Evaluation of Biofilm Formation in Candida tropicalis Using a Silicone-Based Platform with Synthetic Urine Medium
Abstract
1. Introduction
2. Materials and Methods
2.1. Media and Reagents
2.2. Plasmid and Strain Construction
2.3. Biofilm Assay and Biofilm Staining
2.4. Statistical Analyses
3. Results
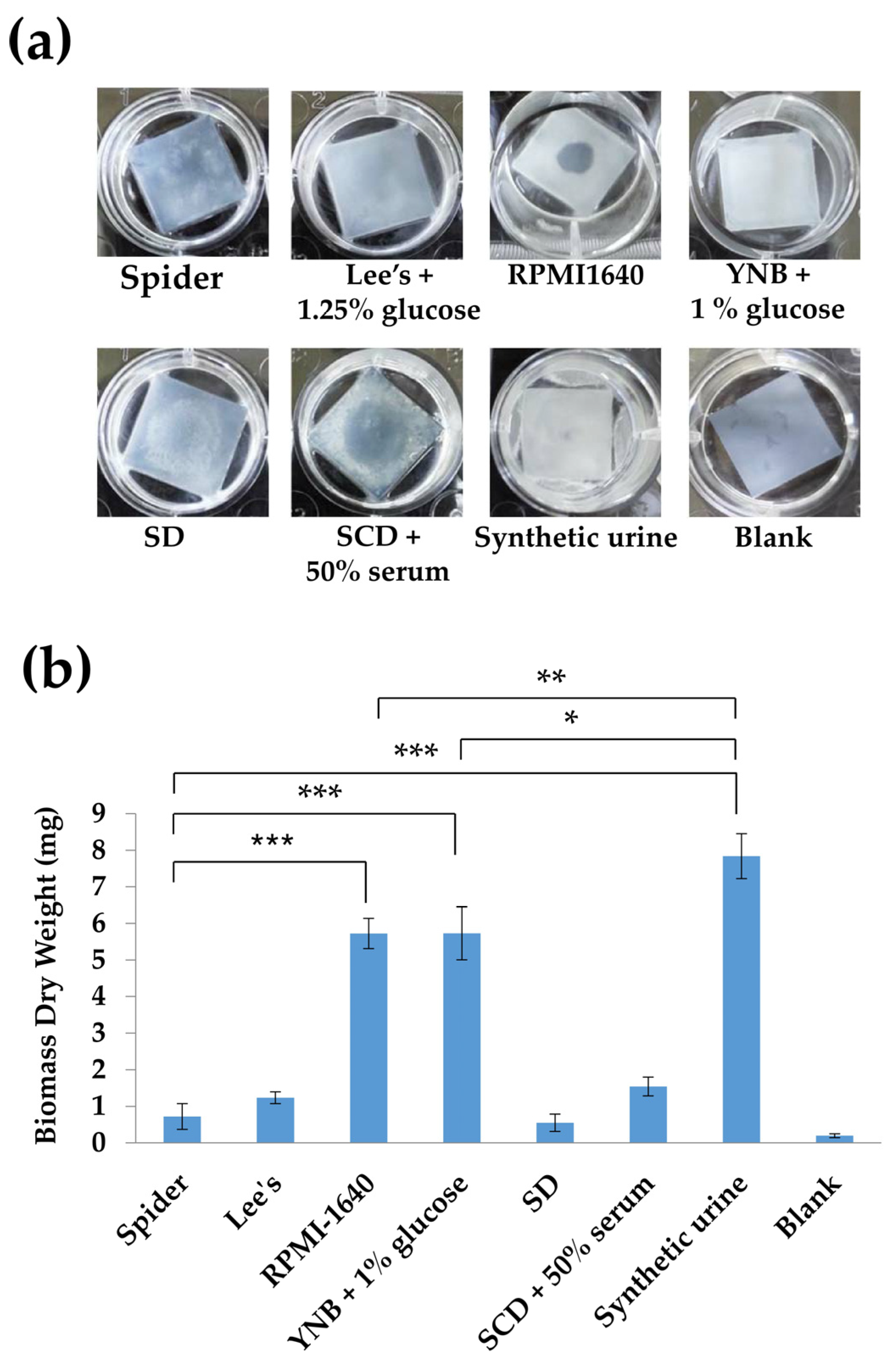
3.1. Effects of Different Culture Conditions on Biofilm Formation in the C. tropicalis Wild-Type Strain (MYA3404)
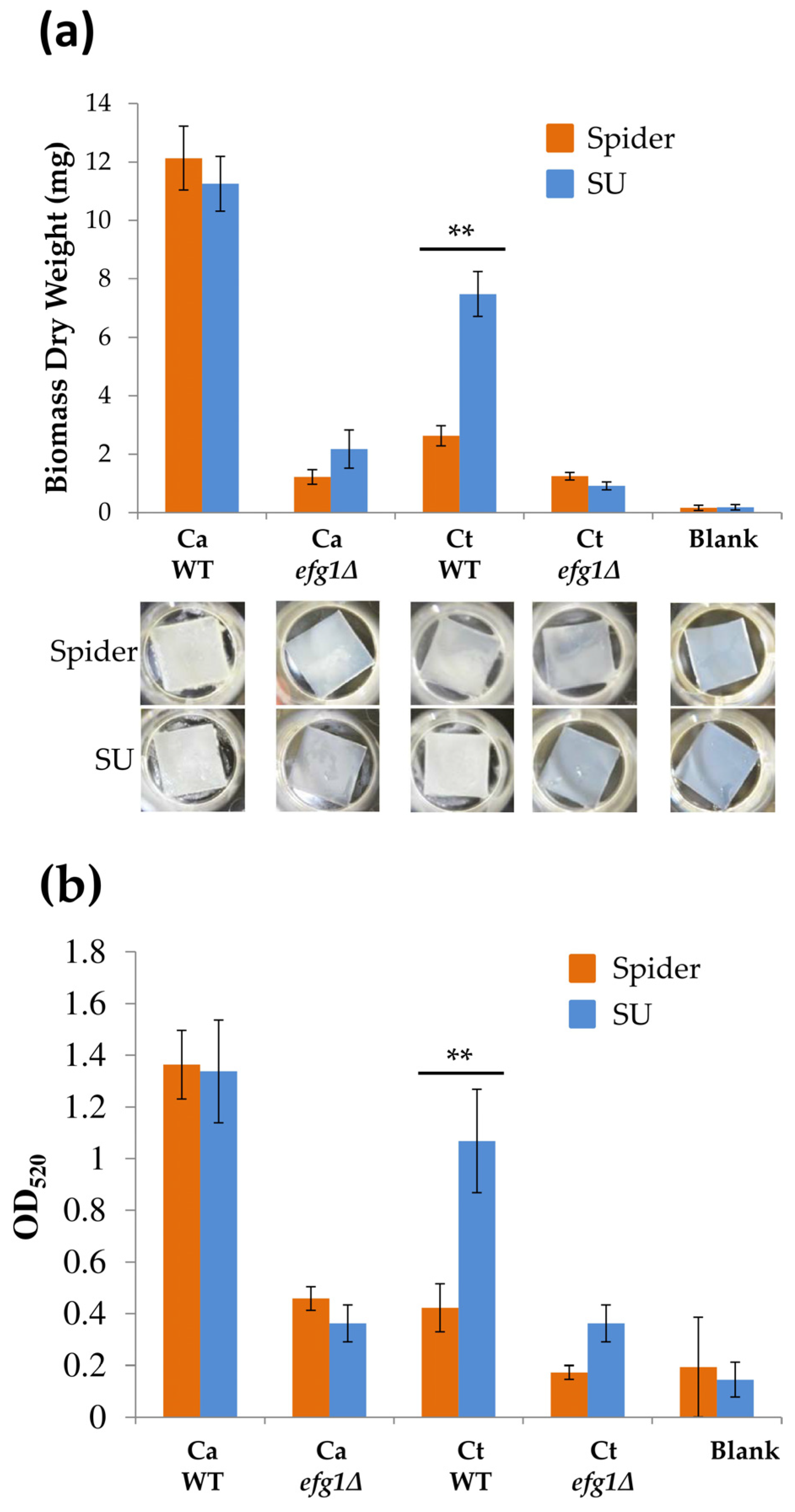
