Production of Raw Starch-Digesting Amylolytic Preparation in Yarrowia lipolytica and Its Application in Biotechnological Synthesis of Lactic Acid and Ethanol
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Basic Culturing Media
2.2. Fed-Batch Bioreactor Cultivations
2.3. Formulation of SoAMY-TlGAMY Preparation
2.4. Purification of SoAMY-TlGAMY and Analysis
2.5. Proof-of-Concept Process 1: EtOH Production by K. marxianus in SSF Process with SoAMY-TlGAMY Crude Preparation
2.5.1. Shake-Flask Production Cultures
2.5.2. Bioreactor Production Cultures
2.6. Proof-of-Concept Process 2: LA Production by Lb. Plantarum in SSF Process with SoAMY-TlGAMY Crude Preparation
2.6.1. Shake-Flask Production Cultures
2.6.2. Bioreactor Production Cultures
2.7. Analytical Methods
2.7.1. Amylolytic Activity Assay (microSIT)
2.7.2. Protein Concentration Assay (microBCA)
2.7.3. Determination of Compounds Concentration—HPLC
Glycerol and Metabolites Concentration
Starch-Decomposition Products Concentration (dp1-dp7)
2.7.4. Microbial Growth Analysis
2.7.4.1. Gravimetric Method—Dry Cellular Biomass Determination
2.7.4.2. Spectrophotometric Measurement at 600 nm Wavelength (OD600)
2.7.4.3. Viable Counts
2.8. Statistical Analysis and Data Managment
3. Results
3.1. Production and Purification of SoAMY-TlGAMY Enzymatic Preparation
3.2. Proof-of-Concept Process 1: Ethanol Production by K. marxianus in SSF Process with SoAMY-TlGAMY
3.2.1. Flask SSF Cultures
3.2.2. Bioreactor SSF Cultures
3.3. Proof-of-Concept Process 2: Lactic Acid Production by Lb. plantarum in SSF Process with SoAMY-TlGAMY
3.3.1. Flask SSF Cultures
3.3.2. Bioreactor SSF Cultures
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cardona, C.A.; Sánchez, Ó.J. Fuel ethanol production: Process design trends and integration opportunities. Bioresour. Technol. 2007, 98, 2415–2457. [Google Scholar] [CrossRef] [PubMed]
- Tsakona, S.; Kopsahelis, N.; Chatzifragkou, A.; Papanikolaou, S.; Kookos, I.K.; Koutinas, A.A. Formulation of fermentation media from flour-rich waste streams for microbial lipid production by Lipomyces starkeyi. J. Biotechnol. 2014, 189, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Smerilli, M.; Neureiter, M.; Wurz, S.; Haas, C.; Frühauf, S.; Fuchs, W. Direct fermentation of potato starch and potato residues to lactic acid by Geobacillus stearothermophilus under non-sterile conditions. J. Chem. Technol. Biotechnol. 2015, 90, 648–657. [Google Scholar] [CrossRef] [PubMed]
