Determination of Loline Alkaloids and Mycelial Biomass in Endophyte-Infected Schedonorus pratensis by Near-Infrared Spectroscopy and Chemometrics
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Reference Analysis of Loline Alkaloids and Mycelial Biomass
2.3. Acquisition of Infrared Spectra
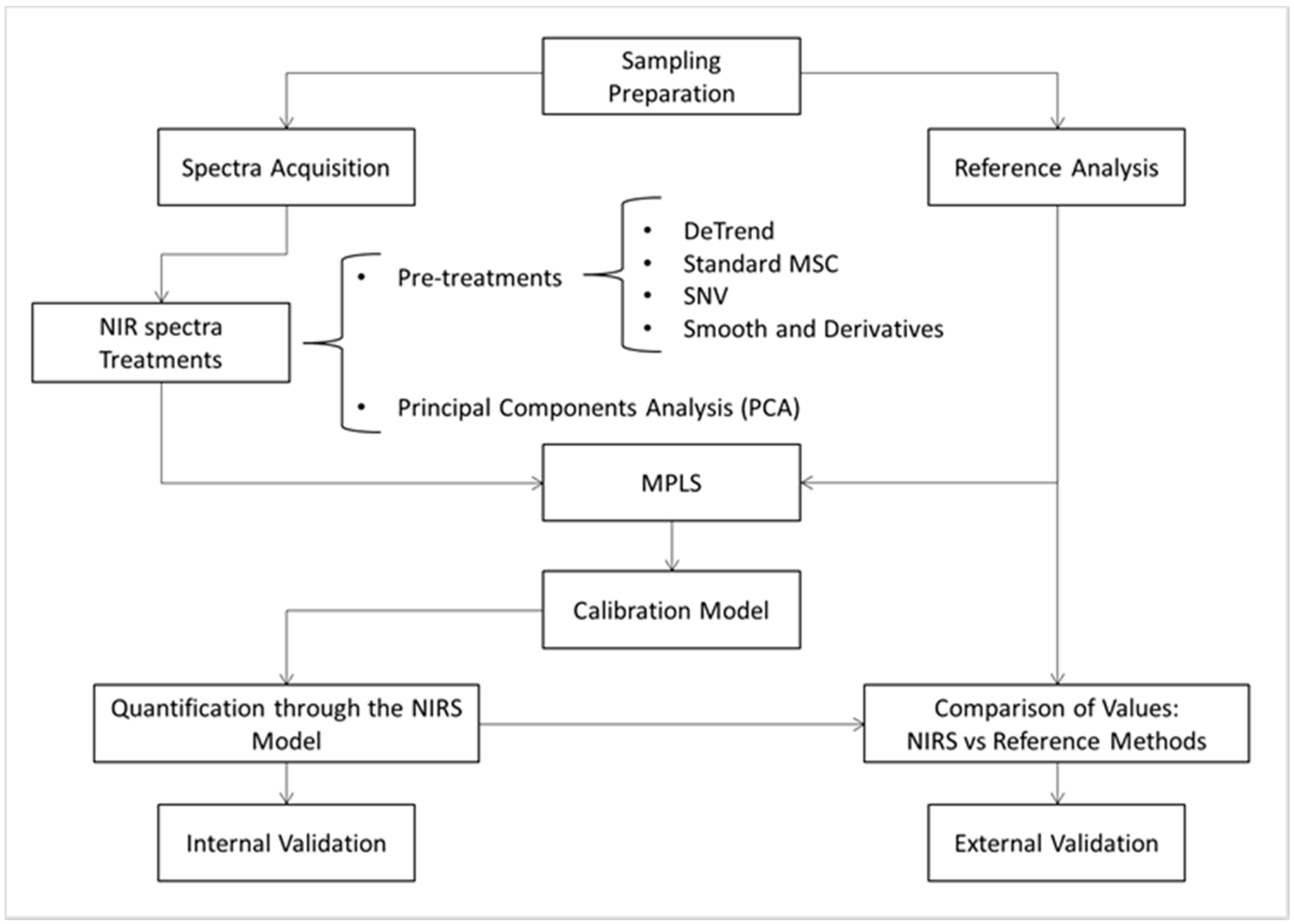
2.4. Training and Calibration of the NIRS Models
3. Results
3.1. Chemical Measurement
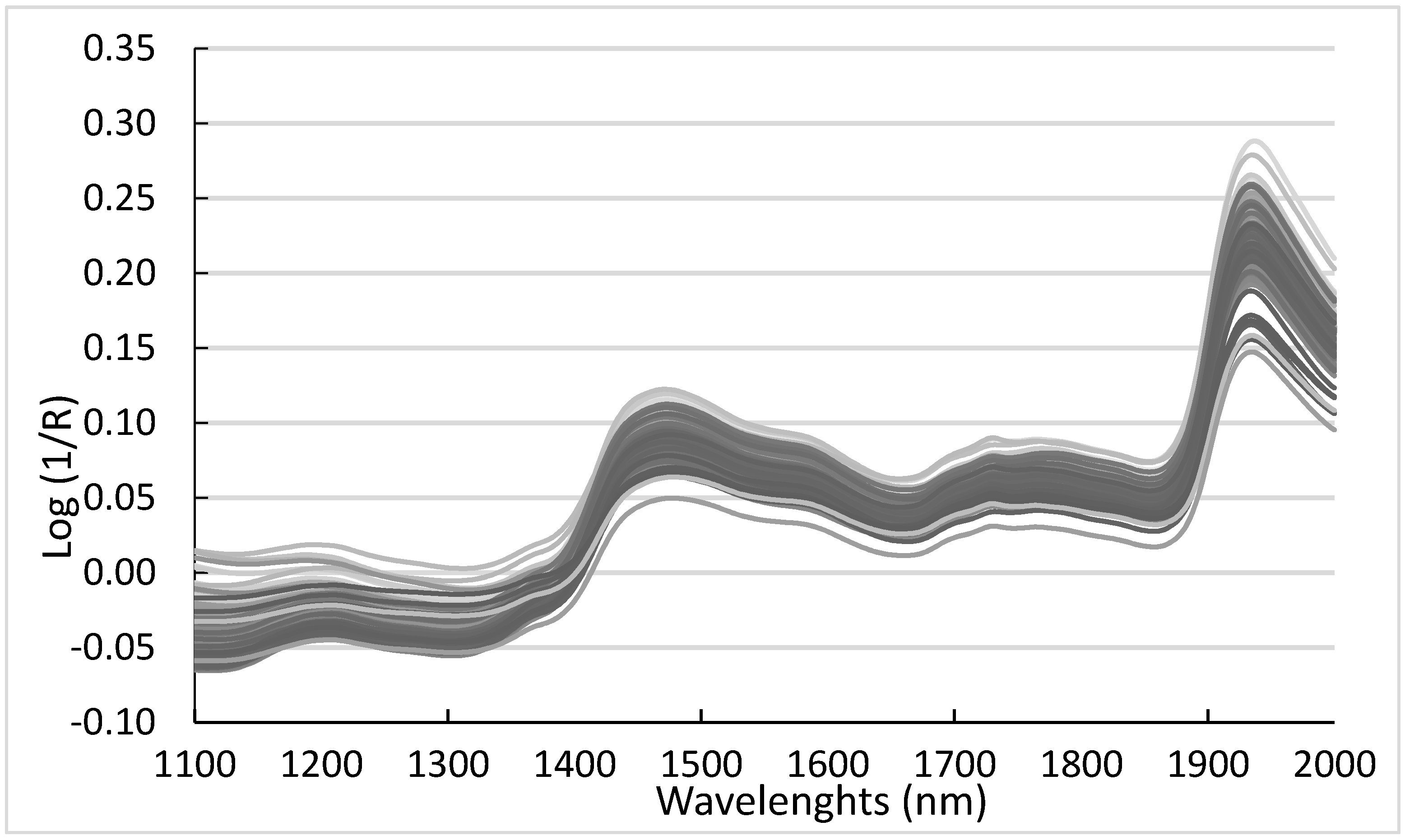
3.2. NIR Analysis
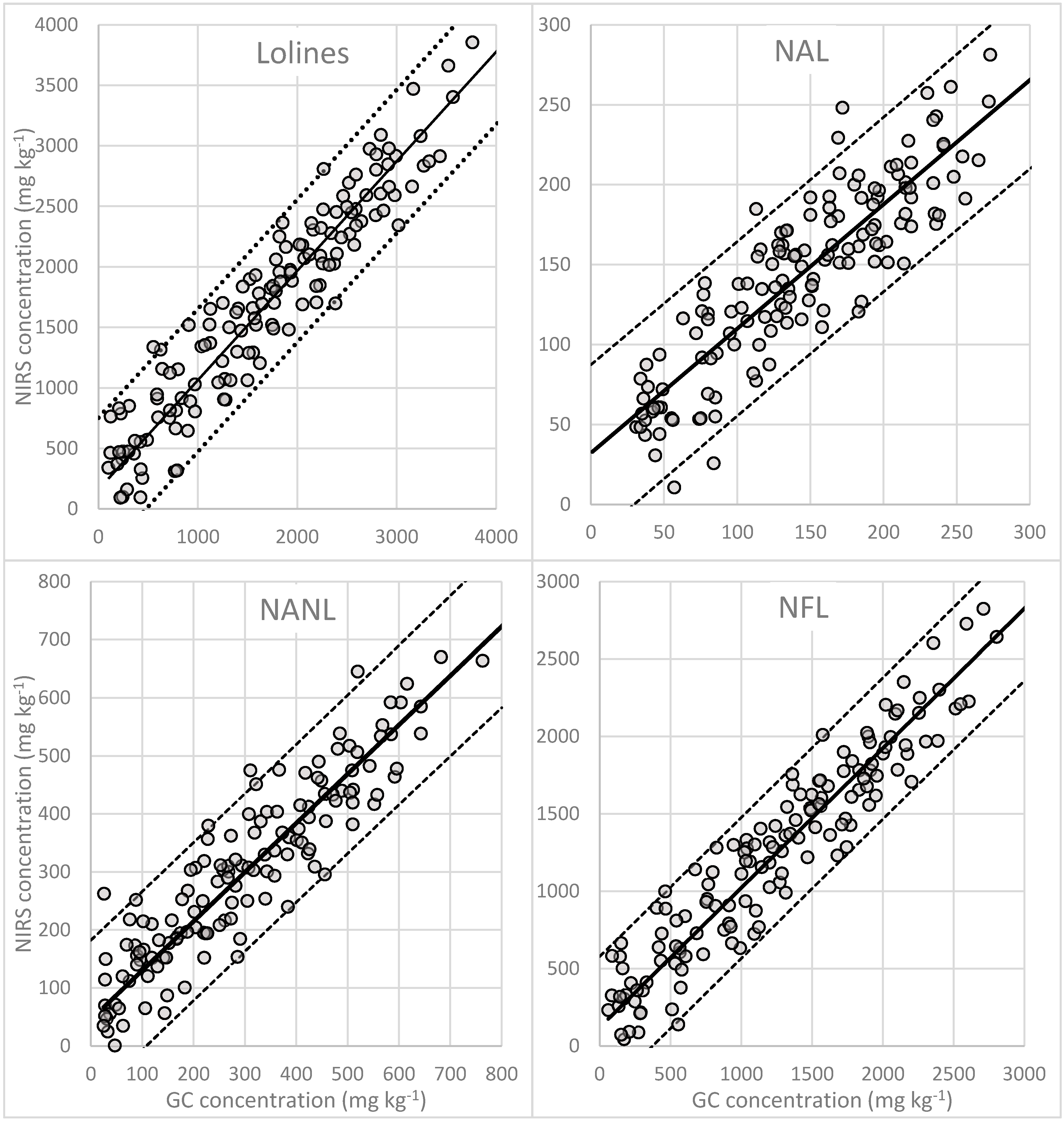
3.3. Quantification of N-Acetylloline (NAL)
3.4. Quantification of N-Acetylnorloline (NANL)
3.5. Quantification of N-Formylloline (NFL)
3.6. Quantification of Total Lolines
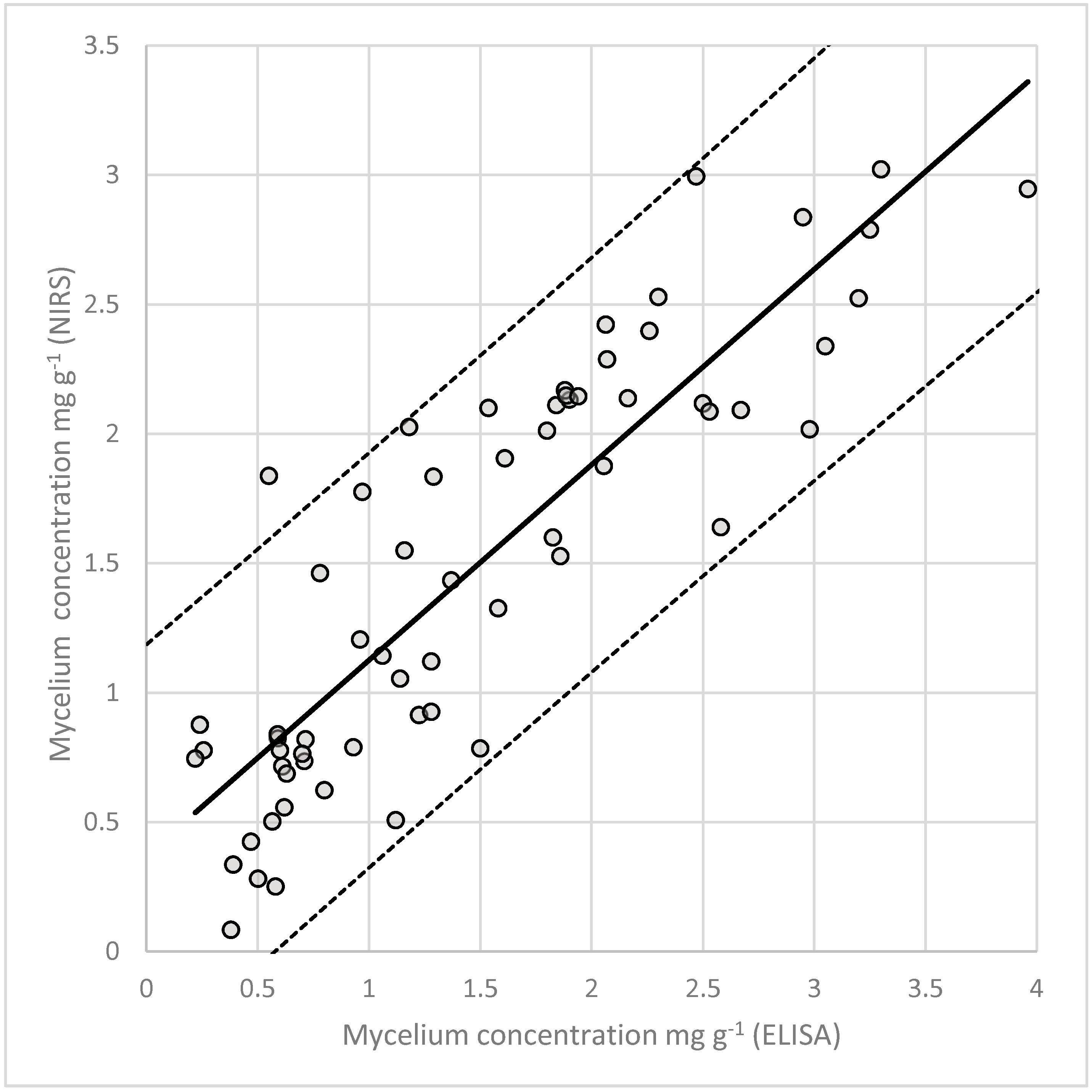
3.7. Quantification of Fungal Mycelium in Planta
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Deaville, E.R.; Flinn, P.C. Near-infrared (NIR) spectroscopy: An alternative approach for the estimation of forage quality and voluntary intake. In Forage Evaluation in Ruminant Nutrition; CABI: Wallingford, CT, USA, 2000; pp. 301–320. [Google Scholar]
- Osborne, B.G. Near-infrared spectroscopy in food analysis. In Encyclopedia of Analytical Chemistry; John Wiley & Sons, Ltd.: Chichester, UK, 2000. [Google Scholar]
- Beever, D.E.; Mould, F.L. Forage evaluation for efficient ruminant livestock production. In Forage Evaluation in Ruminant Nutrition; CABI: Wallingford, CT, USA, 2000; pp. 15–42. [Google Scholar]
- Givens, D.I.; Deaville, E.R. The current and future role of near infrared reflectance spectroscopy in animal nutrition: A review. Aust. J. Agric. Res. 1999, 50, 1131–1145. [Google Scholar] [CrossRef]
- Norris, K.H.; Hart, J.R. 4. Direct spectrophotometric determination of moisture content of grain and seeds. J. Near Infrared Spectrosc. 1996, 4, 23–30. [Google Scholar] [CrossRef]
- Norris, K.H.; Barnes, R.F.; Moore, J.E.; Shenk, J.S. Predicting forage quality by infrared replectance spectroscopy. J. Anim. Sci. 1976, 43, 889–897. [Google Scholar] [CrossRef]
