The Role of Bacterial Membrane Vesicles in the Dissemination of Antibiotic Resistance and as Promising Carriers for Therapeutic Agent Delivery
Abstract
1. Introduction
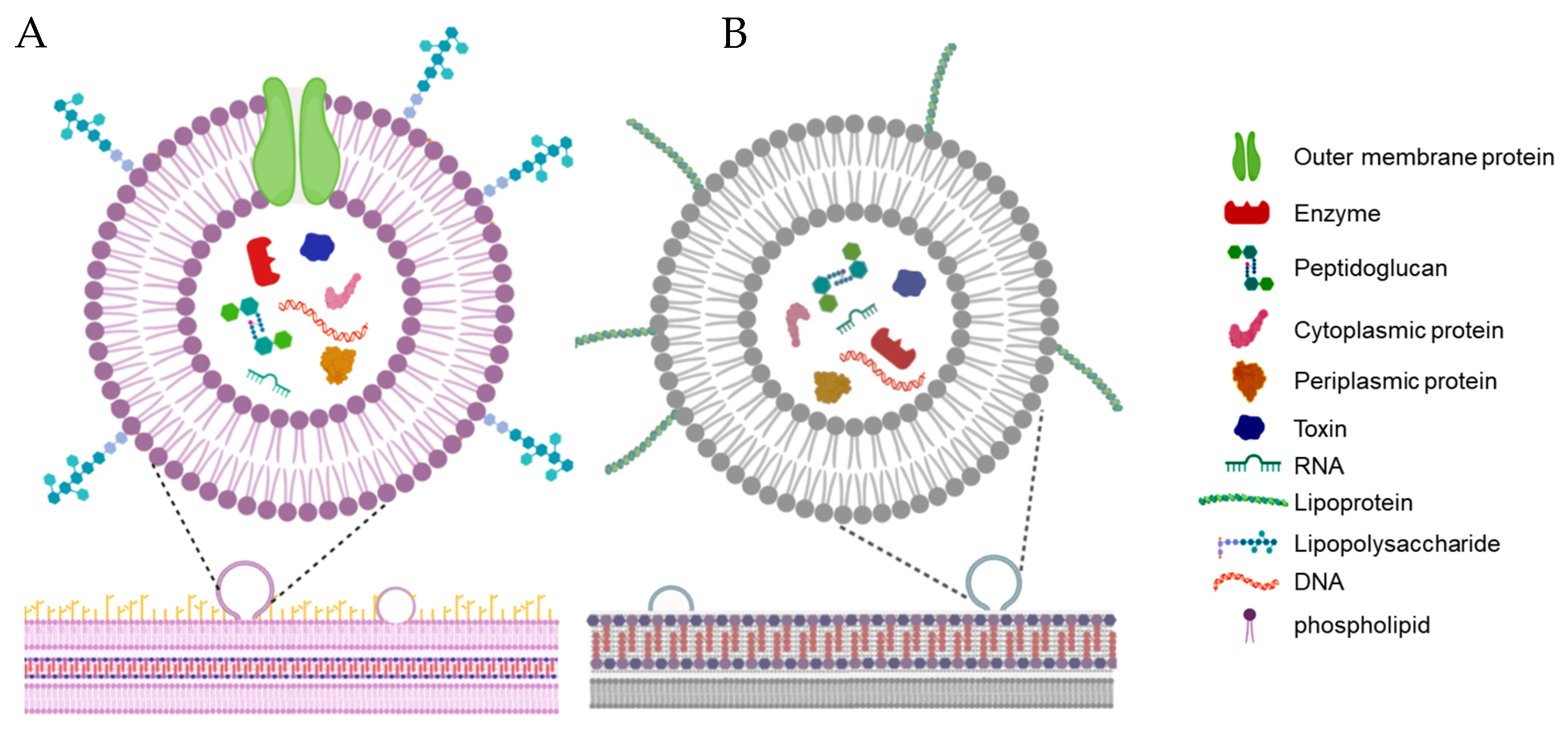
2. Terminology and Characteristics of Bacterial Membrane Vesicles
3. Isolation and Purification of Bacterial Membrane Vesicles
4. Biogenesis of Bacterial Membrane Vesicles
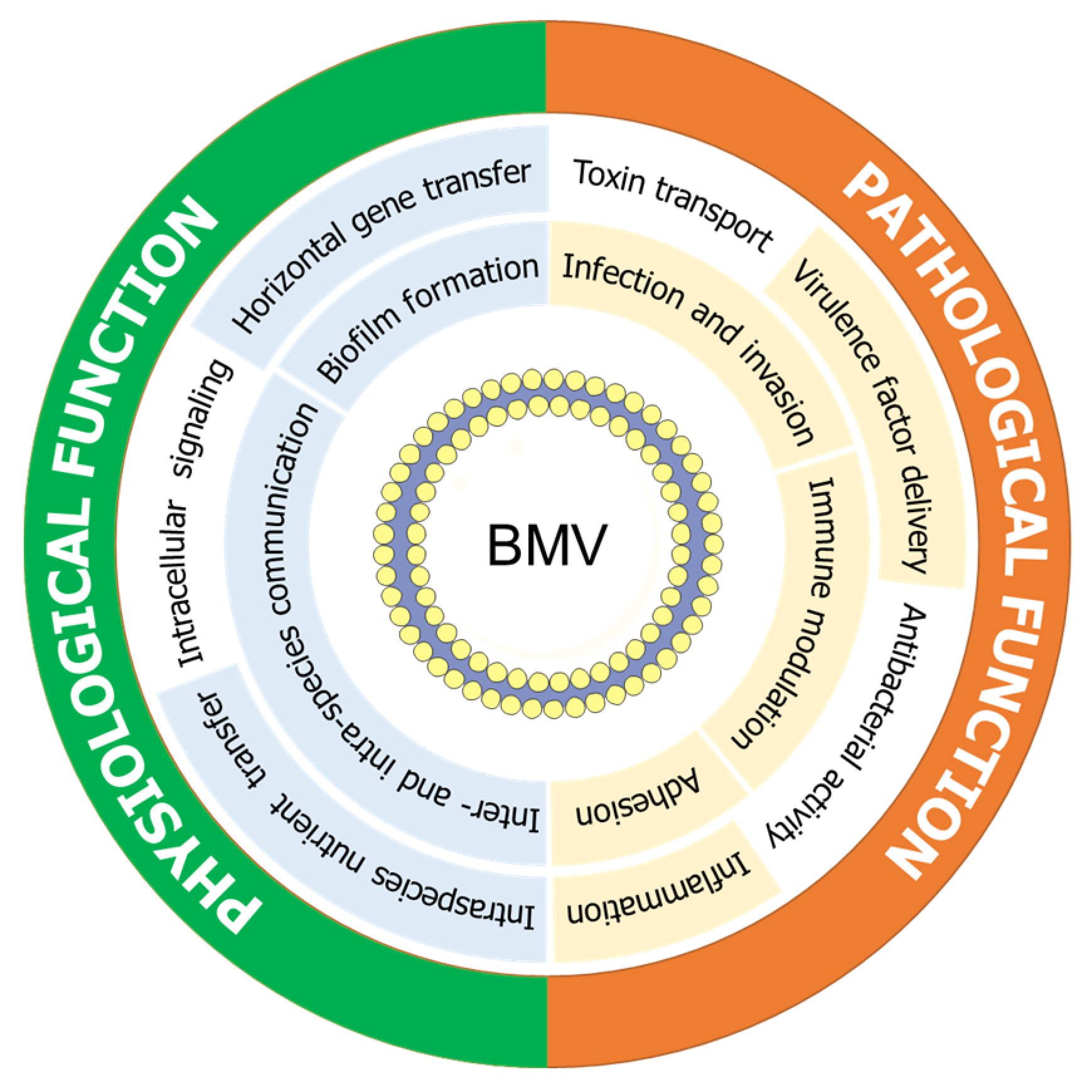
5. Biological Functions of Bacterial Membrane Vesicles
6. Gene Transfer Potential of Bacterial Membrane Vesicles
7. Proteomic Properties of Bacterial Membrane Vesicles
8. Bacterial Membrane Vesicle-Based Therapeutic Approaches
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chattopadhyay, M.K.; Jaganandham, M.V. Vesicles-mediated resistance to antibiotics in bacteria. Front. Microbiol. 2015, 6, 758. [Google Scholar] [CrossRef]
- Ghai, I.; Ghai, S. Understanding antibiotic resistance via outer membrane permeability. Infect. Drug Resist. 2018, 11, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Zgurskaya, H.I.; Rybenkov, V.V.; Krishnamoorthy, G.; Leus, I.V. Trans-envelope multidrug efflux pumps of Gram-negative bacteria and their synergism with the outer membrane barrier. Res. Microbiol. 2018, 169, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Podolsky, S.H. The evolving response to antibiotic resistance (1945–2018). Palgrave Commun. 2018, 4, 124. [Google Scholar] [CrossRef]
- Bonnington, K.E.; Kuehn, M.J. Outer membrane vesicle production facilitates LPS remodeling and outer membrane maintenance in Salmonella during environmental transitions. mBio 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Kim, S.I.; Ryu, S.; Yoon, H. Identification and characterization of outer membrane vesicle-associated proteins in Salmonella enterica serovar Typhimurium. Infect. Immun. 2014, 82, 4001–4010. [Google Scholar] [CrossRef] [PubMed]
- Beceiro, A.; Tomás, M.; Bou, G. Antimicrobial resistance and virulence: A successful or deleterious association in the bacterial world? Clin. Microbiol. Rev. 2013, 26, 185–230. [Google Scholar] [CrossRef]
- Deatherage, B.L.; Cookson, B.T. Membrane vesicle release in bacteria, eukaryotes, and archaea: A Conserved yet underappreciated aspect of microbial life. Infect. Immun. 2012, 80, 1948–1957. [Google Scholar] [CrossRef]
- Camussi, G.; Deregibus, M.C.; Bruno, S.; Cantaluppi, V.; Biancone, L. Exosomes/microvesicles as a mechanism of cell-to-cell communication. Kidney Int. 2010, 78, 838–848. [Google Scholar] [CrossRef]
- Brown, L.; Wolf, J.M.; Prados-Rosales, R.; Casadevall, A. Through the wall: Extracellular vesicles in Gram-positive bacteria, mycobacteria and fungi. Nat. Rev. Microbiol. 2015, 13, 620–630. [Google Scholar] [CrossRef]
- Yang, Y.; Hong, Y.; Cho, E.; Kim, G.B.; Kim, I.-S. Extracellular vesicles as a platform for membrane-associated therapeutic protein delivery. J. Extracell. Ves. 2018, 7, 1440131. [Google Scholar] [CrossRef]
- Dorward, D.W.; Garon, C.F.; Judd, R.C. Export and intercellular transfer of DNA via membrane blebs of Neisseria gonorrhoeae. J. Bacteriol. 1989, 171, 2499–2505. [Google Scholar] [CrossRef] [PubMed]
- Jan, A.T. Outer membrane vesicles (OMVs) of Gram-negative bacteria: A perspective update. Front. Microbiol. 2017, 8, 1053. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Pillai, J. Bacterial membrane vesicles as novel nanosystems for drug delivery. Int. J. Nanomed. 2017, 12, 6329–6341. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.H.; Park, E.C.; Lee, S.-Y.; Lee, H.; Choi, C.-W.; Yi, Y.-S.; Ro, H.-J.; Lee, J.C.; Jun, S.; Kim, H.-Y.; et al. Antibiotic treatment modulates protein components of cytotoxic outer membrane vesicles of multidrug-resistant clinical strain, Acinetobacter baumannii DU202. Clin. Proteom. 2018, 15, 28. [Google Scholar] [CrossRef]
- Dauros Singorenko, P.; Chang, V.; Whitcombe, A.; Simonov, D.; Hong, J.; Phillips, A.; Swift, S.; Blenkiron, C. Isolation of membrane vesicles from prokaryotes: A technical and biological comparison reveals heterogeneity. J. Extracell. Ves. 2017, 6, 1324731. [Google Scholar] [CrossRef]
- Orench-Rivera, N.; Kuehn, M.J. Environmentally controlled bacterial vesicle-mediated export. Cell. Microbiol. 2016, 18, 1525–1536. [Google Scholar] [CrossRef]
- Chutkan, H.; MacDonald, I.; Manning, A.; Kuehn, M.J. Quantitative and qualitative preparations of bacterial outer membrane vesicles. In Bacterial Cell Surfaces; Springer: Berlin/Heidelberg, Germany, 2013; pp. 259–272. [Google Scholar]
- Nühse, T.S.; Stensballe, A.; Jensen, O.N.; Peck, S.C. Large-scale analysis of in vivo phosphorylated membrane proteins by immobilized metal ion affinity chromatography and mass spectrometry. Mol. Cell. Proteom. 2003, 2, 1234–1243. [Google Scholar] [CrossRef]
- Livshits, M.A.; Khomyakova, E.; Evtushenko, E.G.; Lazarev, V.N.; Kulemin, N.A.; Semina, S.E.; Generozov, E.V.; Govorun, V.M. Isolation of exosomes by differential centrifugation: Theoretical analysis of a commonly used protocol. Sci. Rep. 2015, 5, 17319. [Google Scholar] [CrossRef]
- Fernández-Llama, P.; Khositseth, S.; Gonzales, P.A.; Star, R.A.; Pisitkun, T.; Knepper, M.A. Tamm-Horsfall protein and urinary exosome isolation. Kidney Int. 2010, 77, 736–742. [Google Scholar] [CrossRef]
- Abramowicz, A.; Widlak, P.; Pietrowska, M. Proteomic analysis of exosomal cargo: The challenge of high purity vesicle isolation. Mol. Biosyst. 2016, 12, 1407–1419. [Google Scholar] [CrossRef] [PubMed]
- Lobb, R.J.; Becker, M.; Wen Wen, S.; Wong, C.S.; Wiegmans, A.P.; Leimgruber, A.; Möller, A. Optimized exosome isolation protocol for cell culture supernatant and human plasma. J. Extracell. Ves. 2015, 4, 27031. [Google Scholar] [CrossRef] [PubMed]
- Spatz, D.D.; Friedlander, R. Ultrafiltration—The membranes, the process and its application to organic molecule fractionation. In Ultrafiltration Membranes and Applications; Springer: Berlin/Heidelberg, Germany, 1980; p. 603. [Google Scholar]
- Junker, K.; Heinzelmann, J.; Beckham, C.; Ochiya, T.; Jenster, G. Extracellular vesicles and their role in urologic malignancies. Eur. Urol. 2016, 70, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Salih, M.; Zietse, R.; Hoorn, E.J. Urinary extracellular vesicles and the kidney: Biomarkers and beyond. Am. J. Physiol.-Ren. Physiol. 2014, 306, F1251–F1259. [Google Scholar] [CrossRef] [PubMed]
- Nordin, J.Z.; Lee, Y.; Vader, P.; Mäger, I.; Johansson, H.J.; Heusermann, W.; Wiklander, O.P.; Hällbrink, M.; Seow, Y.; Bultema, J.J. Ultrafiltration with size-exclusion liquid chromatography for high yield isolation of extracellular vesicles preserving intact biophysical and functional properties. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 879–883. [Google Scholar] [CrossRef]
- Klimentová, J.; Stulík, J. Methods of isolation and purification of outer membrane vesicles from gram-negative bacteria. Microbiol. Res. 2015, 170, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Shin, H.; Kim, J.; Kim, J.; Park, J. Isolation of high-purity extracellular vesicles by extracting proteins using aqueous two-phase system. PLoS ONE 2015, 10, e0129760. [Google Scholar] [CrossRef]
- Shin, H.; Han, C.; Labuz, J.M.; Kim, J.; Kim, J.; Cho, S.; Gho, Y.S.; Takayama, S.; Park, J. High-yield isolation of extracellular vesicles using aqueous two-phase system. Sci. Rep. 2015, 5, 13103. [Google Scholar] [CrossRef]
