Effects of Atmospheric Plasma Corona Discharges on Soil Bacteria Viability
Abstract
1. Introduction
2. Materials and Methods
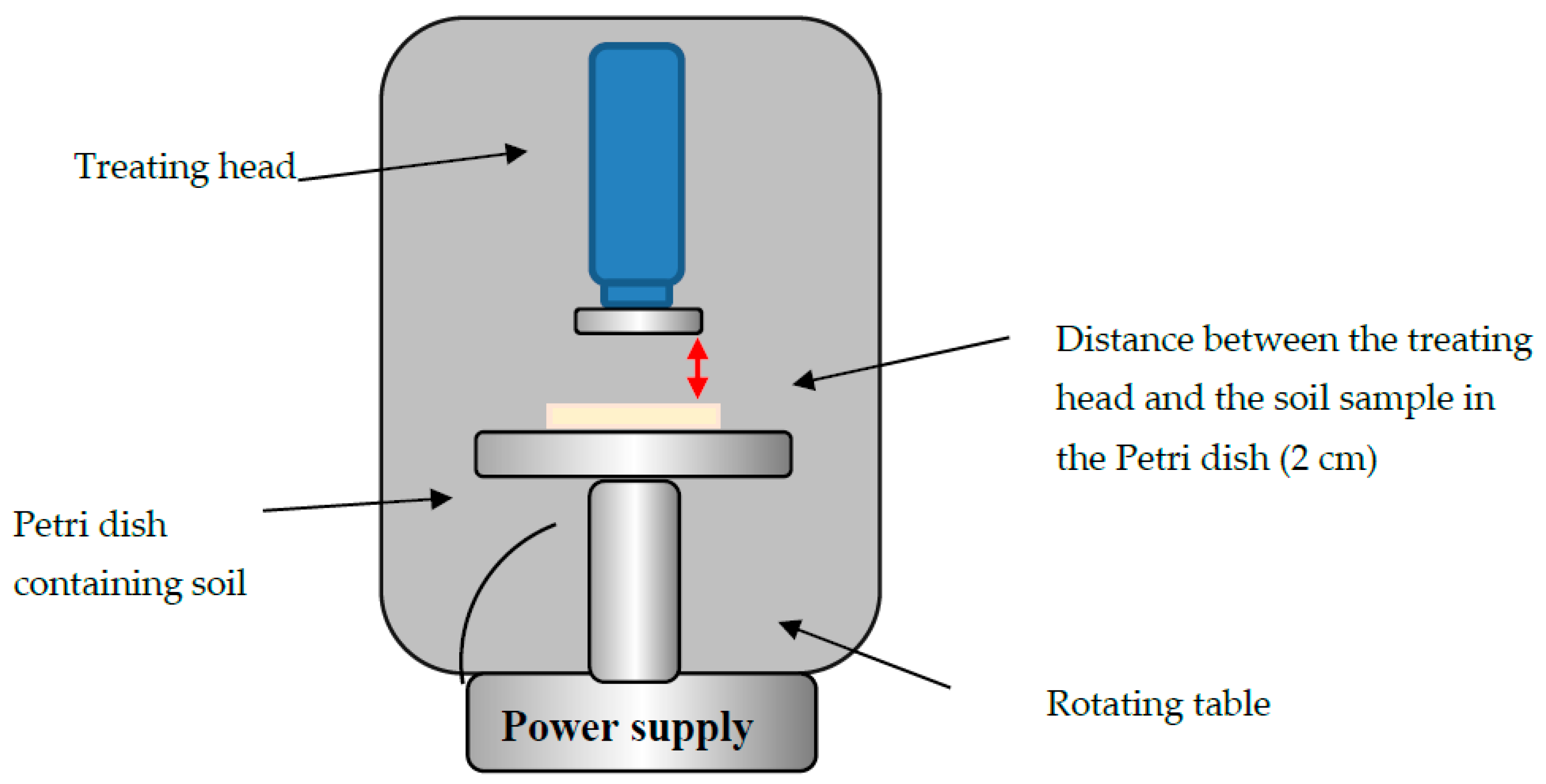
2.1. Plasma Corona Discharge System and Experimental Conditions
2.2. Soil Exposure to Corona Discharge
2.3. Viable Count Assay
2.3.1. Measurement of Bacterial Concentration in Soil as a Function of Plasma Treatment
2.3.2. Measuring the Concentration of Isolated Soil Bacteria as a Function of Plasma Treatment
2.4. Measurements of Soil Bacterial Viability
2.5. Examination of the Relative Bacterial Population Distribution as a Function of Plasma Treatment
2.6. Statistics
3. Results and Discussion
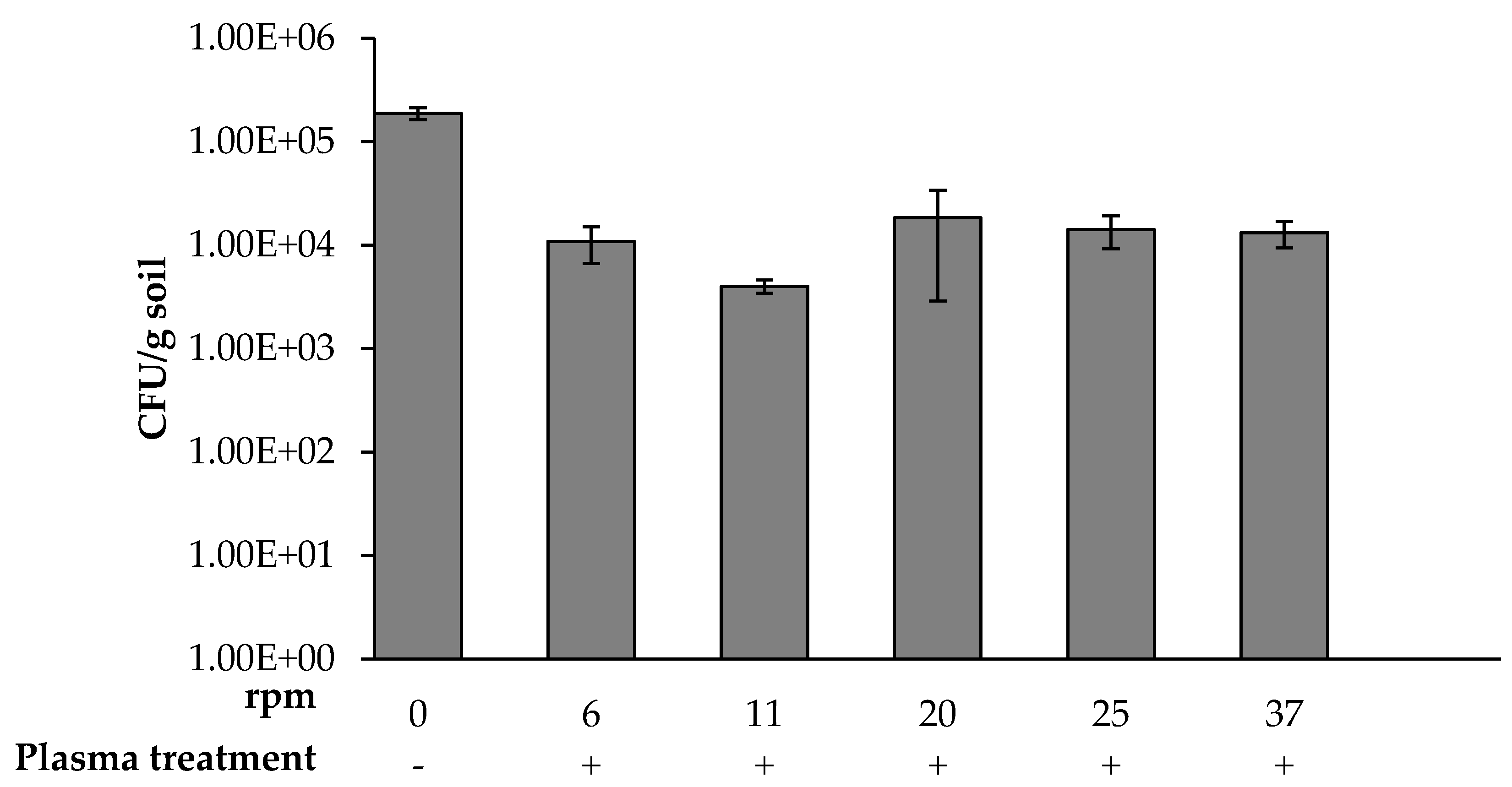
3.1. Bacterial Soil Concentration as a Function of Plasma Corona Discharge Exposure Duration and Rotation Rate
3.2. Investigating Possible Recovery of Bacterial Soil Exposed to Plasma Corona Discharges
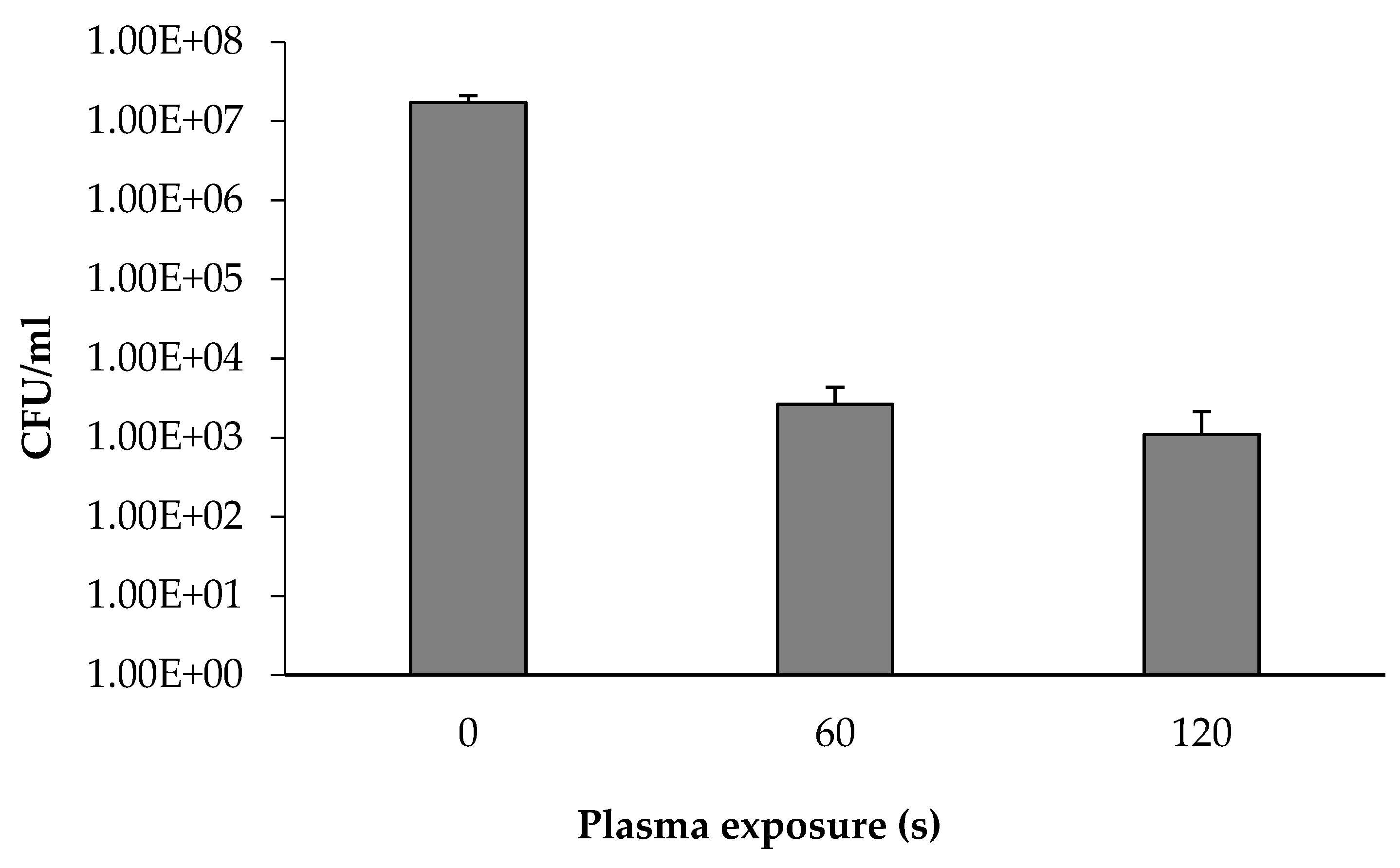
3.3. The Concentration of Isolated Soil Bacteria as a Function of Plasma Corona Discharge Exposure
3.4. Relative Distribution of the Soil Bacterial Population as a Function of Plasma Treatment
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Atidégla, S.C.; Huat, J.; Agbossou, E.K.; Saint-Macary, H.; Glèlè Kakai, R.; Kakai, R.G. Vegetable contamination by the fecal bacteria of poultry manure: Case study of gardening sites in southern Benin. Int. J. Food Sci. 2016, 2016, 1–8. [Google Scholar] [CrossRef] [PubMed]
- D’Antonio, D.; Violante, B.; Farina, C.; Sacco, R.; Angelucci, D.; Masciulli, M.; Iacone, A.; Romano, F. Necrotizing pneumonia caused by Penicillium chrysogenum. J. Clin. Microbiol. 1997, 35, 3335–3337. [Google Scholar] [CrossRef] [PubMed]
- Oerke, E.C. Crop losses to pests. J. Agric. Sci. 2006, 144, 31–43. [Google Scholar] [CrossRef]
- Stryczewska, H.D.; Ebihara, K.; Takayama, M.; Gyoutoku, Y.; Tachibana, M. Non-thermal plasma-based technology for soil treatment. Plasma Process. Polym. 2005, 2, 238–245. [Google Scholar] [CrossRef]