- Tsakona, S.; Papadaki, A.; Kopsahelis, N.; Kachrimanidou, V.; Papanikolaou, S.; Koutinas, A. Development of a circular oriented bioprocess for microbial oil production using diversified mixed confectionery side-streams. Foods 2019, 8, 300. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Soni, S.K. Production of starch-gel digesting amyloglucosidase by Aspergillus oryzae HS-3 in solid state fermentation. Process Biochem. 2001, 37, 453–459. [Google Scholar] [CrossRef]
- Olofsson, K.; Bertilsson, M.; Lidén, G. A short review on SSF—An interesting process option for ethanol production from lignocellulosic feedstocks. Biotechnol. Biofuels 2008, 1, 7. [Google Scholar] [CrossRef]
- Białas, W.; Szymanowska, D.; Grajek, W. Fuel ethanol production from granular corn starch using Saccharomyces cerevisiae in a long term repeated SSF process with full stillage recycling. Bioresour. Technol. 2010, 101, 3126–3131. [Google Scholar] [CrossRef]
- Białas, W.; Czerniak, A.; Szymanowska-Powałowska, D. Kinetic modeling of simultaneous saccharification and fermentation of corn starch for ethanol production. Acta Biochim. Pol. 2014, 61, 153–162. [Google Scholar] [CrossRef]
- Hoshino, K.; Taniguchi, M.; Marumoto, H.; Shimizu, K.; Fujii, M. Continuous lactic acid production from raw starch in a fermentation system using a reversibly soluble-autoprecipitatipg amylase and immobilized cells of Lactobacillus casei. Agric. Biol. Chem. 1991, 55, 479–485. [Google Scholar] [CrossRef]
- Sun, H.; Zhao, P.; Ge, X.; Xia, Y.; Hao, Z.; Liu, J.; Peng, M. Recent advances in microbial raw starch degrading enzymes. Appl. Biochem. Biotechnol. 2010, 160, 988–1003. [Google Scholar] [CrossRef]
- Robertson, G.H.; Wong, D.W.S.; Lee, C.C.; Wagschal, K.; Smith, M.R.; Orts, W.J. Native or raw starch digestion: A key step in energy efficient biorefining of grain. J. Agric. Food Chem. 2006, 54, 353–365. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.G.; Kim, C.H.; Rhee, S.K. A kinetic model and simulation of starch saccharification and simultaneous ethanol fermentation by amyloglucosidase and Zymomonas mobilis. Bioprocess Eng. 1992, 7, 335–341. [Google Scholar] [CrossRef]