- Konoshenko, M.Y.; Lekchnov, E.A.; Vlassov, A.V.; Laktionov, P.P. Isolation of extracellular vesicles: General methodologies and latest trends. Biomed Res. Int. 2018, 2018, 8545347. [Google Scholar] [CrossRef]
- Ingato, D.; Lee, J.U.; Sim, S.J.; Kwon, Y.J. Good things come in small packages: Overcoming challenges to harness extracellular vesicles for therapeutic delivery. J. Control. Release 2016, 241, 174–185. [Google Scholar] [CrossRef]
- Alves, N.J.; Turner, K.B.; DiVito, K.A.; Daniele, M.A.; Walper, S.A. Affinity purification of bacterial outer membrane vesicles (OMVs) utilizing a His-tag mutant. Res. Microbiol. 2017, 168, 139–146. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Zeng, Y. Microfluidic exosome analysis toward liquid biopsy for cancer. J. Lab. Autom. 2016, 21, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.-C.; Tao, S.-C.; Dawn, H. Microfluidics-based on-a-chip systems for isolating and analysing extracellular vesicles. J. Extracell. Ves. 2018, 7, 1508271. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.; Catchpole, R.; Forterre, P. Extracellular membrane vesicles in the three domains of life and beyond. Fems Microbiol. Rev. 2018, 43, 273–303. [Google Scholar] [CrossRef] [PubMed]
- Schwechheimer, C.; Sullivan, C.J.; Kuehn, M.J. Envelope control of outer membrane vesicle production in Gram-negative bacteria. Biochemistry 2013, 52, 3031–3040. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Defourny, K.A.Y.; Smid, E.J.; Abee, T. Gram-positive bacterial extracellular vesicles and their impact on health and disease. Front. Microbiol. 2018, 9, 1502. [Google Scholar] [CrossRef] [PubMed]
- Haurat, M.F.; Elhenawy, W.; Feldman Mario, F. Prokaryotic membrane vesicles: New insights on biogenesis and biological roles. Biol. Chem. 2015, 396, 95. [Google Scholar] [CrossRef]
- Berleman, J.; Auer, M. The role of bacterial outer membrane vesicles for intra- and interspecies delivery. Environ. Microbiol. 2013, 15, 347–354. [Google Scholar] [CrossRef]
- Jagannadham, M.V.; Chattopadhyay, M.K. Role of outer membrane vesicles of bacteria. Resonance 2015, 20, 711–725. [Google Scholar] [CrossRef]
- McBroom, A.J.; Kuehn, M.J. Release of outer membrane vesicles by Gram-negative bacteria is a novel envelope stress response. Mol. Microbiol. 2007, 63, 545–558. [Google Scholar] [CrossRef]
- Bartolini, E.; Ianni, E.; Frigimelica, E.; Petracca, R.; Galli, G.; Scorza, F.B.; Norais, N.; Laera, D.; Giusti, F.; Pierleoni, A.; et al. Recombinant outer membrane vesicles carrying Chlamydia muridarum HtrA induce antibodies that neutralize chlamydial infection in vitro. J. Extracell. Ves. 2013, 2, 20181. [Google Scholar] [CrossRef]
- Lagos, L.; Tandberg, J.I.; Repnik, U.; Boysen, P.; Ropstad, E.; Varkey, D.; Paulsen, I.T.; Winther-Larsen, H.C. Characterization and vaccine potential of membrane vesicles produced by Francisella noatunensis subsp. orientalis in an adult zebrafish model. Clin. Vaccine Immunol. 2017, 24, e00557-16. [Google Scholar]
- Kulp, A.; Kuehn, M.J. Biological functions and biogenesis of secreted bacterial outer membrane vesicles. Ann. Rev. Microbiol. 2010, 64, 163–184. [Google Scholar] [CrossRef] [PubMed]
- Horspool, A.M.; Schertzer, J.W. Reciprocal cross-species induction of outer membrane vesicle biogenesis via secreted factors. Sci. Rep. 2018, 8, 9873. [Google Scholar] [CrossRef] [PubMed]
- Bonnington, K.E.; Kuehn, M.J. Protein selection and export via outer membrane vesicles. Biochim. Biophys. Acta 2014, 1843, 1612–1619. [Google Scholar] [CrossRef] [PubMed]
- McBroom, A.J.; Johnson, A.P.; Vemulapalli, S.; Kuehn, M.J. Outer membrane vesicle production by Escherichia coli is independent of membrane instability. J. Bacteriol. 2006, 188, 5385–5392. [Google Scholar] [CrossRef]
- Raivio, T.L. Envelope stress responses and Gram-negative bacterial pathogenesis. Mol. Microbiol. 2005, 56, 1119–1128. [Google Scholar] [CrossRef]
- Rhodius, V.A.; Suh, W.C.; Nonaka, G.; West, J.; Gross, C.A. Conserved and variable functions of the sigmaE stress response in related genomes. PLoS Biol. 2006, 4, e2. [Google Scholar] [CrossRef]
- Song, T.; Mika, F.; Lindmark, B.; Liu, Z.; Schild, S.; Bishop, A.; Zhu, J.; Camilli, A.; Johansson, J.; Vogel, J.; et al. A new Vibrio cholerae sRNA modulates colonization and affects release of outer membrane vesicles. Mol. Microbiol. 2008, 70, 100–111. [Google Scholar] [CrossRef]
- Udekwu, K.I.; Darfeuille, F.; Vogel, J.; Reimegård, J.; Holmqvist, E.; Wagner, E.G.H. Hfq-dependent regulation of OmpA synthesis is mediated by an antisense RNA. Genes Dev. 2005, 19, 2355–2366. [Google Scholar] [CrossRef]
- Papenfort, K.; Pfeiffer, V.; Mika, F.; Lucchini, S.; Hinton, J.C.D.; Vogel, J. SigmaE-dependent small RNAs of Salmonella respond to membrane stress by accelerating global omp mRNA decay. Mol. Microbiol. 2006, 62, 1674–1688. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.-H.; Choi, C.-W.; Kwon, S.-O.; Park, G.W.; Cho, K.; Kwon, K.-H.; Kim, J.Y.; Yoo, J.S.; Lee, J.C.; Choi, J.-S.; et al. Quantitative proteomic analysis of cell wall and plasma membrane fractions from multidrug-resistant Acinetobacter baumannii. J. Proteome Res. 2011, 10, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Ghai, I.; Ghai, S. Exploring bacterial outer membrane barrier to combat bad bugs. Infect. Drug Resist. 2017, 10, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Trimble, M.J.; Mlynárčik, P.; Kolář, M.; Hancock, R.E.W. Polymyxin: Alternative mechanisms of action and resistance. Cold Spring Harb. Perspect. Med. 2016, 6, a025288. [Google Scholar] [CrossRef]
- Dutta, S.; Iida, K.-i.; Takade, A.; Meno, Y.; Nair, G.B.; Yoshida, S.-I. Release of shiga toxin by membrane vesicles in Shigella dysenteriae serotype 1 strains and in vitro effects of antimicrobials on toxin production and release. Microbiol. Immunol. 2004, 48, 965–969. [Google Scholar] [CrossRef]
- MacDonald, I.A.; Kuehn, M.J. Stress-induced outer membrane vesicle production by Pseudomonas aeruginosa. J. Bacteriol. 2013, 195, 2971–2981. [Google Scholar] [CrossRef]
- Bauwens, A.; Kunsmann, L.; Karch, H.; Mellmann, A.; Bielaszewska, M. Antibiotic-mediated modulations of outer membrane vesicles in Enterohemorrhagic Escherichia coli O104:H4 and O157:H7. Antimicrob. Agents Chemother. 2017, 61, e00937. [Google Scholar] [CrossRef]
- Sabnis, A.; Ledger, E.V.K.; Pader, V.; Edwards, A.M. Antibiotic interceptors: Creating safe spaces for bacteria. PLoS Path. 2018, 14, e1006924. [Google Scholar] [CrossRef]
- Lee, J.; Kim, S.-H.; Choi, D.-S.; Lee, J.S.; Kim, D.-K.; Go, G.; Park, S.-M.; Kim, S.H.; Shin, J.H.; Chang, C.L.; et al. Proteomic analysis of extracellular vesicles derived from Mycobacterium tuberculosis. Proteomics 2015, 15, 3331–3337. [Google Scholar] [CrossRef]
- Pader, V.; Hakim, S.; Painter, K.L.; Wigneshweraraj, S.; Clarke, T.B.; Edwards, A.M. Staphylococcus aureus inactivates daptomycin by releasing membrane phospholipids. Nat. Microbiol. 2016, 2, 16194. [Google Scholar] [CrossRef]
- Ledger, E.V.K.; Pader, V.; Edwards, A.M. Enterococcus faecalis and pathogenic streptococci inactivate daptomycin by releasing phospholipids. Microbiology 2017, 163, 1502–1508. [Google Scholar] [CrossRef]
- Manning, A.J.; Kuehn, M.J. Contribution of bacterial outer membrane vesicles to innate bacterial defense. BMC Microbiol. 2011, 11, 258. [Google Scholar] [CrossRef] [PubMed]
- Marsh, D. Thermodynamics of phospholipid self-assembly. Biophys. J. 2012, 102, 1079–1087. [Google Scholar] [CrossRef] [PubMed]