- Shenk, J.S. Early history of forage and feed analysis by NIR 1972–1983. NIR News 1993, 4, 12–13. [Google Scholar] [CrossRef]
- Park, R.S.; Gordon, F.J.; Agnew, R.E.; Barnes, R.J.; Steen, R.W.J. The use of Near Infrared Reflectance Spectroscopy on dried samples to predict biological parameters of grass silage. Anim. Feed Sci. Technol. 1997, 68, 235–246. [Google Scholar] [CrossRef]
- Soto-Barajas, M.C.; Zabalgogeazcoa, I.; González-Martin, I.; Vázquez-de-Aldana, B.R. Qualitative and quantitative analysis of endophyte alkaloids in perennial ryegrass using near-infrared spectroscopy. J. Sci. Food Agric. 2017. [Google Scholar] [CrossRef]
- Barrière, Y.; Guillet, C.; Goffner, D.; Pichon, M. Genetic variation and breeding strategies for improved cell wall digestibility in annual forage crops. A review. Anim. Res. 2003, 52. [Google Scholar] [CrossRef]
- Soto-Barajas, M.C.; Zabalgogeazcoa, I.; González-Martin, I.; Vázquez-de-Aldana, B.R. Near-infrared spectroscopy allows detection and species identification of Epichloë endophytes in Lolium perenne. J. Sci. Food Agric. 2018, 98, 5037–5044. [Google Scholar] [CrossRef]
- Leuchtmann, A.; Bacon, C.W.; Schardl, C.L.; White, J.F.; Tadych, M. Nomenclatural realignment of Neotyphodium species with genus Epichloë. Mycologia 2014, 106, 202–215. [Google Scholar] [CrossRef]
- Schardl, C.L. Epichloë species: Fungal symbionts of grasses. Annu. Rev. Phytopathol. 1996, 34, 109–130. [Google Scholar] [CrossRef]
- Johnson, L.J.; De Bonth, A.C.M.; Briggs, L.R.; Caradus, J.R.; Finch, S.C.; Fleetwood, D.J.; Fletcher, L.R.; Hume, D.E.; Johnson, R.D.; Popay, A.J.; et al. The exploitation of Epichloë endophytes for agricultural benefit. Fungal Divers. 2013, 60. [Google Scholar] [CrossRef]
- Saikkonen, K.; Young, C.A.; Helander, M.; Schardl, C.L. Endophytic Epichloë species and their grass hosts: From evolution to applications. Plant Mol. Biol. 2016, 90, 665–675. [Google Scholar] [CrossRef] [PubMed]
- Panaccione, D.G.; Beaulieu, W.T.; Cook, D. Bioactive alkaloids in vertically transmitted fungal endophytes. Funct. Ecol. 2014, 28, 299–314. [Google Scholar] [CrossRef]