- Tu, C.; Ristaino, J.B.; Hu, S. Soil microbial biomass and activity in organic tomato farming systems: Effects of organic inputs and straw mulching. Soil Biol. Biochem. 2005, 38, 247–255. [Google Scholar] [CrossRef]
- McCulloch, A.; Midgley, P.M. Stratospheric Chemistry Topics: Halogen Sources, Anthropogenic. In Encyclopedia of Atmospheric Sciences, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2003; pp. 221–227. ISBN 9780123822260. [Google Scholar]
- Zhang, D.; Yan, D.; Fang, W.; Huang, B.; Wang, X.; Wang, X.; Zhu, J.; Liu, J.; Ouyang, C.; Li, Y.; et al. Chloropicrin alternated with biofumigation increases crop yield and modifies soil bacterial and fungal communities in strawberry production. Sci. Total Environ. 2019, 675, 615–622. [Google Scholar] [CrossRef]
- Wolf, D.C.; Dao, T.H.; Scott, H.D.; Lavy, T.L. Influence of sterilization methods on selected soil microbiological, physical, and chemical properties. J. Environ. Qual. 1989, 18, 39–44. [Google Scholar] [CrossRef]
- Trevors, J.T. Sterilization and inhibition of microbial activity in soil. J. Microbiol. Methods 1996, 26, 53–59. [Google Scholar] [CrossRef]
- Lees, K.; Fitzsimons, M.; Snape, J.; Tappin, A.; Comber, S. Soil sterilisation methods for use in OECD 106: How effective are they? Chemosphere 2018, 209, 61–67. [Google Scholar] [CrossRef]
- Lieberman, M.A.; Michael, A.; Lichtenberg, A.J. Principles of Plasma Discharges and Materials Processing; Wiley: Hoboken, NJ, USA, 2005; ISBN 9780471720010. [Google Scholar]
- Thomas, M.; Mittal, K.L. Atmospheric Pressure Plasma Treatment of Polymers; Thomas, M., Mittal, K., Eds.; Scrivener Publishing LLC: Salem, MA, USA, 2013; ISBN 9781118747308. [Google Scholar]
- Svarnas, P.; Giannakopoulos, E.; Kalavrouziotis, I.; Krontiras, C.; Georga, S.; Pasolari, R.S.; Papadopoulos, P.K.; Apostolou, I.; Chrysochoou, D. Sanitary effect of FE-DBD cold plasma in ambient air on sewage biosolids. Sci. Total Environ. 2020, 705, 135940. [Google Scholar] [CrossRef]