- Schaar, V.; Nordström, T.; Mörgelin, M.; Riesbeck, K. Moraxella catarrhalis outer membrane vesicles carry β-lactamase and promote survival of Streptococcus pneumoniae and Haemophilus influenzae by inactivating amoxicillin. Antimicrob. Agents Chemother. 2011, 55, 3845–3853. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, H.M.; Nagaraj, R.; Jagannadham, M.V. Protective role of E. coli outer membrane vesicles against antibiotics. Microbiol. Res. 2015, 181, 1–7. [Google Scholar] [CrossRef]
- Schwechheimer, C.; Kuehn, M.J. Outer-membrane vesicles from Gram-negative bacteria: Biogenesis and functions. Nat. Rev. Microbiol. 2015, 13, 605–619. [Google Scholar] [CrossRef]
- Campos, M.A.; Vargas, M.A.; Regueiro, V.; Llompart, C.M.; Albertí, S.; Bengoechea, J.A. Capsule polysaccharide mediates bacterial resistance to antimicrobial peptides. Infect. Immun. 2004, 72, 7107–7114. [Google Scholar] [CrossRef]
- Jones, A.; Geörg, M.; Maudsdotter, L.; Jonsson, A.-B. Endotoxin, capsule, and bacterial attachment contribute to Neisseria meningitidis resistance to the human antimicrobial peptide LL-37. J. Bacteriol. 2009, 191, 3861–3868. [Google Scholar] [CrossRef]
- Llobet, E.; Tomás, J.M.; Bengoechea, J.A. Capsule polysaccharide is a bacterial decoy for antimicrobial peptides. Microbiology 2008, 154, 3877–3886. [Google Scholar] [CrossRef]
- Geisinger, E.; Isberg, R.R. Antibiotic modulation of capsular exopolysaccharide and virulence in Acinetobacter baumannii. PLoS Path. 2015, 11, e1004691. [Google Scholar] [CrossRef]
- Billings, N.; Ramirez Millan, M.; Caldara, M.; Rusconi, R.; Tarasova, Y.; Stocker, R.; Ribbeck, K. The extracellular matrix component psl provides fast-acting antibiotic defense in Pseudomonas aeruginosa biofilms. PLoS Path. 2013, 9, e1003526. [Google Scholar] [CrossRef] [PubMed]
- Chiang, W.-C.; Nilsson, M.; Jensen, P.Ø.; Høiby, N.; Nielsen, T.E.; Givskov, M.; Tolker-Nielsen, T. Extracellular DNA shields against aminoglycosides in Pseudomonas aeruginosa biofilms. Antimicrob. Agents Chemother. 2013, 57, 2352–2361. [Google Scholar] [CrossRef] [PubMed]
- Doroshenko, N.; Tseng, B.S.; Howlin, R.P.; Deacon, J.; Wharton, J.A.; Thurner, P.J.; Gilmore, B.F.; Parsek, M.R.; Stoodley, P. Extracellular DNA impedes the transport of vancomycin in Staphylococcus epidermidis biofilms preexposed to subinhibitory concentrations of vancomycin. Antimicrob. Agents Chemother. 2014, 58, 7273–7282. [Google Scholar] [CrossRef] [PubMed]
- Elhenawy, W.; Bording-Jorgensen, M.; Valguarnera, E.; Haurat, M.F.; Wine, E.; Feldman, M.F. LPS remodeling triggers formation of outer membrane vesicles in Salmonella. mBio 2016, 7, e00940-16. [Google Scholar] [CrossRef] [PubMed]
- Tashiro, Y.; Inagaki, A.; Shimizu, M.; Ichikawa, S.; Takaya, N.; Nakajima-Kambe, T.; Uchiyama, H.; Nomura, N. Characterization of phospholipids in membrane vesicles derived from Pseudomonas aeruginosa. Biosci. Biotechnol. Biochem. 2011, 75, 605–607. [Google Scholar] [CrossRef] [PubMed]
- Ciofu, O.; Beveridge, T.J.; Kadurugamuwa, J.; Walther-Rasmussen, J.; Høiby, N. Chromosomal β-lactamase is packaged into membrane vesicles and secreted from Pseudomonas aeruginosa. J. Antimicrob. Chemother. 2000, 45, 9–13. [Google Scholar] [CrossRef]
- Eddy, J.L.; Gielda, L.M.; Caulfield, A.J.; Rangel, S.M.; Lathem, W.W. Production of outer membrane vesicles by the plague pathogen Yersinia pestis. PLoS ONE 2014, 9, e107002. [Google Scholar] [CrossRef]
- Chan, K.W.; Shone, C.; Hesp, J.R. Antibiotics and iron-limiting conditions and their effect on the production and composition of outer membrane vesicles secreted from clinical isolates of extraintestinal pathogenic E. coli. Proteomics 2017, 11, 1600091. [Google Scholar] [CrossRef]
- Wang, S.; Gao, J.; Wang, Z. Outer membrane vesicles for vaccination and targeted drug delivery. Nanomed Nanobiotechnol 2018, 11, e1523. [Google Scholar] [CrossRef]
- Baker, S.; Davitt, C.; Morici, L. Gram-negative bacterial outer membrane vesicles inhibit growth of multidrug-resistant organisms and induce wound-healing cytokines. Open Forum Infect. Dis. 2016, 3, 2242. [Google Scholar] [CrossRef]
- Pan, K.-L.; Hsiao, H.-C.; Weng, C.-L.; Wu, M.-S.; Chou, C.P. Roles of DegP in prevention of protein misfolding in the periplasm upon overexpression of penicillin acylase in Escherichia coli. J. Bacteriol. 2003, 185, 3020–3030. [Google Scholar] [CrossRef] [PubMed]
- Tashiro, Y.; Toyofuku, M.; Nakajima-Kambe, T.; Uchiyama, H.; Nomura, N. Bicyclic compounds repress membrane vesicle production and Pseudomonas quinolone signal synthesis in Pseudomonas aeruginosa. FEMS Microbiol. Lett. 2010, 304, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Rollauer, S.E.; Sooreshjani, M.A.; Noinaj, N.; Buchanan, S.K. Outer membrane protein biogenesis in Gram-negative bacteria. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2015, 370, 20150023. [Google Scholar] [CrossRef] [PubMed]
- Bitto, N.; Kaparakis-Liaskos, M. The therapeutic benefit of bacterial membrane vesicles. Int. J. Mol. Sci. 2017, 18, 1287. [Google Scholar] [CrossRef] [PubMed]
- Klieve, A.V.; Yokoyama, M.T.; Forster, R.J.; Ouwerkerk, D.; Bain, P.A.; Mawhinney, E.L. Naturally occurring DNA transfer system associated with membrane vesicles in cellulolytic Ruminococcus spp. of ruminal origin. Appl. Environ. Microbiol. 2005, 71, 4248–4253. [Google Scholar] [CrossRef]
- Chiura, H.X.; Kogure, K.; Hagemann, S.; Ellinger, A.; Velimirov, B. Evidence for particle-induced horizontal gene transfer and serial transduction between bacteria. FEMS Microbiol. Ecol. 2011, 76, 576–591. [Google Scholar] [CrossRef]
- Rumbo, C.; Fernández-Moreira, E.; Merino, M.; Poza, M.; Mendez, J.A.; Soares, N.C.; Mosquera, A.; Chaves, F.; Bou, G. Horizontal transfer of the OXA-24 carbapenemase gene via outer membrane vesicles: A new mechanism of dissemination of carbapenem resistance genes in Acinetobacter baumannii. Antimicrob. Agents Chemother. 2011, 55, 3084–3090. [Google Scholar] [CrossRef]
- Domingues, S.; Nielsen, K.M. Membrane vesicles and horizontal gene transfer in prokaryotes. Curr. Opin. Microbiol. 2017, 38, 16–21. [Google Scholar] [CrossRef]
- Biller, S.J.; Schubotz, F.; Roggensack, S.E.; Thompson, A.W.; Summons, R.E.; Chisholm, S.W. Bacterial vesicles in marine ecosystems. Science 2014, 343, 183–186. [Google Scholar] [CrossRef]
- Sjöström, A.E.; Sandblad, L.; Uhlin, B.E.; Wai, S.N. Membrane vesicle-mediated release of bacterial RNA. Sci. Rep. 2015, 5, 15329. [Google Scholar] [CrossRef]
- Renelli, M.; Matias, V.; Lo, R.Y.; Beveridge, T.J. DNA-containing membrane vesicles of Pseudomonas aeruginosa PAO1 and their genetic transformation potential. Microbiology 2004, 150, 2161–2169. [Google Scholar] [CrossRef] [PubMed]
- Fulsundar, S.; Harms, K.; Flaten, G.E.; Johnsen, P.J.; Chopade, B.A.; Nielsen, K.M. Gene transfer potential of outer membrane vesicles of Acinetobacter baylyi and effects of stress on vesiculation. Appl. Environ. Microbiol. 2014, 80, 3469–3483. [Google Scholar] [CrossRef] [PubMed]
- Blenkiron, C.; Simonov, D.; Muthukaruppan, A.; Tsai, P.; Dauros, P.; Green, S.; Hong, J.; Print, C.G.; Swift, S.; Phillips, A.R. Uropathogenic Escherichia coli releases extracellular vesicles that are associated with RNA. PLoS ONE 2016, 11, e0160440. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Kong, Q.; Roland, K.L.; Curtiss, R., 3rd. Membrane vesicles of Clostridium perfringens type A strains induce innate and adaptive immunity. Int. J. Med. Microbiol. 2014, 304, 431–443. [Google Scholar] [CrossRef]