- Clay, K.; Schardl, C. Evolutionary origins and ecological consequences of endophyte symbiosis with grasses. Am. Nat. 2002, 160, S99–S127. [Google Scholar] [CrossRef] [PubMed]
- Porter, J.K.; Thompson, F.N. Effects of fescue toxicosis on reproduction in livestock. J. Anim. Sci. 1992, 70, 1594–1603. [Google Scholar] [CrossRef]
- Gallagher, R.T.; White, E.P.; Mortimer, P.H. Ryegrass staggers: Isolation of potent neurotoxins lolitrem A and lolitrem B from staggers-producing pastures. N. Z. Vet. J. 1981, 29, 189–190. [Google Scholar] [CrossRef]
- Leuchtmann, A.; Schmidt, D.; Bush, L.P. Different levels of protective alkaloids in grasses with stroma-forming and seed-transmitted Epichloë/Neotyphodium endophytes. J. Chem. Ecol. 2000, 26, 1025–1036. [Google Scholar] [CrossRef]
- Popay, A.J.; Tapper, B.A.; Podmore, C. Endophyte-infected meadow fescue and loline alkaloids affect argentine stem weevil larvae. N. Z. Plant Prot. 2009, 62, 19–27. [Google Scholar]
- Nelli, M.R.; Scheerer, J.R. Synthesis of peramine, an anti-insect defensive alkaloid produced by endophytic fungi of cool season grasses. J. Nat. Prod. 2016, 79, 1189–1192. [Google Scholar] [CrossRef]
- Lee, S.T.; Gardner, D.R.; Cook, D. Identification of indole diterpenes in Ipomoea asarifolia and Ipomoea muelleri, plants tremorgenic to livestock. J. Agric. Food Chem. 2017, 65, 5266–5277. [Google Scholar] [CrossRef]
- Panaccione, D.G.; Ryan, K.L.; Schardl, C.L.; Florea, S. Analysis and modification of ergot alkaloid profiles in fungi. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 2012; Volume 515, pp. 267–290. [Google Scholar]
- Baldauf, M.W.; Mace, W.J.; Richmond, D.S. Endophyte-mediated resistance to black cutworm as a function of plant cultivar and endophyte strain in tall fescue. Environ. Entomol. 2011, 40, 639–647. [Google Scholar] [CrossRef]
- Roberts, C.A.; Joost, R.E.; Rottinghaus, G.E. Quantification of ergovaline in tall fescue by near infrared reflectance spectroscopy. Crop Sci. 1997, 37, 281–284. [Google Scholar] [CrossRef]