- Nwobi, A.; Cybulska, I.; Tesfai, W.; Shatilla, Y.; Rodríguez, J.; Thomsen, M.H. Simultaneous saccharification and fermentation of solid household waste following mild pretreatment using a mix of hydrolytic enzymes in combination with Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2015, 99, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, J.; Singh, S.; Nain, L. Thermotolerant fermenting yeasts for simultaneous saccharification fermentation of lignocellulosic biomass. Electron. J. Biotechnol. 2016, 21, 82–92. [Google Scholar] [CrossRef]
- Gupta, R.; Gigras, P.; Mohapatra, H.; Goswami, V.K.; Chauhan, B. Microbial α-amylases: A biotechnological perspective. Process Biochem. 2003, 38, 1599–1616. [Google Scholar] [CrossRef]
- Xu, Q.S.; Yan, Y.S.; Feng, J.X. Efficient hydrolysis of raw starch and ethanol fermentation: A novel raw starch-digesting glucoamylase from Penicillium oxalicum. Biotechnol. Biofuels 2016, 9, 1–18. [Google Scholar] [CrossRef]
- Ngernyuang, N.; Kobayashi, I.; Promboon, A.; Ratanapo, S.; Tamura, T.; Ngernsiri, L. Cloning and expression analysis of the Bombyx mori α-amylase gene (Amy) from the indigenous Thai silkworm strain, Nanglai. J. Insect Sci. 2011, 11, 1–16. [Google Scholar] [CrossRef]
- Van Zyl, W.H.; Bloom, M.; Viktor, M.J. Engineering yeasts for raw starch conversion. Appl. Microbiol. Biotechnol. 2012, 95, 1377–1388. [Google Scholar] [CrossRef]
- Celińska, E.; Olkowicz, M.; Grajek, W. L-Phenylalanine catabolism and 2-phenylethano synthesis in Yarrowia lipolytica-mapping molecular identities through whole-proteome quantitative mass spectrometry analysis. FEMS Yeast Res. 2015, 15. [Google Scholar] [CrossRef]
- Nicaud, J.M.; Madzak, C.; van den Broek, P.; Gysler, C.; Duboc, P.; Niederberger, P.; Gaillardin, C. Protein expression and secretion in the yeast Yarrowia lipolytica. FEMS Yeast Res. 2002, 2, 371–379. [Google Scholar] [CrossRef]
- Groenewald, M.; Boekhout, T.; Neuvéglise, C.; Gaillardin, C.; Van Dijck, P.W.M.; Wyss, M. Yarrowia lipolytica: Safety assessment of an oleaginous yeast with a great industrial potential. Crit. Rev. Microbiol. 2014, 40, 187–206. [Google Scholar] [CrossRef] [PubMed]
- Madzak, C. Engineering Yarrowia lipolytica for use in biotechnological applications: A review of major achievements and recent innovations. Mol. Biotechnol. 2018, 60, 621–635. [Google Scholar] [CrossRef] [PubMed]