- Ho, M.-H.; Chen, C.-H.; Goodwin, J.S.; Wang, B.-Y.; Xie, H. Functional advantages of Porphyromonas gingivalis vesicles. PLoS ONE 2015, 10, e0123448. [Google Scholar] [CrossRef]
- Blesa, A.; Berenguer, J. Contribution of vesicle-protected extracellular DNA to horizontal gene transfer in Thermus spp. Int. Microbiol. 2015, 18, 177–187. [Google Scholar]
- Pérez-Cruz, C.; Carrión, O.; Delgado, L.; Martinez, G.; López-Iglesias, C.; Mercade, E. New type of outer membrane vesicle produced by the Gram-negative bacterium Shewanella vesiculosa M7T: Implications for DNA content. Appl. Environ. Microbiol. 2013, 79, 1874–1881. [Google Scholar] [CrossRef]
- Rath, P.; Huang, C.; Wang, T.; Wang, T.; Li, H.; Prados-Rosales, R.; Elemento, O.; Casadevall, A.; Nathan, C.F. Genetic regulation of vesiculogenesis and immunomodulation in Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 2013, 110, E4790–E4797. [Google Scholar] [CrossRef]
- Liao, S.; Klein, M.I.; Heim, K.P.; Fan, Y.; Bitoun, J.P.; Ahn, S.-J.; Burne, R.A.; Koo, H.; Brady, L.J.; Wen, Z.T. Streptococcus mutans extracellular DNA is upregulated during growth in biofilms, actively released via membrane vesicles, and influenced by components of the protein secretion machinery. J. Bacteriol. 2014, 196, 2355–2366. [Google Scholar] [CrossRef]
- Yaron, S.; Kolling, G.L.; Simon, L.; Matthews, K.R. Vesicle-mediated transfer of virulence genes from Escherichia coli O157:H7 to other enteric bacteria. Appl. Environ. Microbiol. 2000, 66, 4414–4420. [Google Scholar] [CrossRef]
- Medvedeva, E.S.; Baranova, N.B.; Mouzykantov, A.A.; Grigorieva, T.Y.; Davydova, M.N.; Trushin, M.V.; Chernova, O.A.; Chernov, V.M. Adaptation of Mycoplasmas to antimicrobial agents: Acholeplasma laidlawii extracellular vesicles mediate the export of ciprofloxacin and a mutant gene related to the antibiotic target. Sci. World J. 2014, 2014, 7. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Choi, C.-W.; Lee, T.; Kim, S.I.; Lee, J.-C.; Shin, J.-H. Transcription factor σB plays an important role in the production of extracellular membrane-derived vesicles in Listeria monocytogenes. PLoS ONE 2013, 8, e73196. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Park, S.B.; Im, S.P.; Lee, J.S.; Jung, J.W.; Gong, T.W.; Lazarte, J.M.S.; Kim, J.; Seo, J.-S.; Kim, J.-H.; et al. Outer membrane vesicles from β-lactam-resistant Escherichia coli enable the survival of β-lactam-susceptible E. coli in the presence of β-lactam antibiotics. Sci. Rep. 2018, 8, 5402. [Google Scholar] [CrossRef] [PubMed]
- Devos, S.; Stremersch, S.; Raemdonck, K.; Braeckmans, K.; Devreese, B. Intra- and Interspecies effects of outer membrane vesicles from Stenotrophomonas maltophilia on β-lactam resistance. Antimicrob. Agents Chemother. 2016, 60, 2516–2518. [Google Scholar] [CrossRef]
- Stentz, R.; Horn, N.; Cross, K.; Salt, L.; Brearley, C.; Livermore, D.M.; Carding, S.R. Cephalosporinases associated with outer membrane vesicles released by Bacteroides spp. protect gut pathogens and commensals against β-lactam antibiotics. J. Antimicrob. Chemother. 2015, 70, 701–709. [Google Scholar] [CrossRef]
- Lee, J.; Lee, E.-Y.; Kim, S.-H.; Kim, D.-K.; Park, K.-S.; Kim, K.P.; Kim, Y.-K.; Roh, T.-Y.; Gho, Y.S. Staphylococcus aureus extracellular vesicles carry biologically active β-lactamase. Antimicrob. Agents Chemother. 2013, 57, 2589–2595. [Google Scholar] [CrossRef]
- Webber, M.A.; Piddock, L.J.V. The importance of efflux pumps in bacterial antibiotic resistance. J. Antimicrob. Chemother. 2003, 51, 9–11. [Google Scholar] [CrossRef]
- Rivera, J.; Cordero, R.J.B.; Nakouzi, A.S.; Frases, S.; Nicola, A.; Casadevall, A. Bacillus anthracis produces membrane-derived vesicles containing biologically active toxins. Proc. Natl. Acad. Sci. USA 2010, 107, 19002–19007. [Google Scholar] [CrossRef]
- Kulkarni, H.M.; Swamy, C.V.B.; Jagannadham, M.V. Molecular characterization and functional analysis of outer membrane vesicles from the antarctic bacterium Pseudomonas syringae suggest a possible response to environmental conditions. J. Proteome Res. 2014, 13, 1345–1358. [Google Scholar] [CrossRef]
- Brameyer, S.; Plener, L.; Müller, A.; Klingl, A.; Wanner, G.; Jung, K. Outer membrane vesicles facilitate trafficking of the hydrophobic signaling molecule CAI-1 between Vibrio harveyi cells. J. Bacteriol. 2018, 200, e00740. [Google Scholar] [CrossRef]
- Kim, D.-K.; Kang, B.; Kim, O.Y.; Choi, D.-S.; Lee, J.; Kim, S.R.; Go, G.; Yoon, Y.J.; Kim, J.H.; Jang, S.C.; et al. EVpedia: An integrated database of high-throughput data for systemic analyses of extracellular vesicles. J. Extracell. Ves. 2013, 2, 20383. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, G.; Garaguso, I.; Adu-Bobie, J.; Doro, F.; Taddei, A.R.; Biolchi, A.; Brunelli, B.; Giuliani, M.M.; Pizza, M.; Norais, N.; et al. Outer membrane vesicles from group B Neisseria meningitidis Δgna33 mutant: Proteomic and immunological comparison with detergent-derived outer membrane vesicles. Proteomics 2006, 6, 1856–1866. [Google Scholar] [CrossRef] [PubMed]
- Bernadac, A.; Gavioli, M.; Lazzaroni, J.C.; Raina, S.; Lloubès, R. Escherichia coli tol-pal mutants form outer membrane vesicles. J. Bacteriol. 1998, 180, 4872–4878. [Google Scholar] [CrossRef]
- Lommatzsch, J.; Templin, M.F.; Kraft, A.R.; Vollmer, W.; Höltje, J.V. Outer membrane localization of murein hydrolases: MltA, a third lipoprotein lytic transglycosylase in Escherichia coli. J. Bacteriol. 1997, 179, 5465–5470. [Google Scholar] [CrossRef]
- Oshida, T.; Sugai, M.; Komatsuzawa, H.; Hong, Y.M.; Suginaka, H.; Tomasz, A. A Staphylococcus aureus autolysin that has an N-acetylmuramoyl-L-alanine amidase domain and an endo-b-N-acetylglucosaminidase domain: Cloning, sequence analysis, and characterization. Proc. Natl. Acad. Sci. USA 1995, 92, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Kolling, G.L.; Matthews, K.R. Export of virulence genes and Shiga toxin by membrane vesicles of Escherichia coli O157:H7. Appl. Environ. Microbiol. 1999, 65, 1843–1848. [Google Scholar] [CrossRef]
- Lee, E.Y.; Bang, J.Y.; Park, G.W.; Choi, D.S.; Kang, J.S.; Kim, H.J.; Park, K.S.; Lee, J.O.; Kim, Y.K.; Kwon, K.H. Global proteomic profiling of native outer membrane vesicles derived from Escherichia coli. Proteomics 2007, 7, 3143–3153. [Google Scholar] [CrossRef] [PubMed]
- Olofsson, A.; Vallström, A.; Petzold, K.; Tegtmeyer, N.; Schleucher, J.; Carlsson, S.; Haas, R.; Backert, S.; Wai, S.N.; Gröbner, G.; et al. Biochemical and functional characterization of Helicobacter pylori vesicles. Mol. Microbiol. 2010, 77, 1539–1555. [Google Scholar] [CrossRef]
- Kesty, N.C.; Kuehn, M.J. Incorporation of heterologous outer membrane and periplasmic proteins into Escherichia coli outer membrane vesicles. J. Biol. Chem. 2004, 279, 2069–2076. [Google Scholar] [CrossRef]
- Thay, B.; Wai, S.N.; Oscarsson, J. Staphylococcus aureus α-toxin-dependent induction of host cell death by membrane-derived vesicles. PLoS ONE 2013, 8, e54661. [Google Scholar] [CrossRef]
- Lee, E.Y.; Choi, D.Y.; Kim, D.K.; Kim, J.W.; Park, J.O.; Kim, S.; Kim, S.H.; Desiderio, D.M.; Kim, Y.K.; Kim, K.P. Gram-positive bacteria produce membrane vesicles: Proteomics-based characterization of Staphylococcus aureus-derived membrane vesicles. Proteomics 2009, 9, 5425–5436. [Google Scholar] [CrossRef]
- Choi, D.-S.; Kim, D.-K.; Choi, S.J.; Lee, J.; Choi, J.-P.; Rho, S.; Park, S.-H.; Kim, Y.-K.; Hwang, D.; Gho, Y.S. Proteomic analysis of outer membrane vesicles derived from Pseudomonas aeruginosa. Proteomics 2011, 11, 3424–3429. [Google Scholar] [CrossRef] [PubMed]