- Pan, J.; Bhardwaj, M.; Nagabhyru, P.; Grossman, R.B.; Schardl, C.L. Enzymes from fungal and plant origin required for chemical diversification of insecticidal loline alkaloids in grass-Epichloë symbiota. PLoS ONE 2014. [Google Scholar] [CrossRef] [PubMed]
- Patchett, B.J. Loline alkaloids: Analysis and Effects on Sheep and Pasture Insects. Ph.D. Thesis, Lincoln University, Canterbury, New Zealand, 2007. [Google Scholar]
- Schardl, C.L.; Grossman, R.B.; Nagabhyru, P.; Faulkner, J.R.; Mallik, U.P. Loline alkaloids: Currencies of mutualism. Phytochemistry 2007, 68, 980–996. [Google Scholar] [CrossRef] [PubMed]
- Freitas, P. Crossing the Species Barrier: Investigating Vertical Transmission of a Fungal Endophyte from Tall Fescue within a Novel Ryegrass Association. Ph.D. Thesis, Lincoln University, Canterbury, New Zealand, 2017. [Google Scholar]
- Spiering, M.J.; Greer, D.H.; Schmid, J. Effects of the fungal endophyte, Neotyphodium lolii, on net photosynthesis and growth rates of perennial ryegrass (Lolium perenne) are independent of in planta endophyte concentration. Ann. Bot. 2006, 98, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Panaccione, D.G.; Johnson, R.D.; Wang, J.; Young, C.A.; Damrongkool, P.; Scott, B.; Schardl, C.L. Elimination of ergovaline from a grass-Neotyphodium endophyte symbiosis by genetic modification of the endophyte. Proc. Natl. Acad. Sci. USA 2001, 98, 12820–12825. [Google Scholar] [CrossRef]
- Rasmussen, S.; Parsons, A.J.; Bassett, S.; Christensen, M.J.; Hume, D.E.; Johnson, L.J.; Johnson, R.D.; Simpson, W.R.; Stacke, C.; Voisey, C.R.; et al. High nitrogen supply and carbohydrate content reduce fungal endophyte and alkaloid concentration in Lolium perenne. New Phytol. 2007, 173, 787–797. [Google Scholar] [CrossRef]
- Young, C.A.; Bryant, M.K.; Christensen, M.J.; Tapper, B.A.; Bryan, G.T.; Scott, B. Molecular cloning and genetic analysis of a symbiosis-expressed gene cluster for lolitrem biosynthesis from a mutualistic endophyte of perennial ryegrass. Mol. Genet. Genom. 2005, 274, 13–29. [Google Scholar] [CrossRef]