- Liao, X.; Liu, D.; Xiang, Q.; Ahn, J.; Chen, S.; Ye, X.; Ding, T. Inactivation mechanisms of non-thermal plasma on microbes: A review. Food Control. 2017, 75, 83–91. [Google Scholar] [CrossRef]
- Farber, R.; Dabush-Busheri, I.; Chaniel, G.; Rozenfeld, S.; Bormashenko, E.; Multanen, V.; Cahan, R. Biofilm grown on wood waste pretreated with cold low-pressure nitrogen plasma: Utilization for toluene remediation. Int. Biodeterior. Biodegrad. 2019, 139, 62–69. [Google Scholar] [CrossRef]
- Rozenfeld, S.; Ouaknin Hirsch, L.; Gandu, B.; Farber, R.; Schechter, A.; Cahan, R. Improvement of microbial electrolysis cell activity by using anode based on combined plasma-pretreated carbon cloth and stainless steel. Energies 2019, 12, 1968. [Google Scholar] [CrossRef]
- Bormashenko, E.; Shapira, Y.; Grynyov, R.; Whyman, G.; Bormashenko, Y.; Drori, E. Interaction of cold radiofrequency plasma with seeds of beans (Phaseolus vulgaris). J. Exp. Bot. 2015, 66, 4013–4021. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Patil, S.; Boehm, D.; Milosavljevic´, V.; Cullen, P.J.; Bourke, P. Mechanisms of inactivation by high-voltage atmospheric cold plasma differ for Escherichia coli and Staphylococcus aureus. Appl. Environ. Microbiol. 2016, 82, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Hong, F.; Guo, Y.; Zhang, J.; Shi, J. Sterilization of Staphylococcus aureus by an atmospheric non-thermal plasma jet. Plasma Sci. Technol. 2013, 15, 439–442. [Google Scholar] [CrossRef]
- Lu, H.; Patil, S.; Keener, K.M.; Cullen, P.J.; Bourke, P. Bacterial inactivation by high-voltage atmospheric cold plasma: Influence of process parameters and effects on cell leakage and DNA. J. Appl. Microbiol. 2014, 116, 784–794. [Google Scholar] [CrossRef]
- Sari, A.H.H.; Fadaee, F. Effect of corona discharge on decontamination of Pseudomonas aeruginosa and E-coli. Surf. Coat. Technol. 2010, 205, 385–390. [Google Scholar] [CrossRef]