- Wai, S.N.; Lindmark, B.; Söderblom, T.; Takade, A.; Westermark, M.; Oscarsson, J.; Jass, J.; Richter-Dahlfors, A.; Mizunoe, Y.; Uhlin, B.E. Vesicle-mediated export and assembly of pore-forming oligomers of the enterobacterial ClyA cytotoxin. Cell 2003, 115, 25–35. [Google Scholar] [CrossRef]
- Bomberger, J.M.; Maceachran, D.P.; Coutermarsh, B.A.; Ye, S.; O’Toole, G.A.; Stanton, B.A. Long-distance delivery of bacterial virulence factors by Pseudomonas aeruginosa outer membrane vesicles. PLoS Path. 2009, 5, e1000382. [Google Scholar] [CrossRef]
- Ballok, A.E.; Filkins, L.M.; Bomberger, J.M.; Stanton, B.A.; O’Toole, G.A. Epoxide-mediated differential packaging of Cif and other virulence factors into outer membrane vesicles. J. Bacteriol. 2014, 196, 3633–3642. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.-W.; Choi, E.-B.; Min, T.-K.; Kim, J.-H.; Kim, M.-H.; Jeon, S.G.; Lee, B.-J.; Gho, Y.S.; Jee, Y.-K.; Pyun, B.-Y.; et al. An important role of α-hemolysin in extracellular vesicles on the development of atopic dermatitis induced by Staphylococcus aureus. PLoS ONE 2014, 9, e100499. [Google Scholar] [CrossRef] [PubMed]
- Horstman, A.L.; Kuehn, M.J. Enterotoxigenic Escherichia coli secretes active heat-labile enterotoxin via outer membrane vesicles. J. Biol. Chem. 2000, 275, 12489–12496. [Google Scholar] [CrossRef]
- Kadurugamuwa, J.L.; Beveridge, T.J. Bacteriolytic effect of membrane vesicles from Pseudomonas aeruginosa on other bacteria including pathogens: Conceptually new antibiotics. J. Bacteriol. 1996, 178, 2767–2774. [Google Scholar] [CrossRef]
- Vasilyeva, N.V.; Tsfasman, I.M.; Suzina, N.E.; Stepnaya, O.A.; Kulaev, I.S. Secretion of bacteriolytic endopeptidaseL5 of Lysobacter sp. XL1 into the medium by means of outer membrane vesicles. FEBS J. 2008, 275, 3827–3835. [Google Scholar] [CrossRef]
- Nevot, M.; Deroncelé, V.; Messner, P.; Guinea, J.; Mercadé, E. Characterization of outer membrane vesicles released by the psychrotolerant bacterium Pseudoalteromonas antarctica NF3. Environ. Microbiol. 2006, 8, 1523–1533. [Google Scholar] [CrossRef]
- Lappann, M.; Otto, A.; Becher, D.; Vogel, U. Comparative proteome analysis of spontaneous outer membrane vesicles and purified outer membranes of Neisseria meningitidis. J. Bacteriol. 2013, 195, 4425–4435. [Google Scholar] [CrossRef] [PubMed]
- Olczak, T.; Wójtowicz, H.; Ciuraszkiewicz, J.; Olczak, M. Species specificity, surface exposure, protein expression, immunogenicity, and participation in biofilm formation of Porphyromonas gingivalis HmuY. BMC Microbiol. 2010, 10, 134. [Google Scholar] [CrossRef] [PubMed]
- Ellis, T.N.; Leiman, S.A.; Kuehn, M.J. Naturally produced outer membrane vesicles from Pseudomonas aeruginosa elicit a potent innate immune response via combined sensing of both lipopolysaccharide and protein components. Infect. Immun. 2010, 78, 3822–3831. [Google Scholar] [CrossRef] [PubMed]
- Prados-Rosales, R.; Baena, A.; Martinez, L.R.; Luque-Garcia, J.; Kalscheuer, R.; Veeraraghavan, U.; Camara, C.; Nosanchuk, J.D.; Besra, G.S.; Chen, B.; et al. Mycobacteria release active membrane vesicles that modulate immune responses in a TLR2-dependent manner in mice. J. Clin. Invest. 2011, 121, 1471–1483. [Google Scholar] [CrossRef]
- Kothary, M.H.; Gopinath, G.R.; Gangiredla, J.; Rallabhandi, P.V.; Harrison, L.M.; Yan, Q.Q.; Chase, H.R.; Lee, B.; Park, E.; Yoo, Y.; et al. Analysis and characterization of proteins associated with outer membrane vesicles secreted by Cronobacter spp. Front. Microbiol. 2017, 8, 134. [Google Scholar] [CrossRef]
- Stumpe, S.; Schmid, R.; Stephens, D.L.; Georgiou, G.; Bakker, E.P. Identification of OmpT as the protease that hydrolyzes the antimicrobial peptide protamine before it enters growing cells of Escherichia coli. J. Bacteriol. 1998, 180, 4002–4006. [Google Scholar] [CrossRef]
- Bergman, M.A.; Cummings, L.A.; Barrett, S.L.R.; Smith, K.D.; Lara, J.C.; Aderem, A.; Cookson, B.T. CD4+ T cells and toll-like receptors recognize Salmonella antigens expressed in bacterial surface organelles. Infect. Immun. 2005, 73, 1350–1356. [Google Scholar] [CrossRef]
- Kwon, S.-O.; Gho, Y.S.; Lee, J.C.; Kim, S.I. Proteome analysis of outer membrane vesicles from a clinical Acinetobacter baumannii isolate. Fems Microbiol. Lett. 2009, 297, 150–156. [Google Scholar] [CrossRef]
- Bauman, S.J.; Kuehn, M.J. Purification of outer membrane vesicles from Pseudomonas aeruginosa and their activation of an IL-8 response. Microb. Infect. 2006, 8, 2400–2408. [Google Scholar] [CrossRef]
- Olaya-Abril, A.; Prados-Rosales, R.; McConnell, M.J.; Martín-Peña, R.; González-Reyes, J.A.; Jiménez-Munguía, I.; Gómez-Gascón, L.; Fernández, J.; Luque-García, J.L.; García-Lidón, C.; et al. Characterization of protective extracellular membrane-derived vesicles produced by Streptococcus pneumoniae. J. Proteom. 2014, 106, 46–60. [Google Scholar] [CrossRef]
- Park, A.J.; Surette, M.D.; Khursigara, C.M. Antimicrobial targets localize to the extracellular vesicle-associated proteome of Pseudomonas aeruginosa grown in a biofilm. Front. Microbiol. 2014, 5, 464. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, L.; Toloza, L.; Giménez, R.; Odena, A.; Oliveira, E.; Aguilar, J.; Badia, J.; Baldomà, L. Proteomic analysis of outer membrane vesicles from the probiotic strain Escherichia coli nissle 1917. Proteomics 2014, 14, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.; Kessler, A.; Cabezas-Sanchez, P.; Luque-Garcia, J.L.; Casadevall, A. Extracellular vesicles produced by the Gram-positive bacterium Bacillus subtilis are disrupted by the lipopeptide surfactin. Mol. Microbiol. 2014, 93, 183–198. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.-Y.; Choi, D.-S.; Kim, K.-P.; Gho, Y.S. Proteomics in gram-negative bacterial outer membrane vesicles. Mass Spectrom. Rev. 2008, 27, 535–555. [Google Scholar] [CrossRef] [PubMed]
- Galka, F.; Wai, S.N.; Kusch, H.; Engelmann, S.; Hecker, M.; Schmeck, B.; Hippenstiel, S.; Uhlin, B.E.; Steinert, M. Proteomic characterization of the whole secretome of Legionella pneumophila and functional analysis of outer membrane vesicles. Infect. Immun. 2008, 76, 1825–1836. [Google Scholar] [CrossRef]
- Moon, D.C.; Choi, C.H.; Lee, J.H.; Choi, C.-W.; Kim, H.-Y.; Park, J.S.; Kim, S.I.; Lee, J.C. Acinetobacter baumannii outer membrane protein a modulates the biogenesis of outer membrane vesicles. J. Microbiol. 2012, 50, 155–160. [Google Scholar] [CrossRef]
- Tong, T.T.; Mörgelin, M.; Forsgren, A.; Riesbeck, K. Haemophilus influenzae survival during complement-mediated attacks is promoted by Moraxella catarrhalis outer membrane vesicles. J. Infect. Dis. 2007, 195, 1661–1670. [Google Scholar]
- Jang, K.-S.; Sweredoski, M.J.; Graham, R.L.J.; Hess, S.; Clemons, W.M. Comprehensive proteomic profiling of outer membrane vesicles from Campylobacter jejuni. J. Proteom. 2014, 98, 90–98. [Google Scholar] [CrossRef]
- Veith, P.D.; Chen, Y.-Y.; Gorasia, D.G.; Chen, D.; Glew, M.D.; O’Brien-Simpson, N.M.; Cecil, J.D.; Holden, J.A.; Reynolds, E.C. Porphyromonas gingivalis outer membrane vesicles exclusively contain outer Membrane and periplasmic proteins and carry a cargo enriched with virulence factors. J. Proteome Res. 2014, 13, 2420–2432. [Google Scholar] [CrossRef]