- Faville, M.J.; Briggs, L.; Cao, M.; Koulman, A.; Jahufer, M.Z.Z.; Koolaard, J.; Hume, D.E. A QTL analysis of host plant effects on fungal endophyte biomass and alkaloid expression in perennial ryegrass. Mol. Breed. 2015, 35, 161. [Google Scholar] [CrossRef]
- Cagnano, G.; Roulund, N.; Jensen, C.S.; Forte, F.P.; Asp, T.; Leuchtmann, A. Large scale screening of Epichloë endophytes infecting Schedonorus pratensis and other forage grasses reveals a relation between microsatellite-based haplotypes and loline alkaloid levels. Front. Plant Sci. 2019, 10, 765. [Google Scholar] [CrossRef]
- Hiatt, E.E.; Hill, N.S.; Bouton, J.H.; Stuedemann, J.A. Tall fescue endophyte detection: Commercial immunoblot test kit compared with microscopic analysis. Crop Sci. 1999, 39, 796–799. [Google Scholar] [CrossRef]
- Cagnano, G.; Lenk, I.; Roulund, N.; Jensen, C.S.; Cox, M.P.; Asp, T. Mycelial biomass and concentration of loline alkaloids driven by complex population structure in Epichloë uncinata and meadow fescue (Schedonorus pratensis). Mycologia 2020. [Google Scholar] [CrossRef] [PubMed]
- Barnes, R.J.; Dhanoa, M.S.; Lister, S.J. Standard normal variate transformation and de-trending of near-infrared diffuse reflectance spectra. Appl. Spectrosc. 1989, 43, 772–777. [Google Scholar] [CrossRef]
- Naes, T.; Isakson, T.; Fearn, T.; Davies, T. A User-Friendly Guide to Multivariate Calibration and Classification; IM Publications LLP: West Sussex, UK; Wiley: Hoboken, NJ, USA, 2002; ISBN 0-9528666-2-5. [Google Scholar]
- Shenk, J.S.; Westerhaus, M. Routine Operation, Calibration, Development and Networksystem Management Manual; NIRSystems, Inc.: Silver Spring, MD, USA, 1995. [Google Scholar]
- Martens, H.; Martens, M. Multivariate analysis of quality. An introduction. Meas. Sci. Technol. 2001, 12, 1746. [Google Scholar] [CrossRef]
- Williams, P.; Sobering, D. How do we do it: A brief summary of the methods we use in developing near infrared calibrations. In Near Infrared Spectroscopy: The Future Waves; Davies, A.M.C., Williams, P.C., Eds.; Nir Publications: Chichester, UK, 1996; pp. 185–188. [Google Scholar]