- Banaschik, R.; Burchhardt, G.; Zocher, K.; Hammerschmidt, S.; Kolb, J.F.; Weltmann, K.D. Comparison of pulsed corona plasma and pulsed electric fields for the decontamination of water containing Legionella pneumophila as model organism. Bioelectrochemistry 2016, 112, 83–90. [Google Scholar] [CrossRef]
- Kim, J.W.; Puligundla, P.; Mok, C. Effect of corona discharge plasma jet on surface-borne microorganisms and sprouting of broccoli seeds. J. Sci. Food Agric. 2017, 97, 128–134. [Google Scholar] [CrossRef]
- Nishioka, T.; Takai, Y.; Kawaradani, M.; Okada, K.; Tanimoto, H.; Misawa, T.; Kusakari, S. Seed disinfection effect of atmospheric pressure plasma and low pressure plasma on Rhizoctonia solani. Biocontrol Sci. 2014, 19, 99–102. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nishioka, T.; Takai, Y.; Mishima, T.; Kawaradani, M.; Tanimoto, H.; Okada, K.; Misawa, T.; Kusakari, S. Low-pressure plasma application for the inactivation of the seed-borne pathogen Xanthomonas campestris. Biocontrol Sci. 2016, 21, 37–43. [Google Scholar] [CrossRef]
- Machala, Z.; Tarabova, B.; Hensel, K.; Spetlikova, E.; Sikurova, L.; Lukes, P. Formation of ROS and RNS in water electro-sprayed through transient spark discharge in air and their bactericidal effects. Plasma Process. Polym. 2013, 10, 649–659. [Google Scholar] [CrossRef]
- Lukes, P.; Dolezalova, E.; Sisrova, I.; Clupek, M. Aqueous-phase chemistry and bactericidal effects from an air discharge plasma in contact with water: Evidence for the formation of peroxynitrite through a pseudo-second-order post-discharge reaction of H2O2 and HNO2. Plasma Sources Sci. Technol. 2014, 23, 015019. [Google Scholar] [CrossRef]
- Xu, Z.; Cheng, C.; Shen, J.; Lan, Y.; Hu, S.; Han, W.; Chu, P.K. In vitro antimicrobial effects and mechanisms of direct current air-liquid discharge plasma on planktonic Staphylococcus aureus and Escherichia coli in liquids. Bioelectrochemistry 2018, 121, 125–134. [Google Scholar] [CrossRef]