- Dorward, D.W.; Schwan, T.G.; Garon, C.F. Immune capture and detection of Borrelia burgdorferi antigens in urine, blood, or tissues from infected ticks, mice, dogs, and humans. J. Clin. Microbiol. 1991, 29, 1162–1170. [Google Scholar] [CrossRef]
- Kadurugamuwa, J.L.; Beveridge, T.J. Delivery of the non-membrane-permeative antibiotic gentamicin into mammalian cells by using Shigella flexneri membrane vesicles. Antimicrob. Agents Chemother. 1998, 42, 1476–1483. [Google Scholar] [CrossRef] [PubMed]
- Fiocca, R.; Necchi, V.; Sommi, P.; Ricci, V.; Telford, J.; Cover, T.L.; Solcia, E. Release of Helicobacter pylori vacuolating cytotoxin by both a specific secretion pathway and budding of outer membrane vesicles. Uptake of released toxin and vesicles by gastric epithelium. J. Pathol. 1999, 188, 220–226. [Google Scholar] [CrossRef]
- Post, D.M.B.; Zhang, D.; Eastvold, J.S.; Teghanemt, A.; Gibson, B.W.; Weiss, J.P. Biochemical and functional characterization of membrane blebs purified from Neisseria meningitidis serogroup B. J. Biol. Chem. 2005, 280, 38383–38394. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lee, J.; Park, J.; Gho, Y.S. Gram-negative and Gram-positive bacterial extracellular vesicles. Semin. Cell Dev. Biol. 2015, 40, 97–104. [Google Scholar] [CrossRef]
- Ollinger, J.; Bowen, B.; Wiedmann, M.; Boor, K.J.; Bergholz, T.M. Listeria monocytogenes sigmaB modulates PrfA-mediated virulence factor expression. Infect. Immun. 2009, 77, 2113–2124. [Google Scholar] [CrossRef]
- Altindis, E.; Fu, Y.; Mekalanos, J.J. Proteomic analysis of Vibrio cholerae outer membrane vesicles. Proc. Natl. Acad. Sci. USA 2014, 111, E1548–E1556. [Google Scholar] [CrossRef]
- Allan, N.D.; Kooi, C.; Sokol, P.A.; Beveridge, T.J. Putative virulence factors are released in association with membrane vesicles from Burkholderia cepacia. Can. J. Microbiol. 2003, 49, 613–624. [Google Scholar] [CrossRef]
- Cossart, P.; Vicente, M.F.; Mengaud, J.; Baquero, F.; Perez-Diaz, J.C.; Berche, P. Listeriolysin O is essential for virulence of Listeria monocytogenes: Direct evidence obtained by gene complementation. Infect. Immun. 1989, 57, 3629–3636. [Google Scholar] [CrossRef]
- Hozbor, D.; Rodriguez, M.E.; Fernández, J.; Lagares, A.; Guiso, N.; Yantorno, O. Release of outer membrane vesicles from Bordetella pertussis. Curr. Microbiol. 1999, 38, 273–278. [Google Scholar] [CrossRef]
- Lee, J.C. Staphylococcus aureus membrane vesicles and Its potential role in bacterial pathogenesis. J. Bacteriol. Virol. 2012, 42, 181–188. [Google Scholar] [CrossRef][Green Version]
- Roberts, C.A.; Buikstra, J.E. Bacterial infections. In Ortner’s Identification of Pathological Conditions in Human Skeletal Remains; Buikstra, J.E., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 321–439. [Google Scholar]
- Lim, S.; Yoon, H. Roles in outer membrane vesicles (OMVs) in bacterial virulence. J. Bacteriol. Virol. 2015, 45, 1–10. [Google Scholar] [CrossRef][Green Version]
- Schulz, E.; Goes, A.; Garcia, R.; Panter, F.; Koch, M.; Müller, R.; Fuhrmann, K.; Fuhrmann, G. Biocompatible bacteria-derived vesicles show inherent antimicrobial activity. J. Control. Release 2018, 290, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Kovar, M.; Boyman, O.; Shen, X.; Hwang, I.; Kohler, R.; Sprent, J. Direct stimulation of T cells by membrane vesicles from antigen-presenting cells. Proc. Natl. Acad. Sci. USA 2006, 103, 11671–11676. [Google Scholar] [CrossRef] [PubMed]
- Muralinath, M.; Kuehn, M.J.; Roland, K.L.; Curtiss, R., 3rd. Immunization with Salmonella enterica serovar Typhimurium-derived outer membrane vesicles delivering the pneumococcal protein PspA confers protection against challenge with Streptococcus pneumoniae. Infect. Immun. 2011, 79, 887–894. [Google Scholar] [CrossRef]
- Schroeder, J.; Aebischer, T. Recombinant outer membrane vesicles to augment antigen-specific live vaccine responses. Vaccine 2009, 27, 6748–6754. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, K.S.; Lee, S.R.; Kim, E.; Kim, M.S.; Lee, E.Y.; Gho, Y.S.; Kim, J.W.; Bishop, R.E.; Chang, K.T. Structural modifications of outer membrane vesicles to refine them as vaccine delivery vehicles. Biochim. Biophys. Acta 2009, 1788, 2150–2159. [Google Scholar] [CrossRef]
- Chen, D.J.; Osterrieder, N.; Metzger, S.M.; Buckles, E.; Doody, A.M.; DeLisa, M.P.; Putnam, D. Delivery of foreign antigens by engineered outer membrane vesicle vaccines. Proc. Natl. Acad. Sci. USA 2010, 107, 3099–3104. [Google Scholar] [CrossRef]
- Embry, A.; Meng, X.; Cantwell, A.; Dube, P.H.; Xiang, Y. Enhancement of immune response to an antigen delivered by vaccinia virus by displaying the antigen on the surface of intracellular mature virion. Vaccine 2011, 29, 5331–5339. [Google Scholar] [CrossRef]
- Persson, G.; Pors, S.E.; Thøfner, I.C.N.; Bojesen, A.M. Vaccination with outer membrane vesicles and the fimbrial protein FlfA offers improved protection against lesions following challenge with Gallibacterium anatis. Vet. Microbiol. 2018, 217, 104–111. [Google Scholar] [CrossRef]
- Schaar, V.; Paulsson, M.; Mörgelin, M.; Riesbeck, K. Outer membrane vesicles shield Moraxella catarrhalis β-lactamase from neutralization by serum IgG. J. Antimicrob. Chemother. 2013, 68, 593–600. [Google Scholar] [CrossRef]
- Stevenson, T.C.; Cywes-Bentley, C.; Moeller, T.D.; Weyant, K.B.; Putnam, D.; Chang, Y.-F.; Jones, B.D.; Pier, G.B.; DeLisa, M.P. Immunization with outer membrane vesicles displaying conserved surface polysaccharide antigen elicits broadly antimicrobial antibodies. Proc. Natl. Acad. Sci. USA 2018, 115, E3106–E3115. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Wang, S.; Yao, Y.; Xia, Y.; Yang, X.; Li, K.; Sun, P.; Liu, C.; Sun, W.; Bai, H.; et al. Employing Escherichia coli-derived outer membrane vesicles as an antigen delivery platform elicits protective immunity against Acinetobacter baumannii infection. Sci. Rep. 2016, 6, 37242. [Google Scholar] [CrossRef] [PubMed]
- Roier, S.; Leitner, D.R.; Iwashkiw, J.; Schild-Prufert, K.; Feldman, M.F.; Krohne, G.; Reidl, J.; Schild, S. Intranasal immunization with nontypeable Haemophilus influenzae outer membrane vesicles induces cross-protective immunity in mice. PLoS ONE 2012, 7, e42664. [Google Scholar] [CrossRef]
- Roier, S.; Fenninger, J.C.; Leitner, D.R.; Rechberger, G.N.; Reidl, J.; Schild, S. Immunogenicity of Pasteurella multocida and Mannheimia haemolytica outer membrane vesicles. Int. J. Med. Microbiol. 2013, 303, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Petersen, H.; Nieves, W.; Russell-Lodrigue, K.; Roy, C.J.; Morici, L.A. Evaluation of a Burkholderia pseudomallei Outer Membrane Vesicle Vaccine in Nonhuman Primates. Procedia Vaccinol 2014, 8, 38–42. [Google Scholar] [CrossRef]
- Choi, H.-I.; Kim, M.; Jeon, J.; Han, J.K.; Kim, K.-S. Overexpression of MicA induces production of OmpC-enriched outer membrane vesicles that protect against Salmonella challenge. Biochem. Biophys. Res. Commun. 2017, 490, 991–996. [Google Scholar] [CrossRef]
- Acevedo, R.; Fernandez, S.; Zayas, C.; Acosta, A.; Sarmiento, M.; Ferro, V.; Rosenqvist, E.; Campa, C.; Cardoso, D.; Garcia, L.; et al. Bacterial outer membrane vesicles and vaccine applications. Front. Immunol. 2014, 5, 121. [Google Scholar] [CrossRef]
- Fuhrmann, G.; Neuer, A.L.; Herrmann, I.K. Extracellular vesicles – A promising avenue for the detection and treatment of infectious diseases? Eur. J. Pharm. Biopharm. 2017, 118, 56–61. [Google Scholar] [CrossRef]
