Assessment of the Immunomodulatory Properties of the Probiotic Strain Lactobacillus paracasei K5 In Vitro and In Vivo
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Bacterial Strains and Culture Conditions
2.3. Animals
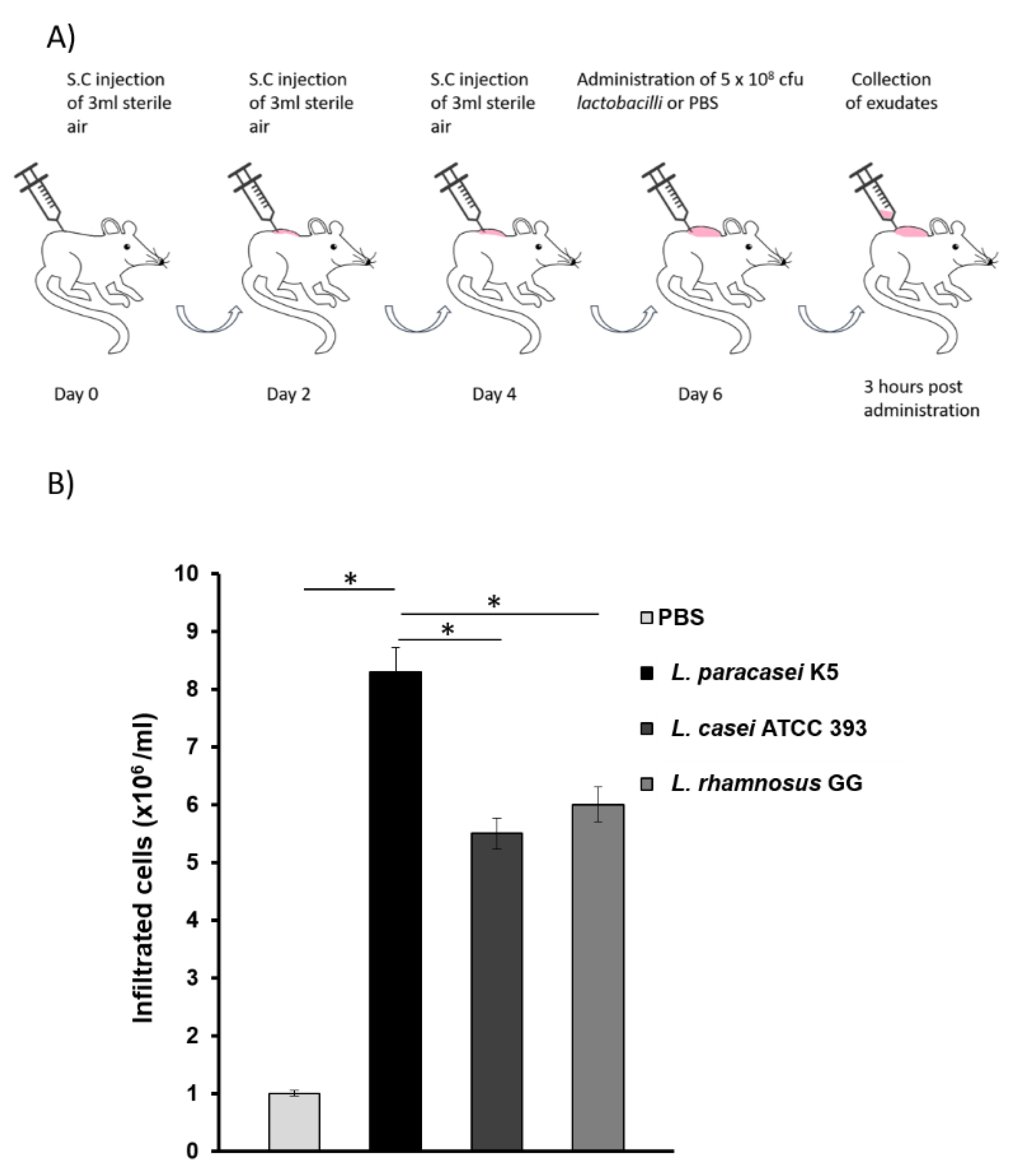
2.4. Air-Pouch Formation and Exudate Collection
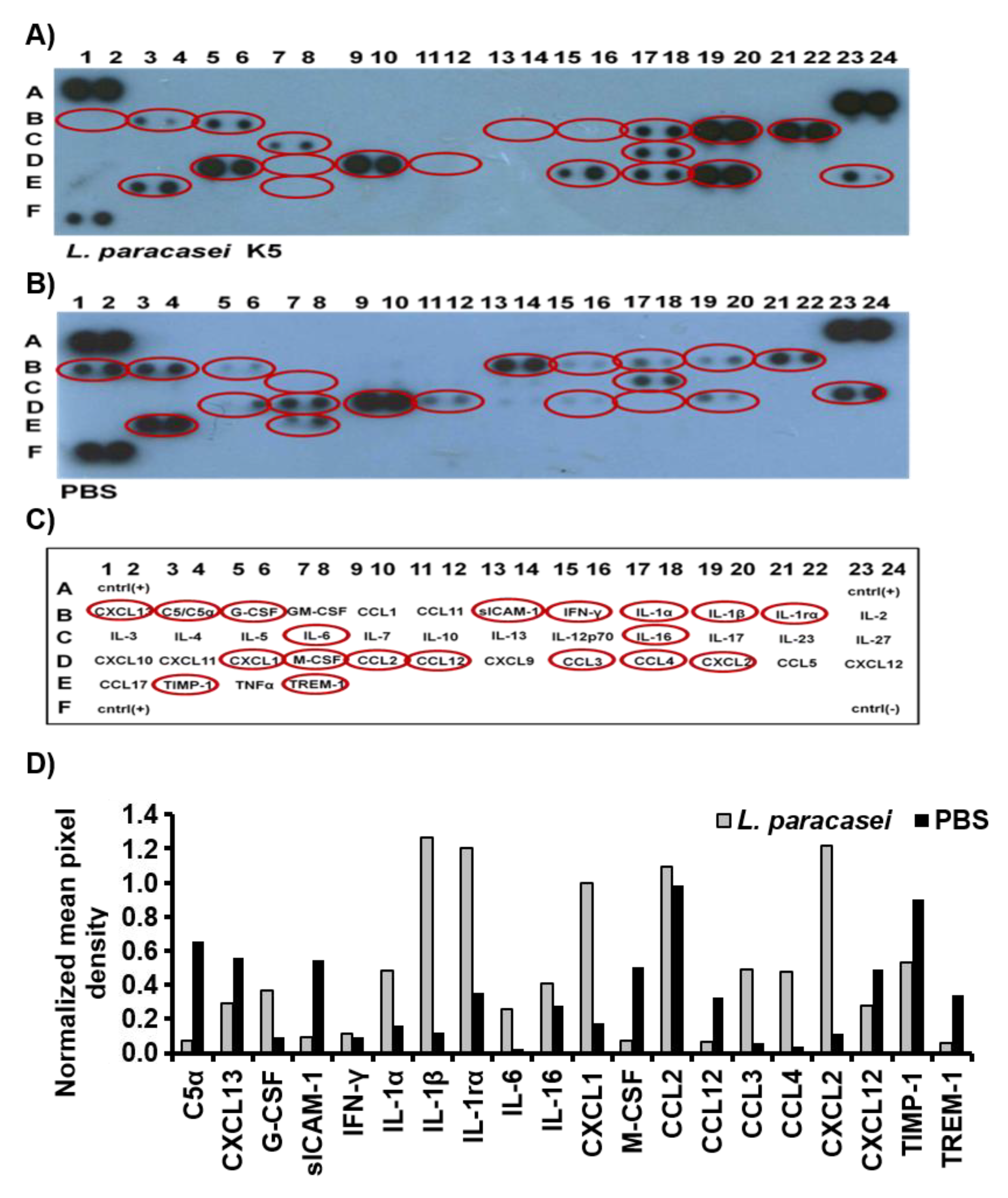
2.5. Detection of Cytokines and Chemokines in the Air-Pouch Exudates
2.6. Treatment of Caco-2 Cells with Probiotic Bacteria
2.7. RNA Extraction and cDNA Synthesis
2.8. Real Time PCR
2.9. Statistical Analysis
3. Results and Discussion
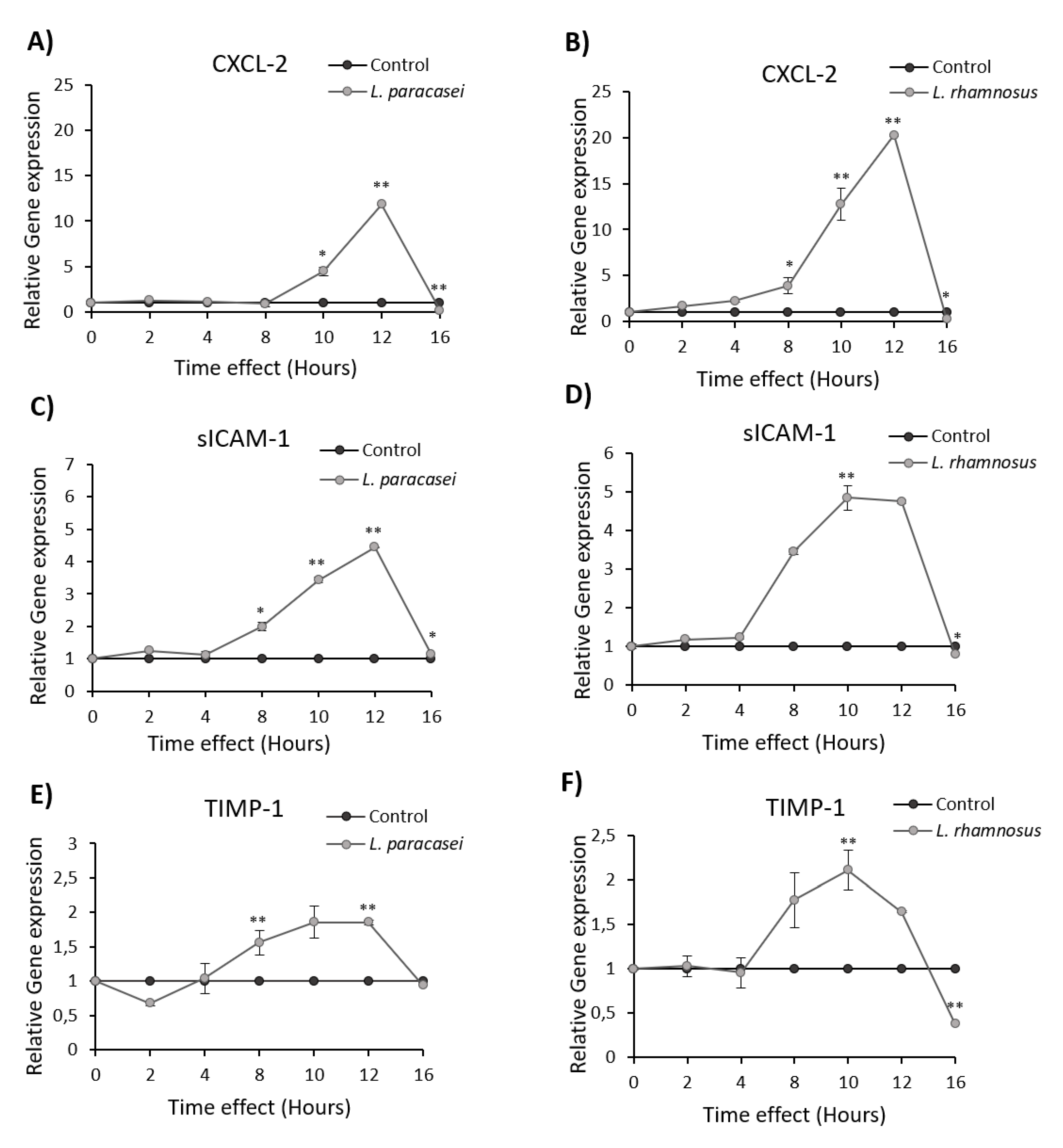
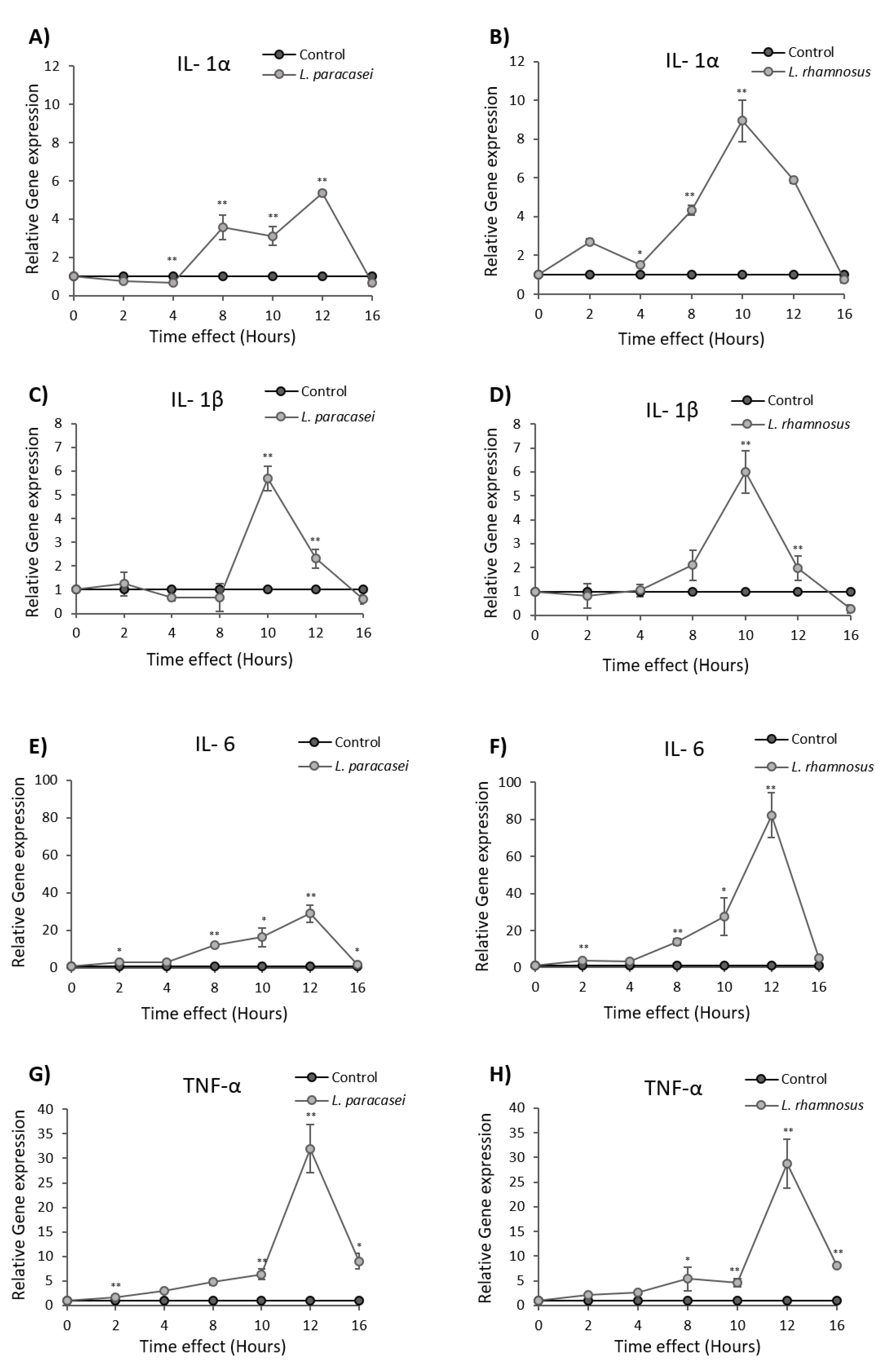
3.1. L. paracasei K5 Triggered the Infiltration of Leukocytes and the Expression of Cytokines and Chemokines in the Exudates of the Air Pouches
3.2. Induction of Cytokine and Chemokine Expression in Caco-2 cells upon Treatment with L. paracasei K5
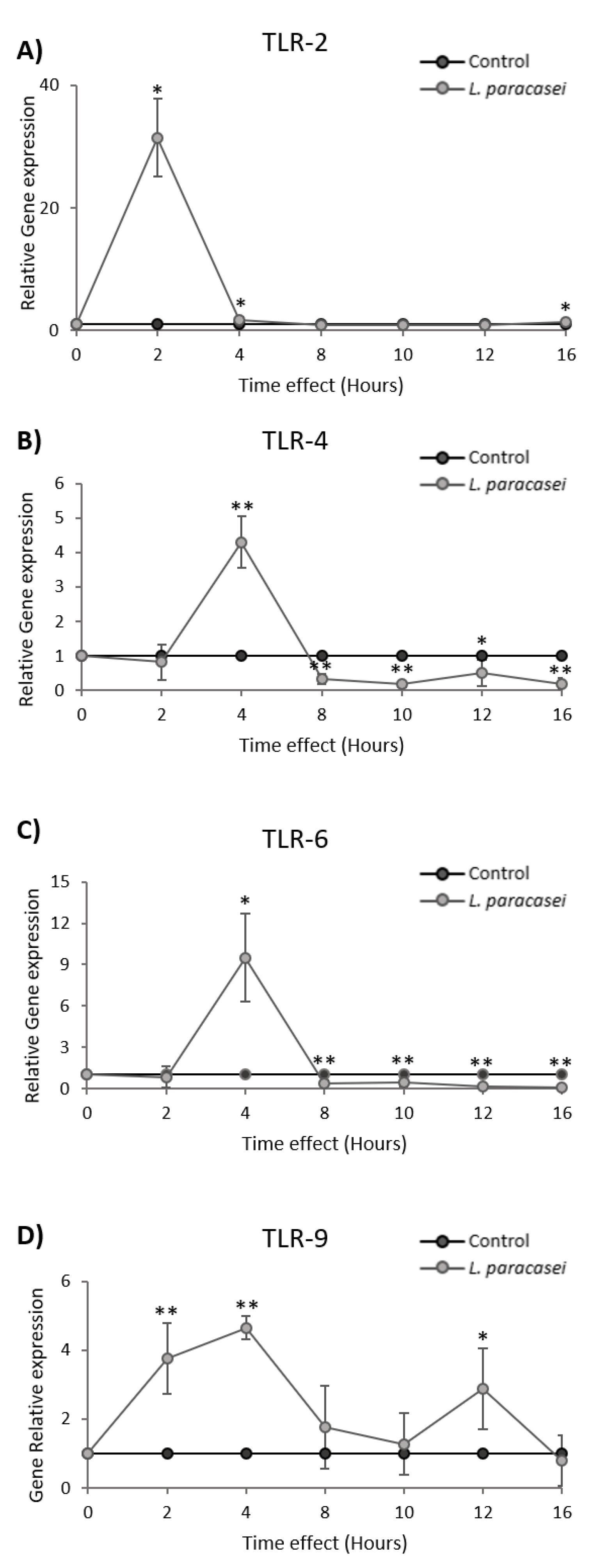
3.3. L. paracasei K5 Induced the Transient Expression of TLRs in Caco-2 Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations/World Health Organization (FAO/WHO). Evaluation of Health and Nutritional Properties of Powder Milk and Live Lactic Acid Bacteria. 2002. Available online: www.fao.org/3/a-a0512e.pdf (accessed on 30 September 2019).
- Moayyedi, P.; Ford, A.C.; Talley, N.J.; Cremonini, F.; Foxx-Orenstein, A.E.; Brandt, L.J.; Quigley, E.M.M. The Efficacy of Probiotics in the Treatment of Irritable Bowel Syndrome: A Systematic Review. Gut 2010, 59, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, J.Z.; Yap, C.; Lytvyn, L.; Ka-Fung Lo, C.; Beardsley, J.; Mertz, D.; Johnston, B.C. Probiotics for the Prevention of Clostridium Difficile-Associated Diarrhea in Adults and Children. Cochrane Database Syst. Rev. 2017, 12, CD006095. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.T.; Anvari, S.; Anagnostou, K. The Role of Probiotics in Preventing Allergic Disease. Children 2019, 5, 24. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, H.; Chen, X.; Chen, Y.; Bao, M.Q. Selection of potential probiotic lactobacilli for cholesterol-lowering properties and their effect on cholesterol metabolism in rats fed a high-lipid diet. J. Dairy Sci. 2012, 95, 1645–1654. [Google Scholar] [CrossRef] [PubMed]
- Monteagudo-Mera, A.; Rastall, R.A.; Gibson, G.R.; Charalampopoulos, D.; Chatzifragkou, A. Adhesion mechanisms mediated by probiotics and prebiotics and their potential impact on human health. Appl. Microbiol. Biotechnol. 2019, 103, 6463–6472. [Google Scholar] [CrossRef]
- Lazar, V.; Bezirtzoglou, E.; Balotescu, C.; Cernat, R.; Ilina, L.; Bulai, D.; Vadineanu, E.; Tache, E. Study of adhesive and invasion capacity of some opportunistic enterobacterial strains and interaction with probiotics. Roum. Biotechnol. Lett. 2004, 9, 1705–1711. [Google Scholar] [CrossRef]
- Plaza-Díaz, J.; Ruiz-Ojeda, F.J.; Gil-Campos, M.; Gil, A. Mechanisms of Action of Probiotics. Adv. Nutr. 2019, 10, S49–S66. [Google Scholar] [CrossRef]
- Galdeano, M.C.; Cazorla, S.I.; Lemme Dumit, J.M.; Vélez, E.; Perdigón, G. Beneficial Effects of Probiotic Consumption on the Immune System. Ann. Nutr. Metab. 2019, 74, 115–124. [Google Scholar] [CrossRef]