- Theron, C.W.; Vandermies, M.; Telek, S.; Steels, S.; Fickers, P. Comprehensive comparison of Yarrowia lipolytica and Pichia pastoris for production of Candida antarctica lipase B. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef]
- Kim, J.W.; Park, T.J.; Ryu, D.D.Y.; Kim, J.Y. High cell density culture of Yarrowia lipolytica using a one-step feeding process. Biotechnol. Prog. 2000, 16, 657–660. [Google Scholar] [CrossRef] [PubMed]
- Kubiak, M.; Borkowska, M.; Bialas, W.; Korpys, P.; Celinska, E. Feeding strategy impacts heterologous protein production in Yarrowia lipolytica fed-batch cultures-Insight into the role of osmolarity. Yeast 2019, 36, 305–318. [Google Scholar] [CrossRef] [PubMed]
- Celińska, E.; Nicaud, J.M. Filamentous fungi-like secretory pathway strayed in a yeast system: Peculiarities of Yarrowia lipolytica secretory pathway underlying its extraordinary performance. Appl. Microbiol. Biotechnol. 2019, 103, 39–52. [Google Scholar] [CrossRef]
- Balat, M.; Balat, H.; Öz, C. Progress in bioethanol processing. Prog. Energy Combust. Sci. 2008, 34, 551–573. [Google Scholar] [CrossRef]
- Celińska, E.; Borkowska, M.; Korpys-Woźniak, P.; Kubiak, M.; Nicaud, J.; Kubiak, P.; Gorczyca, M.; Białas, W. Optimization of Yarrowia lipolytica-based consolidated biocatalyst through synthetic biology approach: Transcription units and signal peptides shuffling. Appl. Microbiol. Biotechnol. 2020. [Google Scholar] [CrossRef]
- Celińska, E.; Ledesma-Amaro, R.; Larroude, M.; Rossignol, T.; Pauthenier, C.; Nicaud, J.M. Golden Gate Assembly system dedicated to complex pathway manipulation in Yarrowia lipolytica. Microb. Biotechnol. 2017, 10, 450–455. [Google Scholar] [CrossRef]
- Celińska, E.; Borkowska, M.; Białas, W.; Korpys, P.; Nicaud, J.M. Robust signal peptides for protein secretion in Yarrowia lipolytica: Identification and characterization of novel secretory tags. Appl. Microbiol. Biotechnol. 2018, 102, 5221–5233. [Google Scholar] [CrossRef]
- Celińska, E.; Borkowska, M.; Białas, W. Evaluation of heterologous α-amylase production in two expression platforms dedicated for Yarrowia lipolytica: Commercial Po1g-pYLSC (php4d) and custom-made A18-pYLTEF (pTEF). Yeast 2016, 33, 165–181. [Google Scholar] [CrossRef] [PubMed]