- Tan, K.; Li, R.; Huang, X.; Liu, Q. Outer membrane vesicles: Current status and future direction of these novel vaccine adjuvants. Front. Microbiol. 2018, 9, 783. [Google Scholar] [CrossRef]
- Edelman, R. Vaccine adjuvants. Rev. Infect. Dis. 1980, 2, 370–383. [Google Scholar] [CrossRef]
- Exley, C. Aluminium adjuvants and adverse events in sub-cutaneous allergy immunotherapy. AllergyAsthma Clin. Immunol. 2014, 10, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Yoo, J.K.; Sohn, H.J.; Kang, H.K.; Kim, D.; Shin, H.J.; Kim, J.H. Protective immunity against Naegleria fowleri infection on mice immunized with the rNfa1 protein using mucosal adjuvants. Parasitol. Res. 2015, 114, 1377–1385. [Google Scholar] [CrossRef] [PubMed]
- Mutsch, M.; Zhou, W.; Rhodes, P.; Bopp, M.; Chen, R.T.; Linder, T.; Spyr, C.; Steffen, R. Use of the inactivated intranasal influenza vaccine and the risk of Bell’s palsy in Switzerland. N. Engl. J. Med. 2004, 350, 896–903. [Google Scholar] [CrossRef] [PubMed]
- Sierra, G.V.; Campa, H.C.; Varcacel, N.M.; Garcia, I.L.; Izquierdo, P.L.; Sotolongo, P.F.; Casanueva, G.V.; Rico, C.O.; Rodriguez, C.R.; Terry, M.H. Vaccine against group B Neisseria meningitidis: Protection trial and mass vaccination results in Cuba. Niph Ann. 1991, 14, 195–207. [Google Scholar]
- Haneberg, B.; Dalseg, R.; Oftung, F.; Wedege, E.; Hoiby, E.A.; Haugen, I.; Holst, J.; Andersen, S.; Aase, A.; Naess, L.; et al. Towards a nasal vaccine against meningococcal disease, and prospects for its use as a mucosal adjuvant. Dev. Biol. Stand. 1998, 92, 127–133. [Google Scholar]
- Estevez, F.; Carr, A.; Solorzano, L.; Valiente, O.; Mesa, C.; Barroso, O.; Sierra, G.V.; Fernandez, L.E. Enhancement of the immune response to poorly immunogenic gangliosides after incorporation into very small size proteoliposomes (VSSP). Vaccine 1999, 18, 190–197. [Google Scholar] [CrossRef]
- Sardinas, G.; Reddin, K.; Pajon, R.; Gorringe, A. Outer membrane vesicles of Neisseria lactamica as a potential mucosal adjuvant. Vaccine 2006, 24, 206–214. [Google Scholar] [CrossRef]
- Gurung, M.; Moon, D.C.; Choi, C.W.; Lee, J.H.; Bae, Y.C.; Kim, J.; Lee, Y.C.; Seol, S.Y.; Cho, D.T.; Kim, S.I.; et al. Staphylococcus aureus produces membrane-derived vesicles that induce host cell death. PLoS ONE 2011, 6, e27958. [Google Scholar] [CrossRef]
- Yuan, J.; Yang, J.; Hu, Z.; Yang, Y.; Shang, W.; Hu, Q.; Zheng, Y.; Peng, H.; Zhang, X.; Cai, X.; et al. Safe Staphylococcal Platform for the Development of Multivalent Nanoscale Vesicles against Viral Infections. Nano Lett. 2018, 18, 725–733. [Google Scholar] [CrossRef]
- Diaz-Garrido, N.; Fábrega, M.-J.; Vera, R.; Giménez, R.; Badia, J.; Baldomà, L. Membrane vesicles from the probiotic Nissle 1917 and gut resident Escherichia coli strains distinctly modulate human dendritic cells and subsequent T cell responses. J. Funct. Foods 2019, 61, 103495. [Google Scholar] [CrossRef]
- Kim, M.H.; Choi, S.J.; Choi, H.I.; Choi, J.P.; Park, H.K.; Kim, E.K.; Kim, M.J.; Moon, B.S.; Min, T.K.; Rho, M.; et al. Lactobacillus plantarum-derived Extracellular Vesicles Protect Atopic Dermatitis Induced by Staphylococcus aureus-derived Extracellular Vesicles. Allergy Asthma Immunol. Res. 2018, 10, 516–532. [Google Scholar] [CrossRef] [PubMed]
| Bacterium | Receptor | Ligand | Reference |
|---|---|---|---|
| Staphylococcus aureus; Enterococcus faecalis; Streptococcus spp. | Monomeric membrane phospholipids | Daptomycin, nisin, pexiganan, melittin | [62,63] |
| Escherichia coli | Lipid and protein | Polymyxin B and E | [64,65] |
| Moraxella catarrhalis; Escherichia coli | Hydrolytic enzymes | Amoxicillin, cefaclor Melittin, penicillin, methicillin | [66] [67,68] |
| Burkholderia cenocepacia | Hydrophobic lipocalins | Rifampicin, norfloxacin, ceftazidime, polymyxin B | [60] |
| Pseudomonas aeruginosa; Streptococcus pneumoniae; Klebsiella pneumoniae | Capsular polysaccharides | Polymyxin B | [69,70,71,72,73] |
| Pseudomonas aeruginosa; Staphylococcus epidermidis; Haemophilus influenzae | eDNA | Kanamycin, tobramycin, vancomycin, human β-defensin-3, gentamicin, amikacin | [70,74,75] |
| Genetic Material | Species | Reference |
|---|---|---|
| Chromosomal DNA | Escherichia coli | [91] |
| Clostridium perfringens | [96] | |
| Neisseria gonorrhoeae | [12] | |
| Porphyromonas gingivalis | [97] | |
| Prochlorococcus sp. | [98] | |
| Ruminococcus spp. | [87] | |
| Shewanella vesiculosa | [99] | |
| Mycobacterium tuberculosis | [100] | |
| Streptococcus mutans | [101] | |
| Plasmid DNA | Acinetobacter baumanni | [94] |
| Acinetobacter baylyi | [89] | |
| Escherichia coli | [102] | |
| Pseudomonas aeruginosa | [93] | |
| Neisseria gonorrhoeae | [12] | |
| Viral DNA | Escherichia coli | [102] |
| Not specified DNA | Acholeplasma laidlawii | [103] |
| mRNA | Escherichia coli | [95] |
| Porphyromonas gingivalis | [97] | |
| rRNA | Escherichia coli | [95] |
| Porphyromonas gingivalis | [97] | |
| sRNA | Escherichia coli | [95] |
| Vibrio cholera | [90] | |
| Clostridium perfringens | [96] | |
| Mycobacterium tuberculosis | [100] | |
| Listeria monocytogenes | [104] | |
| tRNA | Escherichia coli | [95] |
| Not specified RNA | Neisseria gonorrhoeae | [12] |
| Proteins | Function | Species | Reference |
|---|---|---|---|
| Outer membrane porins | |||
| OmpA and OmpX | Binding to host cell receptors | Cronobacter sakazakii | [137] |
| Cronobacter turicensis | [137] | ||
| Cronobacter malonaticus | [137] | ||
| OmpA, OmpC, and OmpF | Binding to host cells | Escherichia coli | [119] |
| Escherichia coli△tolR | [138] | ||
| OmpC | Pore-forming activity | Salmonella typhi | [139] |
| AbOmpA | Binding to host tissue | Acinetobacter baumannii | [140] |
| OprE and OprF | Porin | Pseudomonas aeruginosa | [141] |
| Pseudoalteromonas antarctica NF3 | [132] | ||
| PorA and PorB | Adherence to host cells | Neisseria meningitis | [133] |
| Neisseria meningitis △gna33 | [114] | ||
| PspA | Binding to human lactoferrin | Streptococcus pneumoniae | [142] |
| Antibiotic resistance | |||
| β-lactamase | β-lactamase activity | Pseudomonas aeruginosa | [78] |
| Streptococcus pneumoniae | [108] | ||
| Moraxella catarrhalis | [66] | ||
| Carbapenemase | Hydrolysis of carbapenem | Acinetobacter baumannii | [89] |
| Cephalosporinases | β-lactamase activity | Bacteroides spp. | [107] |
| Penicillin-binding proteins | Peptidoglycan-based cell wall biogenesis | Streptococcus pneumoniae | [123] |
| TolC | Multidrug efflux pumps | Escherichia coli | [67] |
| Escherichia coli△tolR | [138] | ||
| Mex | Multidrug efflux pumps | Pseudomonas aeruginosa | [143] |
| Pseudoalteromonas antarctica NF3 | [132] | ||
| Mtr | Multidrug efflux pumps | Neisseria meningitis | [133] |
| Neisseria meningitis△gna33 | [114] | ||
| ABC Transporters | |||
| BtuB | Vitamin B12 Transporter | Escherichia coli | [144] |
| Escherichia coli△tolR | [138] | ||
| Tsx | Nucleoside-specific channel-forming protein | Escherichia coli | [119] |
| Escherichia coli△tolR | [138] | ||
| FecA, FhuA, FhuE, FiuA, FptA | Siderophore transporter | Neisseria meningitis△gna33 | [114] |
| Escherichia coli | [144] | ||
| Clostridium perfringens | [96] | ||
| Bacillus subtilis | [145] | ||
| Escherichia coli△tolR | [138] | ||
| FadL | Long-chain fatty acid transporter | Escherichia coli | [119] |
| Escherichia coli△tolR | [138] | ||
| Maltoporin LamB | ABC Transporters | Pseudoalteromonas antarctica NF3 | [119] |
| Escherichia coli△tolR | [138] | ||
| Escherichia coli | [144] | ||