- Stoffels, E.; Sakiyama, Y.; Graves, D.B. Cold Atmospheric plasma: Charged species and their interactions with cells and tissues. IEEE Trans. Plasma Sci. 2008, 36, 1441–1457. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, Z.; Shen, J.; Li, X.; Ding, L.; Ma, J.; Lan, Y.; Xia, W.; Cheng, C.; Sun, Q.; et al. Effects and mechanism of atmospheric-pressure dielectric barrier discharge cold plasma on lactate dehydrogenase (LDH) enzyme. Sci. Rep. 2015, 5, 10031. [Google Scholar] [CrossRef]
- Xu, Z.; Wei, J.; Shen, J.; Liu, Y.; Ma, R.; Zhang, Z.; Qian, S.; Ma, J.; Lan, Y.; Zhang, H.; et al. Genetic effects of an air discharge plasma on Staphylococcus aureus at the gene transcription level. Appl. Phys. Lett. 2015, 106, 213701. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Lozupone, C.A.; Turnbaugh, P.J.; Fierer, N.; Knight, R. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc. Natl. Acad. Sci. USA 2011, 108, 4516–4522. [Google Scholar] [CrossRef]
- Deng, X.; Shi, J.; Kong, M.G. Physical mechanisms of inactivation of Bacillus subtilis spores using cold atmospheric plasmas. IEEE Trans. Plasma Sci. 2006, 34, 1310–1316. [Google Scholar] [CrossRef]
- Purevdorj, D.; Igura, N.; Ariyada, O.; Hayakawa, I. Effect of feed gas composition of gas discharge plasmas on Bacillus pumilus spore mortality. Lett. Appl. Microbiol. 2003, 37, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F.; Kylián, O.; Rauscher, H.; Gilliland, D.; Sirghi, L. Use of a low-pressure plasma discharge for the decontamination and sterilization of medical devices. Pure Appl. Chem. 2008, 80, 1939–1951. [Google Scholar] [CrossRef]
- Dobrynin, D.; Fridman, G.; Friedman, G.; Fridman, A. Physical and biological mechanisms of direct plasma interaction with living tissue. New J. Phys. 2009, 11, 115020. [Google Scholar] [CrossRef]
- Kovalova, Z.; Leroy, M.; Kirkpatrick, M.J.; Odic, E.; Machala, Z. Corona discharges with water electrospray for Escherichia coli biofilm eradication on a surface. Bioelectrochemistry 2016, 112, 91–99. [Google Scholar] [CrossRef]