- Fong, F.L.; Shah, N.P.; Kirjavainen, P.; El-Nezami, H. Mechanism of Action of Probiotic Bacteria on Intestinal and Systemic Immunities and Antigen-Presenting Cells. Int. Rev. Immunol. 2016, 35, 179–188. [Google Scholar] [CrossRef]
- Kawasaki, T.; Kawai, T. Toll-like receptor signaling pathways. Front. Immunol. 2014, 5, 461. [Google Scholar] [CrossRef]
- Plessas, S.; Nouska, C.; Karapetsas, A.; Kazakos, S.; Alexopoulos, A.; Mantzourani, I.; Chondrou, P.; Fournomiti, M.; Galanis, A.; Bezirtzoglou, E. Isolation, Characterization and Evaluation of the Probiotic Potential of a Novel Lactobacillus Strain Isolated from Feta-Type Cheese. Food Chem. 2017, 226, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Terpou, A.; Mantzourani, I.; Galanis, A.; Kanellaki, M.; Bezirtzoglou, E.; Bekatorou, A.; Koutinas, A.A.; Plessas, S. Employment of L. paracasei K5 as a Novel Potentially Probiotic Freeze-Dried Starter for Feta-Type Cheese Production. Microorganisms 2018, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Mantzourani, I.; Terpou, A.; Bekatorou, A.; Mallouchos, A.; Alexopoulos, A.; Kimbaris, A.; Bezirtzoglou, E.; Koutinas, A.A.; Plessas, S. Functional pomegranate beverage production by fermentation with a novel synbiotic L. paracasei biocatalyst. Food Chem. 2020, 308, 125658. [Google Scholar] [CrossRef] [PubMed]
- Mantzourani, I.; Plessas, S.; Odatzidou, M.; Alexopoulos, A.; Galanis, A.; Bezirtzoglou, E. Effect of a novel Lactobacillus paracasei starter on sourdough bread quality. Food Chem. 2019, 271, 259–265. [Google Scholar] [CrossRef]