| ArtI, BraC, FliY, GlnH, HisJ | Amino acid transporter | Neisseria meningitis | [133] |
| Maltose/maltodextrin | Sugar transporter | Streptococcus pneumoniae | |
| Sugar ABC transporter | [142] | ||
| Motility-related proteins | |||
| Pilus-associated protein | Motility-related proteins | Neisseria meningitis | [133] |
| Neisseria meningitis△gna33 | [114] | ||
| Flagellin FliC | Motility-related proteins | Pseudoalteromonas antarctica NF3 | [132] |
| Escherichia coli | [144] | ||
| Pseudomonas aeruginosa | [146] | ||
| Protease/chaperone | |||
| MSP | Protease | Legionella pneumophila | [147] |
| Protease Pla | Toxicity | Yersinia pestis | [79] |
| Proteases | Enzyme activity | Streptococcus pneumoniae | [142] |
| Acinetobacter baumanni | [148] | ||
| Yersinia pestis | [79] | ||
| Chaperone SurA | Chaperone | Pseudoalteromonas antarctica NF3 | [132] |
| Chaperone | Escherichia coli | [144] | |
| Chaperone | Escherichia coli△tolR | [138] | |
| Tail-specific peptidase Prc | Protease | Escherichia coli | [144] |
| Protease | Neisseria meningitis△gna33 | [114] | |
| Protease DegQ | Protease | Pseudoalteromonas antarctica NF3 | [146] |
| Protease | Escherichia coli△tolR | [146] | |
| Protease | Escherichia coli | [144] | |
| Adhesion/invasion | |||
| F1 outer fimbrial antigen | Complement binding | Yersinia pestis | [79] |
| Adhesin Ail | Adhesion | Yersinia pestis | [79] |
| UspA1, UspA2 | Complement binding | Moraxella catarrhalis | [149] |
| CDT | Toxicity, invasion | Campylobacter jejuni | [150] |
| RgpA, RgpB, Kqp | Host tissue invasion | Porphyromonas gingivalis | [151] |
| Opacity protein | Adhesion and invasion | Neisseria meningitis | [133] |
| OspA, OspB, OspD | Adherence to host cells | Borrelia burgdorferi | [152] |
| IpaB, C, D | Invasion of plasmid antigens | Shigellaflexneri | [153] |
| Staphopain A | Invasion | Streptococcus pneumoniae | [108] |
| SabA | Adherence | Helicobacter pylori | [154] |
| Killing of competing bacteria | |||
| Endopeptidase L5 | Peptidoglycan hydrolyse | Lysobacter sp. | [131] |
| N-acetylmuramoyl-L-alanine amidase | Peptidoglycan hydrolyse | Streptococcus pneumoniae | [117] |
| SLT | Murein hydrolyses | Neisseria meningitis | [155] |
| Neisseria meningitis△gna33 | [132] | ||
| Escherichia coli△tolR | [119] | ||
| Escherichia coli | [138] | ||
| Mlt | Murein hydrolyse | Neisseria meningitis | [155] |
| Pseudoalteromonas antarctica NF3 | [114] | ||
| Escherichia coli△tolR | [119] | ||
| Escherichia coli | [138] | ||
| Host cell modulation | |||
| α-Hemolysin | Hemolysis | Pseudomonas aeruginosa | [153] |
| Pseudoalteromonas antarctica NF3 | [132] | ||
| Staphylococcus aureus | [128] | ||
| Neisseria meningitis△gna33 | [114] | ||
| Cytolysin A (ClyA) | Pore-forming ability | Enterohemorrhagic E. coli | [125] |
| Salmonella typhi | [125] | ||
| Heat labile enterotoxin (LT) | Toxicity | Enterotoxigenic E. coli | [129] |
| Shiga toxin (Stx) | Toxicity | Shiga toxin producing E. coli | [13] |
| Toxicity | Shigella dysenteriae | [13] | |
| Cif | Decrease of apical CFTR expression | Pseudomonas aeruginosa | [127] |
| VacA | Vacuolating activity | Helicobacter pylori | [154] |
| Proteolysin | Proteolysis | Streptococcus pneumoniae | [156] |
| β2 toxin | Toxicity | Streptococcus mutans | [156] |
| SEQ, SSaA1, and SSaA2 | Evade the host immune system | Streptococcus pneumoniae | [123] |
| Lmo2785 | Catalase | Listeria monocytogenes | [157] |
| SOD | Immunomodulatory effect | Acinetobacter baumannii | [140] |
| Virulence factors | |||
| Phospholipase C Protease | Hydrolyzes of phospholipids | Pseudomonas aeruginosa | [13] |
| Hcp | Adherence | Helicobacter pylori | [154] |
| Rtx toxin | Cytotoxicity, depolymerizing actin | Vibrio cholera | [158] |
| Macrophage infectivity potentiator (MIP) | Cytotoxicity | Neisseria meningitis | [133] |
| Neisseria meningitis△gna33 | [114] | ||
| Hemagglutinin | Enzyme activities | Burkholderia cepacia | [159] |
| IgA protease | Protease activity | Neisseria meningitis | [133] |
| Pseudoalteromonas antartica NF3 | [132] | ||
| InlB and LLO8 | Cellular invasion | Listeria monocytogenes | [160] |
| Pertussis toxin (Ptx), | Cytotoxicity | Bordetella pertussis | [161] |
| Adenylate cyclase, hemolysin | |||
| SbI | IgG-binding protein | Staphylococcus aureus | [162] |
| Protective antigen, | Toxicity | Bacillus anthracis | [110] |
| Lethal factor, Edema toxin | |||
| Anthrolysin | |||
| Cytoplasmic proteins | |||
| GroEL | 60 KDa chaperonin | Neisseria meningitis | [133] |
| Escherichia coli | [144] | ||
| ATP-dependent DNA helicase | Interaction | Staphylococcus aureus | [123] |
| EF-Tu | Elongation factor | Neisseria meningitis | [133] |
| Staphylococcus aureus | [123] | ||
| Clostridium perfringens | [96] | ||
| Pyruvate kinase | Glycolysis | Staphylococcus aureus | [123] |
| Acetate kinase | Phosphorylation | Staphylococcus aureus | [123] |
| Type-3 secretion proteins | Cytoplasmic proteins | Acinetobacter baumannii | [140] |
| Alkaline phosphatase | In vitro enzyme activities | Pseudomonas aeruginosa | [143] |
| DNA gyrase subunit A | Stimulate to antibiotics | Staphylococcus aureus | [123] |
| Hsp60 | Heat shock protein | Legionella pneumophila | [13] |
| DnaK | Heat shock 70 kDa protein | Neisseria meningitis△gna33 | [114] |
| 30S ribosomal protein S1 (RpsA) | Cytoplasmic proteins | Neisseria meningitis△gna33 | [114] |
| Cytoplasmic proteins | Escherichia coli | [144] | |
| 50S ribosomal protein L7/L12 (RplL) | Cytoplasmic proteins | Escherichia coli | [144] |
| Coagulation | |||
| Staphylocoagulase precursor [COL] | coagulation | Staphylococcus aureus | [123] |
| Staphylocoagulase precursor | coagulation | Staphylococcus aureus | [123] |
| Truncated secreted von Willebrand | coagulation | Staphylococcus aureus | [123] |
| Factor-binding protein VWbp | coagulation | Staphylococcus aureus | [123] |
| Others | |||
| Iss | Increased serum survival | Escherichia coli | [144] |
| OstA | Organic solvent tolerance protein | Pseudoalteromonas antartica NF3 | [132] |
| Organic solvent tolerance protein | Escherichia coli△tolR | [138] | |
| Organic solvent tolerance protein | Escherichia coli | [144] | |
| NADH dehydrogenase-like protein | Oxidation reduction | Staphylococcus aureus | [123] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uddin, M.J.; Dawan, J.; Jeon, G.; Yu, T.; He, X.; Ahn, J. The Role of Bacterial Membrane Vesicles in the Dissemination of Antibiotic Resistance and as Promising Carriers for Therapeutic Agent Delivery. Microorganisms 2020, 8, 670. https://doi.org/10.3390/microorganisms8050670
Uddin MJ, Dawan J, Jeon G, Yu T, He X, Ahn J. The Role of Bacterial Membrane Vesicles in the Dissemination of Antibiotic Resistance and as Promising Carriers for Therapeutic Agent Delivery. Microorganisms. 2020; 8(5):670. https://doi.org/10.3390/microorganisms8050670
Chicago/Turabian StyleUddin, Md Jalal, Jirapat Dawan, Gibeom Jeon, Tao Yu, Xinlong He, and Juhee Ahn. 2020. "The Role of Bacterial Membrane Vesicles in the Dissemination of Antibiotic Resistance and as Promising Carriers for Therapeutic Agent Delivery" Microorganisms 8, no. 5: 670. https://doi.org/10.3390/microorganisms8050670
APA StyleUddin, M. J., Dawan, J., Jeon, G., Yu, T., He, X., & Ahn, J. (2020). The Role of Bacterial Membrane Vesicles in the Dissemination of Antibiotic Resistance and as Promising Carriers for Therapeutic Agent Delivery. Microorganisms, 8(5), 670. https://doi.org/10.3390/microorganisms8050670