- Patchett, B.; Gooneratne, R.; Chapman, B.; Fletcher, L. Effects of loline-producing endophyte-infected meadow fescue ecotypes on New Zealand grass grub (Costelytra zealandica). N. Z. J. Agric. Res. 2011, 54, 303–313. [Google Scholar] [CrossRef]
- Patchett, B.J.; Chapman, R.B.; Fletcher, L.R.; Gooneratne, S.R. Endophyte infected Festuca pratensis containing loline alkaloids deters feeding by Listronotus bonariensis. N. Z. Plant Prot. 2008, 61, 205–209. [Google Scholar] [CrossRef][Green Version]
- Wilkinson, H.H.; Siegel, M.R.; Blankenship, J.D.; Mallory, A.C.; Bush, L.P.; Schardl, C.L. Contribution of Fungal Loline Alkaloids to Protection from Aphids in a Grass-Endophyte Mutualism. Mol. Plant Microbe Interact. 2000, 13, 1027–1033. [Google Scholar] [CrossRef]
- Patchett, B.; Gooneratne, R.; Fletcher, L.; Chapman, B. Seasonal changes in leaf and stem loline alkaloids in meadow fescue. Crop Pasture Sci. 2009, 62, 261–267. [Google Scholar] [CrossRef]
- Barker, G.M.G.; Patchett, B.J.; Cameron, N.E.N. Epichloë uncinata infection and loline content afford Festulolium grasses protection from black beetle (Heteronychus arator). N. Z. J. Agric. Res. 2015, 58, 35–56. [Google Scholar] [CrossRef]
- Levasseur, C.; Pinson-Gadais, L.; Kleiber, D.; Surel, O. Near infrared spectroscopy used as a support to the diagnostic of Fusarium species. Rev. Med. Vet. 2010, 161, 438–444. [Google Scholar]
- Tamburini, E.; Mamolini, E.; De Bastiani, M.; Marchetti, M.G. Quantitative determination of Fusarium proliferatum concentration in intact garlic cloves using near-infrared spectroscopy. Sensors 2016, 16, 1099. [Google Scholar] [CrossRef] [PubMed]
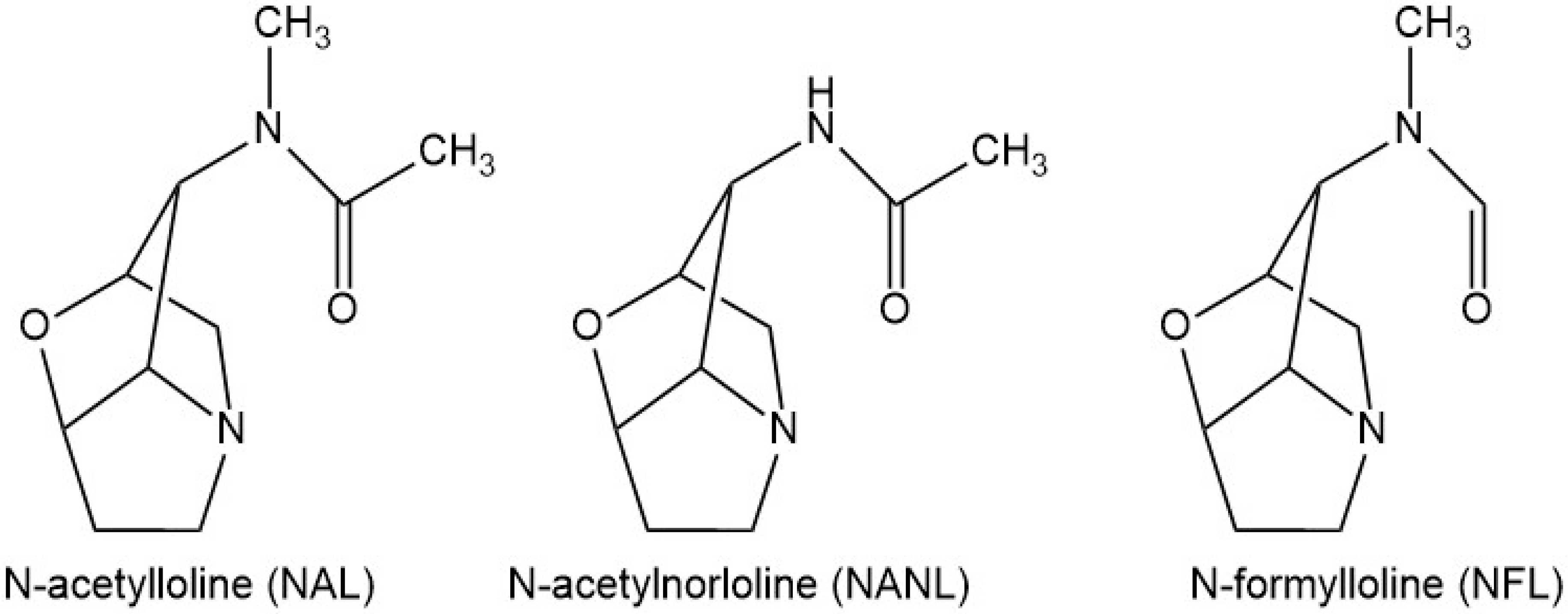
| Parameter | Statistical Descriptor | Training/Calibration Set | Validation Set |
|---|---|---|---|
| N-acetylloline (NAL) | n | 143 | 46 |
| Range (mg∙kg−1) | 31–320 | 25–339 | |
| Mean (mg∙kg−1) | 145 | 157 | |
| SD (mg∙kg−1) | 66 | 82 | |
| N-acetylnorloline (NANL) | n | 142 | 38 |
| Range (mg∙kg−1) | 25–982 | 43–804 | |
| Mean (mg∙kg−1) | 295 | 332 | |