- Chondrou, P.; Karapetsas, A.; Kiousi, D.E.; Tsela, D.; Tiptiri-Kourpeti, A.; Anestopoulos, I.; Kotsianidis, I.; Bezirtzoglou, E.; Pappa, A.; Galanis, A. Lactobacillus Paracasei K5 Displays Adhesion, Anti-Proliferative Activity and Apoptotic Effects in Human Colon Cancer Cells. Benef. Microbes 2018, 9, 975–983. [Google Scholar] [CrossRef]
- Kotzamanidis, C.; Kourelis, A.; Litopoulou-Tzanetaki, E.; Tzanetakis, N.; Yiangou, M. Evaluation of adhesion capacity, cell surface traits and immunomodulatory activity of presumptive probiotic Lactobacillus strains. Int. J. Food Microbiol. 2010, 140, 154–163. [Google Scholar] [CrossRef]
- Saxami, G.; Karapetsas, A.; Chondrou, P.; Vasiliadis, S.; Lamprianidou, E.; Kotsianidis, I.; Ypsilantis, P.; Botaitis, S.; Simopoulos, C.; Galanis, A. Potentially Probiotic Lactobacillus Strains with Anti-Proliferative Activity Induce Cytokine/Chemokine Production and Neutrophil Recruitment in Mice. Benef. Microbes 2017, 8, 615–623. [Google Scholar] [CrossRef]
- Lenoir, M.; Del Carmen, S.; Cortes-Perez, N.G.; Lozano-Ojalvo, D.; Muñoz-Provencio, D.; Chain, F.; Langella, P.; de Moreno de LeBlanc, A.; LeBlanc, J.G.; Bermúdez-Humarán, L.G. Lactobacillus casei BL23 regulates Treg and Th17 T-cell populations and reduces DMH-associated colorectal cancer. J. Gastroenterol. 2016, 51, 862–873. [Google Scholar] [CrossRef]
- Garcia-Castillo, V.; Komatsu, R.; Clua, P.; Indo, Y.; Takagi, M.; Salva, S.; Aminul Islam, M.; Alvarez, S.; Takahashi, H.; Garcia-Cancino, A.; et al. Evaluation of the Immunomodulatory Activities of the Probiotic Strain Lactobacillus fermentum UCO-979C. Front. Immunol. 2019, 10, 1376. [Google Scholar] [CrossRef]