- Celińska, E.; Białas, W.; Borkowska, M.; Grajek, W. Cloning, expression, and purification of insect (Sitophilus oryzae) alpha-amylase, able to digest granular starch, in Yarrowia lipolytica host. Appl. Microbiol. Biotechnol. 2015, 99, 2727–2739. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Borkowska, M.; Białas, W.; Kubiak, M.; Celińska, E. Rapid micro-assays for amylolytic activities determination: Customization and validation of the tests. Appl. Microbiol. Biotechnol. 2019, 1–13. [Google Scholar] [CrossRef]
- Guyot, J.P.; Calderon, M.; Morlon-Guyot, J. Effect of pH control on lactic acid fermentation of starch by Lactobacillus manihotivorans LMG 18010(T). J. Appl. Microbiol. 2000, 88, 176–182. [Google Scholar] [CrossRef]
- Bujang, K.; Sujang, S.; Salwani, D.; Adeni, A. Effects of calcium carbonate in fermentation of L-lactic acid from hydrolyzed sago starch. IC Biotech 2004, 26, 637–643. [Google Scholar]
- Liu, S. An overview of chemical reaction analysis. In Bioprocess Engineering, 2nd ed.; Liu, S., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; Chapter 3; pp. 81–137. ISBN 978-0-444-63783-3. [Google Scholar]
- Celińska, E.; Borkowska, M.; Białas, W. Enhanced production of insect raw-starch-digesting alpha-amylase accompanied by high erythritol synthesis in recombinant Yarrowia lipolytica fed-batch cultures at high-cell-densities. Process Biochem. 2017, 52, 78–85. [Google Scholar] [CrossRef]
- Celińska, E.; Borkowska, M.; Białas, W. Evaluation of a recombinant insect-derived amylase performance in simultaneous saccharification and fermentation process with industrial yeasts. Appl. Microbiol. Biotechnol. 2016, 100, 2693–2707. [Google Scholar] [CrossRef]
- Yang, C.H.; Huang, Y.C.; Chen, C.Y.; Wen, C.Y. Heterologous expression of Thermobifida fusca thermostable alpha-amylase in Yarrowia lipolytica and its application in boiling stable resistant sago starch preparation. J. Ind. Microbiol. Biotechnol. 2010, 37, 953–960. [Google Scholar] [CrossRef]
- Thorsen, T.S.; Johnsen, A.H.; Josefsen, K.; Jensen, B. Identification and characterization of glucoamylase from the fungus Thermomyces lanuginosus. Biochim. Biophys. Acta Proteins Proteom. 2006, 1764, 671–676. [Google Scholar] [CrossRef]
- Raimondi, S.; Zanni, E.; Amaretti, A.; Palleschi, C.; Uccelletti, D.; Rossi, M. Thermal adaptability of Kluyveromyces marxianus in recombinant protein production. Microb. Cell Fact. 2013, 12. [Google Scholar] [CrossRef] [PubMed]