- Vleugels, M.; Shama, G.; Deng, X.T.; Greenacre, E.; Brocklehurst, T.; Kong, M.G. Atmospheric plasma inactivation of biofilm-forming bacteria for food safety control. IEEE Trans. Plasma Sci. 2005, 33, 824–828. [Google Scholar] [CrossRef]
- Laroussi, M. Low Temperature plasma-based sterilization: Overview and state-of-the-art. Plasma Process. Polym. 2005, 2, 391–400. [Google Scholar] [CrossRef]
- Moreau, S.; Moisan, M.; Tabrizian, M.; Barbeau, J.; Pelletier, J.; Ricard, A.; Yahia, L. Using the flowing afterglow of a plasma to inactivate Bacillus subtilis spores: Influence of the operating conditions. J. Appl. Phys. 2000, 88, 1166–1174. [Google Scholar] [CrossRef]
- Kayes, M.M.; Critzer, F.J.; Kelly-Wintenberg, K.; Roth, J.R.; Montie, T.C.; Golden, D.A. Inactivation of foodborne pathogens using A one atmosphere uniform glow discharge plasma. Foodborne Pathog. Dis. 2007, 4, 50–59. [Google Scholar] [CrossRef]
- Muranyi, P.; Wunderlich, J.; Heise, M. Influence of relative gas humidity on the inactivation efficiency of a low temperature gas plasma. J. Appl. Microbiol. 2008, 104, 1659–1666. [Google Scholar] [CrossRef]
- Perni, S.; Shama, G.; Kong, M.G. Cold atmospheric plasma disinfection of cut fruit surfaces contaminated with migrating microorganisms. J. Food Prot. 2008, 71, 1619–1625. [Google Scholar] [CrossRef]
- Yong, H.I.; Kim, H.-J.; Park, S.; Alahakoon, A.U.; Kim, K.; Choe, W.; Jo, C. Evaluation of pathogen inactivation on sliced cheese induced by encapsulated atmospheric pressure dielectric barrier discharge plasma. Food Microbiol. 2015, 46, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Edelblute, C.M.; Malik, M.A.; Heller, L.C. Antibacterial efficacy of a novel plasma reactor without an applied gas flow against methicillin resistant Staphylococcus aureus on diverse surfaces. Bioelectrochemistry 2016, 112, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Rumbach, P.; Bartels, D.M.; Sankaran, R.M.; Go, D.B. The solvation of electrons by an atmospheric-pressure plasma. Nat. Commun. 2015, 6, 8248. [Google Scholar] [CrossRef]