- Aindelis, G.; Tiptiri-Kourpeti, A.; Lampri, E.; Spyridopoulou, K.; Lamprianidou, E.; Kotsianidis, I.; Ypsilantis, P.; Pappa, A.; Chlichlia, K. Immune Responses Raised in an Experimental Colon Carcinoma Model Following Oral Administration of Lactobacillus casei. Cancers 2020, 12, 368. [Google Scholar] [CrossRef]
- Hu, J.; Wang, C.; Ye, L.; Yang, W.; Huang, H.; Meng, F.; Shi, S.; Ding, Z. Anti-tumour immune effect of oral administration of Lactobacillus plantarum to CT26 tumour-bearing mice. J. Biosci. 2015, 40, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Sheng, J.; Wang, M.; Luo, H.; Zhu, J.; Zhang, B.; Liu, Z.; Yang, X. Combination Therapy of TGF-β Blockade and Commensal-derived Probiotics Provides Enhanced Antitumor Immune Response and Tumor Suppression. Theranostics 2019, 9, 4115–4129. [Google Scholar] [CrossRef] [PubMed]
- Kourelis, A.; Zinonos, I.; Kakagianni, M.; Christidou, A.; Christoglou, N.; Yiannaki, E.; Testa, T.; Kotzamanidis, C.; Litopoulou-Tzanetaki, E.; Tzanetakis, N.; et al. Validation of the dorsal air pouch model to predict and examine immunostimulatory responses in the gut. J. Appl. Microbiol. 2010, 108, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Lü, X.; Man, C.; Han, L.; Shan, Y.; Qu, X.; Liu, Y.; Yang, S.; Xue, Y.; Zhang, Y. Lactobacillus acidophilus induces cytokine and chemokine production via NF-κB and p38 mitogen-activated protein kinase signaling pathways in intestinal epithelial cells. Clin. Vaccine Immunol. 2012, 19, 603–608. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, L.; Sun, H.; Shan, Y.; Liu, Y.; Li, L.; Qu, B.; Man, C. Induction of cytokines via NF-κB and p38 MAP kinase signalling pathways associated with the immunomodulation by Lactobacillus plantarum NDC 75017 in vitro and in vivo. J. Funct. Foods 2016, 20, 215–225. [Google Scholar] [CrossRef]
- Belguesmia, Y.; Domenger, D.; Caron, J.; Dhulster, P.; Ravallec, R.; Drider, D.; Cudennec, B. Novel probiotic evidence of lactobacilli on immunomodulation and regulation of satiety hormones release in intestinal cells. J. Funct. Foods 2016, 24, 276–286. [Google Scholar] [CrossRef]
- Fernández-Tomé, S.; Marin, A.C.; Ortega Moreno, L.; Baldan-Martin, M.; Mora-Gutiérrez, I.; Lanas-Gimeno, A.; Moreno-Monteagudo, J.A.; Santander, C.; Sánchez, B.; Chaparro, M.; et al. Immunomodulatory Effect of Gut Microbiota-Derived Bioactive Peptides on Human Immune System from Healthy Controls and Patients with Inflammatory Bowel Disease. Nutrients 2019, 11, 2605. [Google Scholar] [CrossRef]
- Delgado, S.; Sánchez, B.; Margolles, A.; Ruas-Madiedo, P.; Ruiz, L. Molecules Produced by Probiotics and Intestinal Microorganisms with Immunomodulatory Activity. Nutrients 2020, 12, 391. [Google Scholar] [CrossRef]
- Vizoso Pinto, M.G.; Rodriguez Gómez, M.; Seifert, S.; Watzl, B.; Holzapfel, W.H.; Franz, C.M. Lactobacilli stimulate the innate immune response and modulate the TLR expression of HT29 intestinal epithelial cells in vitro. Int. J. Food Microbiol. 2009, 133, 86–93. [Google Scholar] [CrossRef]