- Kuloyo, O.O.; du Preez, J.C.; del García-Aparicio, M.P.; Kilian, S.G.; Steyn, L.; Görgens, J. Opuntia ficus-indica cladodes as feedstock for ethanol production by Kluyveromyces marxianus and Saccharomyces cerevisiae. World J. Microbiol. Biotechnol. 2014, 30, 3173–3183. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.H.; Hung, W.C.; Lo, K.Y.; Chen, Y.H.; Wan, H.P.; Cheng, K.C. Bioethanol production from taro waste using thermo-tolerant yeast Kluyveromyces marxianus K21. Bioresour. Technol. 2016, 201, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Hoshino, T.; Matsushika, A. High-temperature ethanol production by a series of recombinant xylose-fermenting Kluyveromyces marxianus strains. Enzym. Microb. Technol. 2019, 129, 109359. [Google Scholar] [CrossRef]
- Zafar, S.; Owais, M. Ethanol production from crude whey by Kluyveromyces marxianus. Biochem. Eng. J. 2006, 27, 295–298. [Google Scholar] [CrossRef]
- Ballesteros, M.; Oliva, J.M.; Negro, M.J.; Manzanares, P.; Ballesteros, I. Ethanol from lignocellulosic materials by a simultaneous saccharification and fermentation process (SFS) with Kluyveromyces marxianus CECT 10875. Process Biochem. 2004, 39, 1843–1848. [Google Scholar] [CrossRef]
- Yu, C.Y.; Jiang, B.H.; Duan, K.J. Production of bioethanol from carrot pomace using the thermotolerant yeast Kluyveromyces marxianus. Energies 2013, 6, 1794–1801. [Google Scholar] [CrossRef]
- Giraud, E.; Champailler, A.; Raimbault, M. Degradation of raw starch by a wild amylolytic strain of Lactobacillus plantarum. Appl. Environ. Microbiol. 1994, 60, 4319–4323. [Google Scholar] [CrossRef]
- Chookietwattana, K. Lactic acid production from simultaneous saccharification and fermentation of cassava starch by Lactobacillus plantarum MSUL 903. APCBEE Procedia 2014, 8, 156–160. [Google Scholar] [CrossRef]
- Yumoto, I.; Ikeda, K. Direct fermentation of starch to L-(+)-lactic acid using Lactobacillus amylophilus. Biotechnol. Lett. 1995, 17, 543–546. [Google Scholar] [CrossRef]
- Vishnu, C.; Seenayya, G.; Reddy, G. Direct fermentation of various pure and crude starchy substrates to L(+) lactic acid using Lactobacillus amylophilus GV6. World J. Microbiol. Biotechnol. 2002, 18, 429–433. [Google Scholar] [CrossRef]
- Ohkouchi, Y.; Inoue, Y. Direct production of l(+)-lactic acid from starch and food wastes using Lactobacillus manihotivorans LMG18011. Bioresour. Technol. 2006, 97, 1554–1562. [Google Scholar] [CrossRef] [PubMed]