- Paredes-Sabja, D.; Setlow, P.; Sarker, M.R. Germination of spores of Bacillales and Clostridiales species: Mechanisms and proteins involved. Trends Microbiol. 2011, 19, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Piggot, P.J.; Hilbert, D.W. Sporulation of Bacillus subtilis. Curr. Opin. Microbiol. 2004, 7, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Hugenholtz, P. Exploring prokaryotic diversity in the genomic era. Genome Biol. 2002, 3. [Google Scholar] [CrossRef] [PubMed]
- Remize, F. Spore-Forming Bacteria. In The Microbiological Quality of Food: Foodborne Spoilers; Elsevier: Amsterdam, The Netherlands, 2017; pp. 99–120. ISBN 9780081005033. [Google Scholar]
- Tocheva, E.I.; Ortega, D.R.; Jensen, G.J. Sporulation, bacterial cell envelopes and the origin of life. Nat. Rev. Microbiol. 2016, 14, 535–542. [Google Scholar] [CrossRef]
- Decker, A.R.; Ramamurthi, K.S. Cell death pathway that monitors spore morphogenesis. Trends Microbiol. 2017, 25, 637–647. [Google Scholar] [CrossRef]
- Tseng, S.; Abramzon, N.; Jackson, J.O.; Lin, W.-J. Gas discharge plasmas are effective in inactivating Bacillus and Clostridium spores. Appl. Microbiol. Biotechnol. 2012, 93, 2563–2570. [Google Scholar] [CrossRef]
- Lunov, O.; Zablotskii, V.; Churpita, O.; Jäger, A.; Polívka, L.; Syková, E.; Dejneka, A.; Kubinová, Š. The interplay between biological and physical scenarios of bacterial death induced by non-thermal plasma. Biomaterials 2016, 82, 71–83. [Google Scholar] [CrossRef]
- Venezia, R.A.; Orrico, M.; Houston, E.; Yin, S.-M.; Naumova, Y.Y. Lethal activity of nonthermal plasma sterilization against microorganisms. Infect. Control Hosp. Epidemiol. 2008, 29, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Agrios, G.N. Plant Pathology, 5th ed.; Elsevier Academic Press: Burlington, NJ, USA, 2005; p. 922. [Google Scholar]