- Kingma, S.D.; Li, N.; Sun, F.; Valladares, R.B.; Neu, J.; Lorca, G.L. Lactobacillus johnsonii N6.2 stimulates the innate immune response through Toll-like receptor 9 in Caco-2 cells and increases intestinal crypt Paneth cell number in biobreeding diabetes-prone rats. J. Nutr. 2011, 141, 1023–1028. [Google Scholar] [CrossRef]
- Seifert, S.; Rodriguez Gómez, M.; Watzl, B.; Holzapfel, W.H.; Franz, C.M.; Vizoso Pinto, M.G. Differential Effect of Lactobacillus johnsonii BFE 6128 on Expression of Genes Related to TLR Pathways and Innate Immunity in Intestinal Epithelial Cells. Probiotics Antimicrob. Proteins 2010, 2, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Wachi, S.; Kanmani, P.; Tomosada, Y.; Kobayashi, H.; Yuri, T.; Egusa, S.; Shimazu, T.; Suda, Y.; Aso, H.; Sugawara, M.; et al. Lactobacillus delbrueckii TUA4408L and its extracellular polysaccharides attenuate enterotoxigenic Escherichia coli-induced inflammatory response in porcine intestinal epitheliocytes via Toll-like receptor-2 and 4. Mol. Nutr. Food Res. 2014, 58, 2080–2093. [Google Scholar] [CrossRef] [PubMed]
- Murofushi, Y.; Villena, J.; Morie, K.; Kanmani, P.; Tohno, M.; Shimazu, T.; Aso, H.; Suda, Y.; Hashiguchi, K.; Saito, T.; et al. The toll-like receptor family protein RP105/MD1 complex is involved in the immunoregulatory effect of exopolysaccharides from Lactobacillus plantarum N14. Mol. Immunol. 2015, 64, 63–75. [Google Scholar] [CrossRef] [PubMed]
| Markers | L. paracasei K5 |
|---|---|
| IL-1α | ↑ a |
| IL-1β | ↑ |
| IL-1rα | ↑ |
| IL-16 | ↑ |
| IL-6 | ↑ |
| C5a | ↓ |
| CCL2 | nd b |
| CCL3 | ↑ |
| CCL4 | ↑ |
| CCL12 | ↓ c |
| IFN-γ | nd |
| sICAM-1 | ↓ |
| CXCL1 | ↑ |
| CXCL2 | ↑ |
| CXCL12 | ↓ |