- Adthalungrong, C.; Saechua, N.; Doungkaew, K. Lactic acid fermentation from tapioca starch by Lactobacillus casei TISTR 453 using simultaneous saccharification and fermentation process. KKU Res. J. 2014, 19, 125–133. [Google Scholar]
- Löser, C.; Urit, T.; Nehl, F.; Bley, T. Screening of Kluyveromyces strains for the production of ethyl acetate: Design and evaluation of a cultivation system. Eng. Life Sci. 2011, 11, 369–381. [Google Scholar] [CrossRef]
- Löser, C.; Urit, T.; Stukert, A.; Bley, T. Formation of ethyl acetate from whey by Kluyveromyces marxianus on a pilot scale. J. Biotechnol. 2013, 163, 17–23. [Google Scholar] [CrossRef]
- Silveira, W.B.; Passos, F.J.V.; Mantovani, H.C.; Passos, F.M.L. Ethanol production from cheese whey permeate by Kluyveromyces marxianus UFV-3: A flux analysis of oxido-reductive metabolism as a function of lactose concentration and oxygen levels. Enzym. Microb. Technol. 2005, 36, 930–936. [Google Scholar] [CrossRef]
- Masiero, S.S.; Peretti, A.; Trierweiler, L.F.; Trierweiler, J.O. Simultaneous cold hydrolysis and fermentation of fresh sweet potato. Biomass Bioenergy 2014, 70, 174–183. [Google Scholar] [CrossRef]
- Sivarathnakumar, S.; Jayamuthunagai, J.; Baskar, G.; Praveenkumar, R.; Selvakumari, I.A.E.; Bharathiraja, B. Bioethanol production from woody stem Prosopis juliflora using thermo tolerant yeast Kluyveromyces marxianus and its kinetics studies. Bioresour. Technol. 2019, 293. [Google Scholar] [CrossRef]
- Mithra, M.G.; Jeeva, M.L.; Sajeev, M.S.; Padmaja, G. Comparison of ethanol yield from pretreated lignocellulo-starch biomass under fed-batch SHF or SSF modes. Heliyon 2018, 4, e00885. [Google Scholar] [CrossRef]
- Szambelan, K.; Nowak, J.; Szwengiel, A.; Jeleń, H.; Łukaszewski, G. Separate hydrolysis and fermentation and simultaneous saccharification and fermentation methods in bioethanol production and formation of volatile by-products from selected corn cultivars. Ind. Crop. Prod. 2018, 118, 355–361. [Google Scholar] [CrossRef]
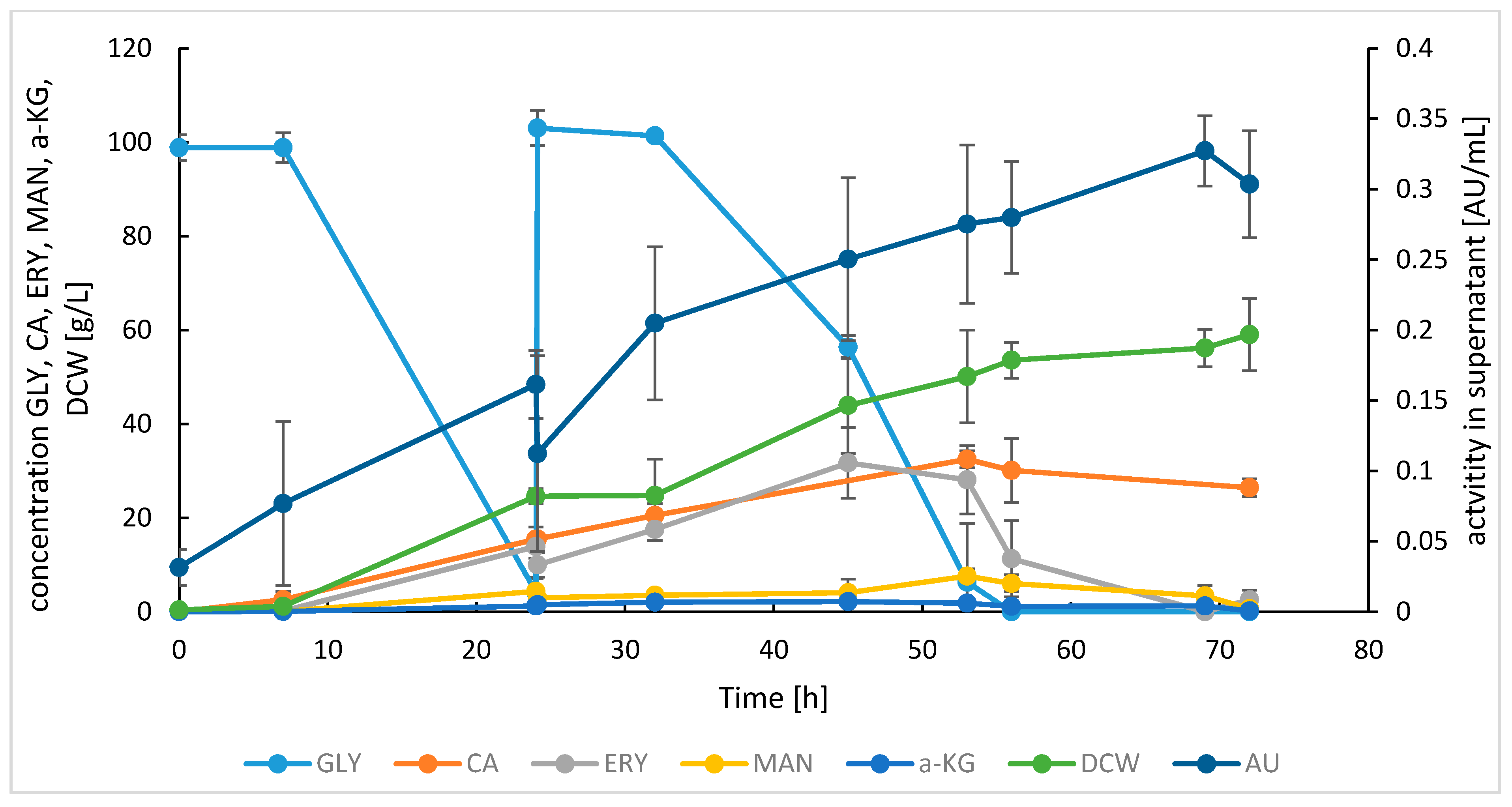
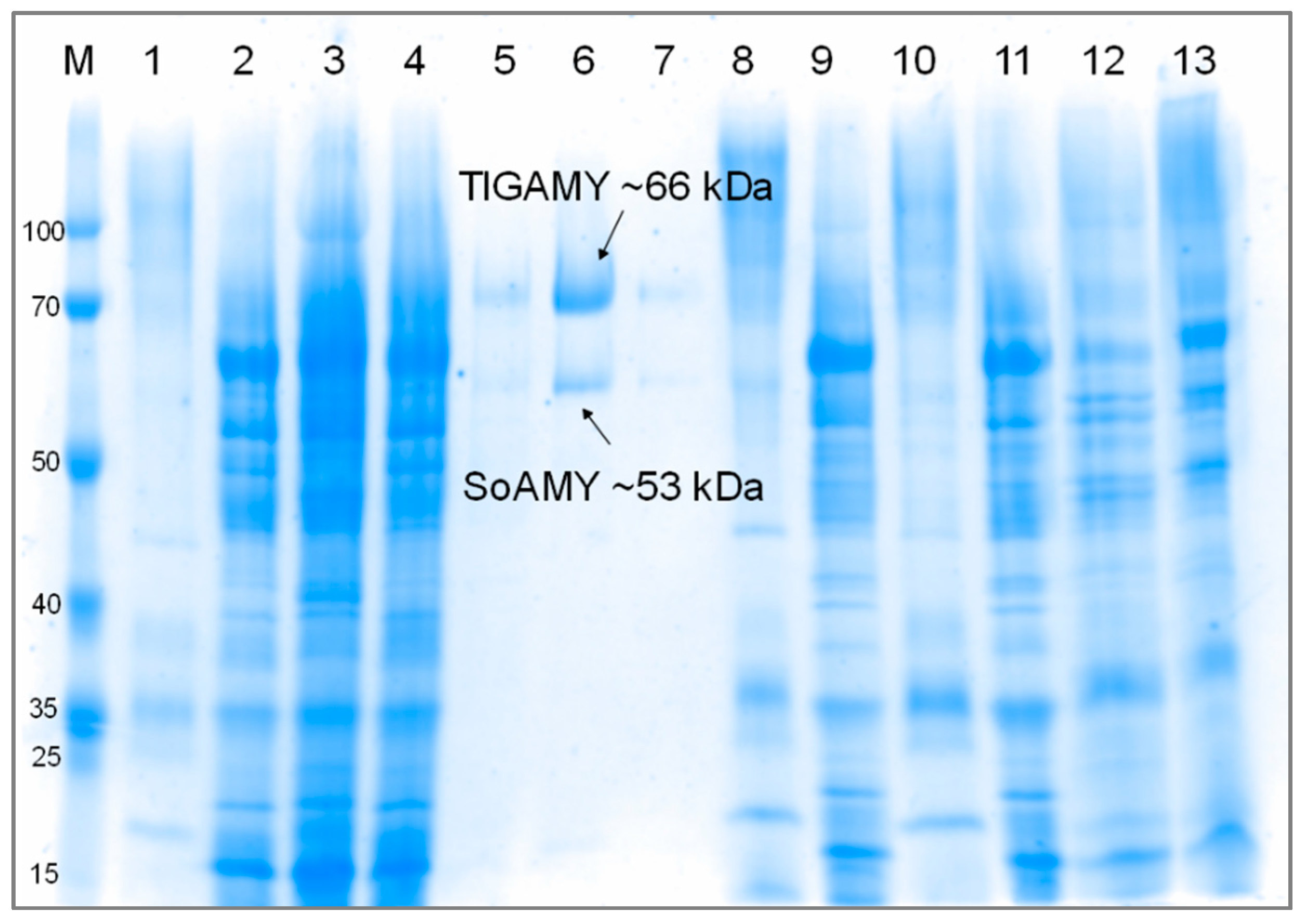





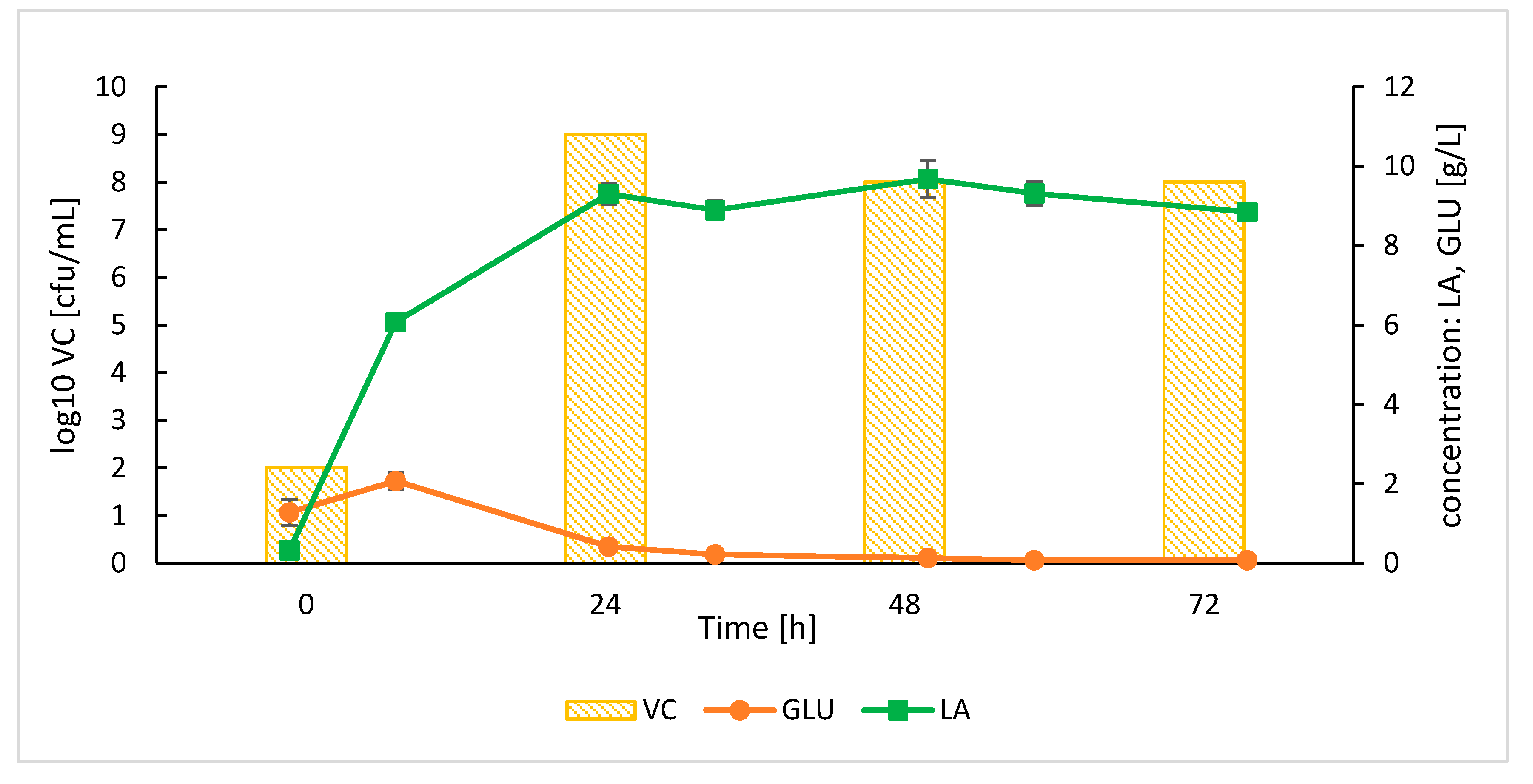
| Protein Fraction | Activity [AU/mL] | Total Protein [mg/mL] | Specific ACT [AU/mg] | |||
|---|---|---|---|---|---|---|
| AU/mL | ±SD | mg/mL | ±SD | AU/mg | ±SD | |
| AS supernatant | 0.18 | 0.16 * | 4.12 | 1.31 * | 0.06 | 0.05 * |
| AS pellet | 317.83 | 21.57 * | 40.22 | 4.83 * | 8.01 | 1.40 * |
