Using a Metabolomic Approach. Microorganisms 2020, 8, 792. https://doi.org/10.3390/microorganisms8060792
Nissen L, Rollini M, Picozzi C, Musatti A, Foschino R, Gianotti A. Yeast-Free Doughs by Zymomonas mobilis: Evaluation of Technological and Fermentation Performances by Using a Metabolomic Approach. Microorganisms. 2020; 8(6):792. https://doi.org/10.3390/microorganisms8060792
Chicago/Turabian StyleNissen, Lorenzo, Manuela Rollini, Claudia Picozzi, Alida Musatti, Roberto Foschino, and Andrea Gianotti. 2020. "Yeast-Free Doughs by Zymomonas mobilis: Evaluation of Technological and Fermentation Performances by Using a Metabolomic Approach" Microorganisms 8, no. 6: 792. https://doi.org/10.3390/microorganisms8060792
APA StyleNissen, L., Rollini, M., Picozzi, C., Musatti, A., Foschino, R., & Gianotti, A. (2020). Yeast-Free Doughs by Zymomonas mobilis: Evaluation of Technological and Fermentation Performances by Using a Metabolomic Approach. Microorganisms, 8(6), 792. https://doi.org/10.3390/microorganisms8060792