| CXCL13 | ↓ |
| G-CSF | ↑ |
| M-CSF | ↓ |
| TIMP-1 | ↓ |
| TREM-1 | ↓ |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chondrou, P.; Karapetsas, A.; Kiousi, D.E.; Vasileiadis, S.; Ypsilantis, P.; Botaitis, S.; Alexopoulos, A.; Plessas, S.; Bezirtzoglou, E.; Galanis, A. Assessment of the Immunomodulatory Properties of the Probiotic Strain Lactobacillus paracasei K5 In Vitro and In Vivo. Microorganisms 2020, 8, 709. https://doi.org/10.3390/microorganisms8050709
Chondrou P, Karapetsas A, Kiousi DE, Vasileiadis S, Ypsilantis P, Botaitis S, Alexopoulos A, Plessas S, Bezirtzoglou E, Galanis A. Assessment of the Immunomodulatory Properties of the Probiotic Strain Lactobacillus paracasei K5 In Vitro and In Vivo. Microorganisms. 2020; 8(5):709. https://doi.org/10.3390/microorganisms8050709
Chicago/Turabian StyleChondrou, Pelagia, Athanasios Karapetsas, Despoina Eugenia Kiousi, Stavros Vasileiadis, Petros Ypsilantis, Sotiris Botaitis, Athanasios Alexopoulos, Stavros Plessas, Eugenia Bezirtzoglou, and Alex Galanis. 2020. "Assessment of the Immunomodulatory Properties of the Probiotic Strain Lactobacillus paracasei K5 In Vitro and In Vivo" Microorganisms 8, no. 5: 709. https://doi.org/10.3390/microorganisms8050709
APA StyleChondrou, P., Karapetsas, A., Kiousi, D. E., Vasileiadis, S., Ypsilantis, P., Botaitis, S., Alexopoulos, A., Plessas, S., Bezirtzoglou, E., & Galanis, A. (2020). Assessment of the Immunomodulatory Properties of the Probiotic Strain Lactobacillus paracasei K5 In Vitro and In Vivo. Microorganisms, 8(5), 709. https://doi.org/10.3390/microorganisms8050709