| F-T | 64.05 | 0.92 | 26.67 | 3.41 | 2.40 | 0.03 |
| W-U | 328.94 | 14.62 | 4.08 | 0.17 | 80.65 | 3.58 |
| F1 | 366.19 | 10.50 | 0.74 | 0.47 | 493.30 | 14.14 |
| F2 | 5609.29 | 199.70 | 0.43 | 0.01 | 13159.51 | 468.49 |
| Purification factor F2 vs. AS pellet [fold] | 1642.97× | |||||
| Culturing Variant | Yield [g/g of Provided Substrate] at 24 h | Product Concentration (g/L) at 24 h |
|---|---|---|
| EtOH Production by K. marxianus in SSF | ||
| Flask | ||
| 32 °C 20 AU | 0.179 | 7.18 |
| 32 °C 25 AU | 0.196 | 7.86 |
| 36 °C 20 AU | 0.177 | 7.09 |
| 36 °C 25 AU | 0.193 | 7.73 |
| 40 °C 20 AU | 0.158 | 6.34 |
| 40 °C 25 AU | 0.191 | 7.66 |
| Bioreactor | ||
| IS | 0.144 | 5.76 |
| HS | 0.192 | 7.68 |
| IS21 | 0.173 | 6.91 |
| control G | 0.340 | 13.59 |
| STARGEN | 0.515 | 20.59 |
| SHF | 0.164 | 6.561 |
| Lactic acid production by Lb. plantarum in SSF | ||
| Flask | ||
| 30° 60 AU raw starch | 0.228 | 4.570 |
| 30° 60 AU cooked starch | 0.654 | 13.079 |
| Bioreactor | ||
| 30° 60 AU cooked starch | 0.465 | 9.301 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gęsicka, A.; Borkowska, M.; Białas, W.; Kaczmarek, P.; Celińska, E. Production of Raw Starch-Digesting Amylolytic Preparation in Yarrowia lipolytica and Its Application in Biotechnological Synthesis of Lactic Acid and Ethanol. Microorganisms 2020, 8, 717. https://doi.org/10.3390/microorganisms8050717
Gęsicka A, Borkowska M, Białas W, Kaczmarek P, Celińska E. Production of Raw Starch-Digesting Amylolytic Preparation in Yarrowia lipolytica and Its Application in Biotechnological Synthesis of Lactic Acid and Ethanol. Microorganisms. 2020; 8(5):717. https://doi.org/10.3390/microorganisms8050717
Chicago/Turabian StyleGęsicka, Aleksandra, Monika Borkowska, Wojciech Białas, Paulina Kaczmarek, and Ewelina Celińska. 2020. "Production of Raw Starch-Digesting Amylolytic Preparation in Yarrowia lipolytica and Its Application in Biotechnological Synthesis of Lactic Acid and Ethanol" Microorganisms 8, no. 5: 717. https://doi.org/10.3390/microorganisms8050717
APA StyleGęsicka, A., Borkowska, M., Białas, W., Kaczmarek, P., & Celińska, E. (2020). Production of Raw Starch-Digesting Amylolytic Preparation in Yarrowia lipolytica and Its Application in Biotechnological Synthesis of Lactic Acid and Ethanol. Microorganisms, 8(5), 717. https://doi.org/10.3390/microorganisms8050717