| SD (mg∙kg−1) | 189 | 201 | |
| N-formylloline (NFL) | n | 146 | 47 |
| Range (mg∙kg−1) | 60–4327 | 77–2990 | |
| Mean (mg∙kg−1) | 1222 | 1208 | |
| SD (mg∙kg−1) | 767 | 773 | |
| Total Lolines | n | 146 | 51 |
| Range (mg∙kg−1) | 101–5629 | 107–3893 | |
| Mean (mg∙kg−1) | 1658 | ||
| SD (mg∙kg−1) | 1009 | 1038 | |
| Mycelial biomass | n | 64 | 22 |
| Range (mg g−1) | 0.220–3.960 | 0.360–3.970 | |
| Mean (mg g−1) | 1.500 | 1.355 | |
| SD (mg g−1) | 0.914 | 0.783 |
| Mathematical Treatment | Principal Components | Variability Explained (%) | Spectral Outliers |
|---|---|---|---|
| n0 | 9 | 99.99 | 4 |
| n1 | 13 | 99.64 | 6 |
| n2 | 23 | 99.02 | 2 |
| n3 | 13 | 99.83 | 6 |
| n4 | 15 | 99.68 | 6 |
| s0 | 12 | 100 | 8 |
| s1 | 15 | 99.55 | 8 |
| s2 | 27 | 98.91 | 4 |
| s3 | 16 | 99.78 | 8 |
| s4 | 17 | 99.66 | 8 |
| d0 | 10 | 99.95 | 7 |
| d1 | 12 | 99.72 | 6 |
| d2 | 23 | 99.02 | 2 |
| d3 | 13 | 99.83 | 6 |
| d4 | 15 | 99.68 | 6 |
| m0 | 13 | 99.97 | 9 |
| m1 | 15 | 99.66 | 8 |
| m2 | 27 | 98.88 | 4 |
| m3 | 16 | 99.78 | 8 |
| m4 | 17 | 99.65 | 8 |
| NAL | NANL | NFL | LOLINES | MYCELIUM | |
|---|---|---|---|---|---|
| Principal Component Analysis (PCA) | |||||
| Pre-treatment † | n3 | s2 | n0 | n0 | s0 |
| Number of principal components (PCs) | 13 | 27 | 9 | 9 | 4 |
| Explained variability (%) | 99.75 | 98.91 | 99.99 | 99.99 | 98.91 |
| Spectral outliers (H > 3.0) | 6 | 4 | 4 | 4 | 4 |
| Modified Partial Least Squares (MPLS) | |||||
| Pre-treatment † | s2 | m2 | m2 | s2 | d2 |
| Number of samples | 143 | 142 | 146 | 146 | 65 |
| Standard deviation (SD) (mg∙kg−1) | 65 | 189 | 767 | 1008 | 0.907 |
| Range (mg∙kg−1) | 31–320 | 25–982 | 60–4327 | 101–5629 | 0.22–3.96 |
| Chemical outliers (T > 2.5) | 6 | 5 | 7 | 7 | 2 |
| Multiple correlation coefficient (RSQ) | 0.765 | 0.836 | 0.893 | 0.897 | 0.729 |
| Standard error of calibration (SEC) (mg∙kg−1) | 32 | 77 | 251 | 324 | 0.473 |
| Standard error of cross validation (SECV) (mg∙kg−1) | 51 | 141 | 520 | 667 | 0.78 |
| Number of PLS factors | 10 | 10 | 10 | 10 | 10 |
| Groups in cross-validation | 6 | 6 | 6 | 6 | 6 |