- Katan, J.; Greenbeger, A.; Alon, H.; Grinstein, A. Solar heating by polyethylene mulching for the control of disease caused by soil-borne pathogen. Phytopathology 1976, 66, 683–688. [Google Scholar] [CrossRef]
- Simmons, C.W.; Claypool, J.T.; Marshall, M.N.; Jabusch, L.K.; Reddy, A.P.; Simmons, B.A.; Singer, S.W.; Stapleton, J.J.; Vander Gheynst, J.S. Characterization of bacterial communities in solarized soil amended with lignocellulosic organic matter. Appl. Soil Ecol. 2014, 73, 97–104. [Google Scholar] [CrossRef]
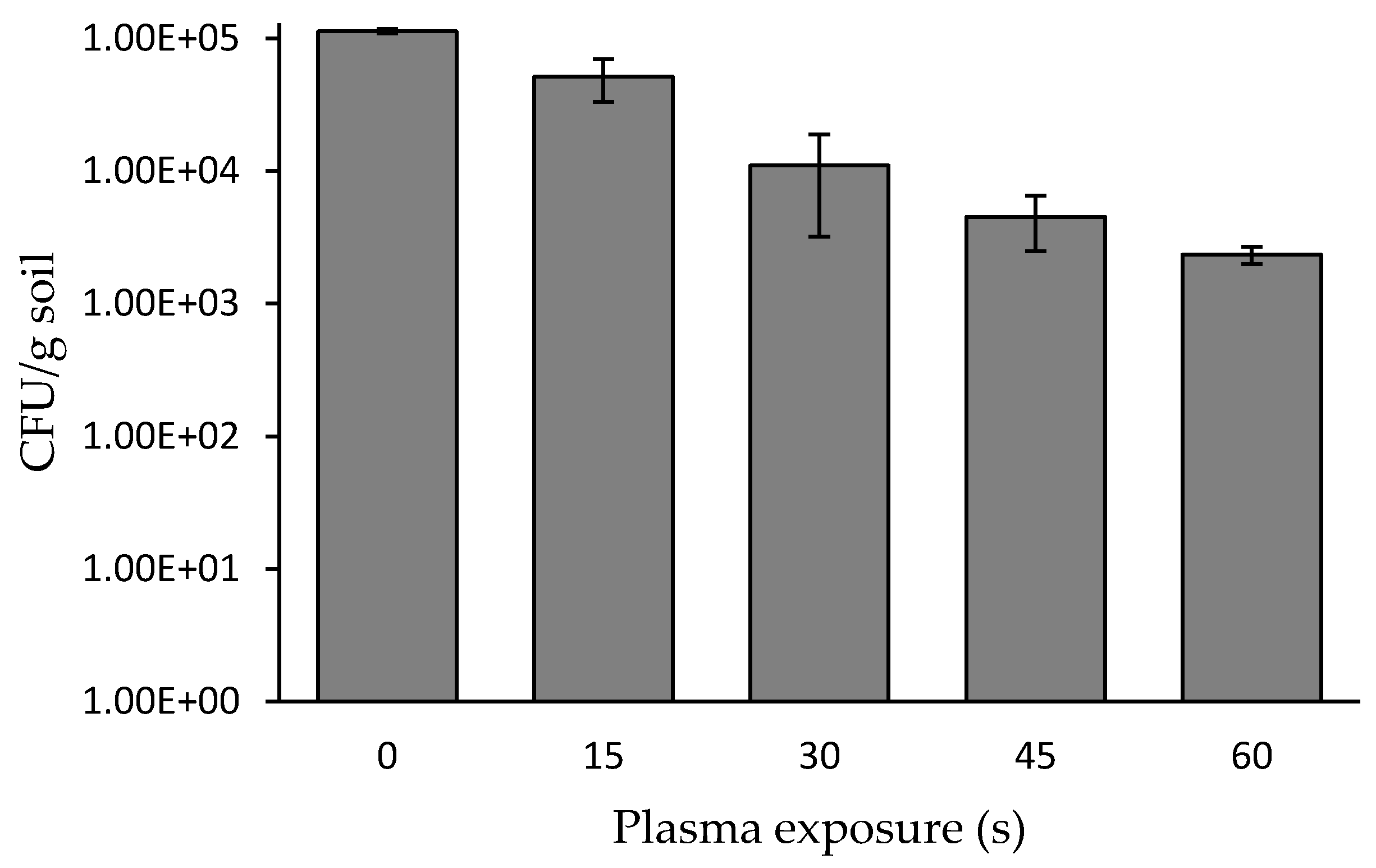
 ), and the control sample not exposed to plasma (
), and the control sample not exposed to plasma (  ). * p < 0.005 by Student’s t-test.
). * p < 0.005 by Student’s t-test.
 ), and the control sample not exposed to plasma (
), and the control sample not exposed to plasma (  ). * p < 0.005 by Student’s t-test.
). * p < 0.005 by Student’s t-test.

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazra, Y.; Dubrovin, I.; Multanen, V.; Bormashenko, E.; Bormashenko, Y.; Cahan, R. Effects of Atmospheric Plasma Corona Discharges on Soil Bacteria Viability. Microorganisms 2020, 8, 704. https://doi.org/10.3390/microorganisms8050704
Lazra Y, Dubrovin I, Multanen V, Bormashenko E, Bormashenko Y, Cahan R. Effects of Atmospheric Plasma Corona Discharges on Soil Bacteria Viability. Microorganisms. 2020; 8(5):704. https://doi.org/10.3390/microorganisms8050704
Chicago/Turabian StyleLazra, Yulia, Irina Dubrovin, Victor Multanen, Edward Bormashenko, Yelena Bormashenko, and Rivka Cahan. 2020. "Effects of Atmospheric Plasma Corona Discharges on Soil Bacteria Viability" Microorganisms 8, no. 5: 704. https://doi.org/10.3390/microorganisms8050704
APA StyleLazra, Y., Dubrovin, I., Multanen, V., Bormashenko, E., Bormashenko, Y., & Cahan, R. (2020). Effects of Atmospheric Plasma Corona Discharges on Soil Bacteria Viability. Microorganisms, 8(5), 704. https://doi.org/10.3390/microorganisms8050704