| Internal Validation | |||||
| Standard error of prediction (SEP) (mg∙kg−1) | 31 | 74 | 240 | 310 | 0.449 |
| Medium value of the residuals (BIAS) (mg∙kg−1) | 0 | 0 | 0 | 0 | −0.003 |
| SEP corrected by the Bias (SEPc) (mg∙kg−1) | 31 | 74 | 241 | 311 | 0.453 |
| Multiple correlation coefficient (RSQ) | 0.778 | 0.846 | 0.901 | 0.905 | 0.754 |
| Ratio performance deviation (RPD) | 2.129 | 2.559 | 3.195 | 3.252 | 2.036 |
| External Validation | |||||
| Root mean standard error (RMSE = SEP) (mg∙kg−1) | 84 | 184 | 718 | 894 | 0.979 |
| Average residual (mg∙kg−1) | 66 | 152 | 535 | 665 | 0.796 |
| Student’s t-test (p) | 0.018 | 0.048 | 0.265 | 0.157 | 0.894 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cagnano, G.; Vázquez-de-Aldana, B.R.; Asp, T.; Roulund, N.; Jensen, C.S.; Soto-Barajas, M.C. Determination of Loline Alkaloids and Mycelial Biomass in Endophyte-Infected Schedonorus pratensis by Near-Infrared Spectroscopy and Chemometrics. Microorganisms 2020, 8, 776. https://doi.org/10.3390/microorganisms8050776
Cagnano G, Vázquez-de-Aldana BR, Asp T, Roulund N, Jensen CS, Soto-Barajas MC. Determination of Loline Alkaloids and Mycelial Biomass in Endophyte-Infected Schedonorus pratensis by Near-Infrared Spectroscopy and Chemometrics. Microorganisms. 2020; 8(5):776. https://doi.org/10.3390/microorganisms8050776
Chicago/Turabian StyleCagnano, Giovanni, Beatriz R. Vázquez-de-Aldana, Torben Asp, Niels Roulund, Christian S. Jensen, and Milton Carlos Soto-Barajas. 2020. "Determination of Loline Alkaloids and Mycelial Biomass in Endophyte-Infected Schedonorus pratensis by Near-Infrared Spectroscopy and Chemometrics" Microorganisms 8, no. 5: 776. https://doi.org/10.3390/microorganisms8050776
APA StyleCagnano, G., Vázquez-de-Aldana, B. R., Asp, T., Roulund, N., Jensen, C. S., & Soto-Barajas, M. C. (2020). Determination of Loline Alkaloids and Mycelial Biomass in Endophyte-Infected Schedonorus pratensis by Near-Infrared Spectroscopy and Chemometrics. Microorganisms, 8(5), 776. https://doi.org/10.3390/microorganisms8050776