3.2. Replacement of Spider Medium with Synthetic Urine in the Adherence Step Profoundly Affected C. tropicalis Biofilm Growth but not that of C. albicans
3.3. Effects of Depletion of Each Ingredient in SU on C. tropicalis Biofilm Growth
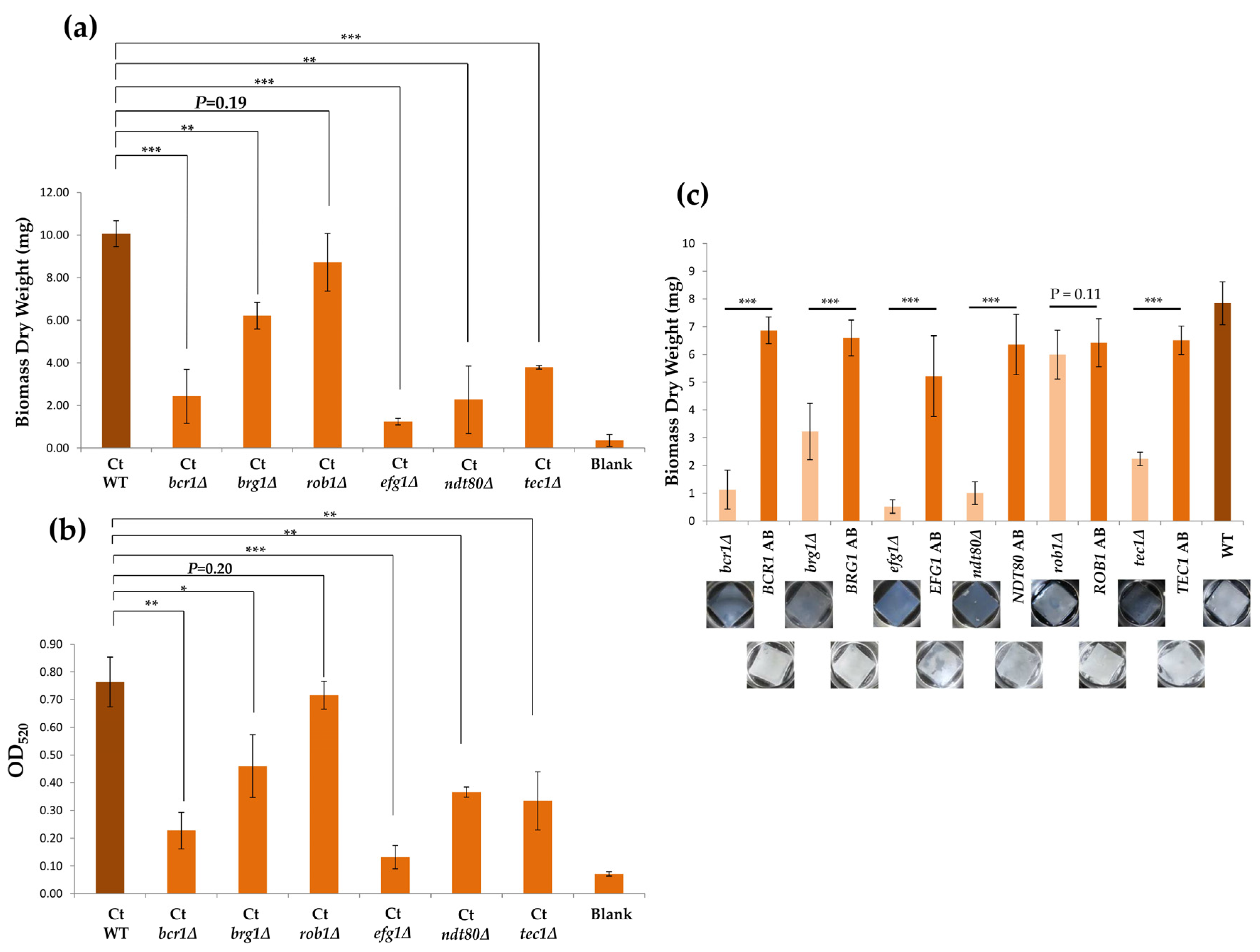
3.4. ROB1 is not Required for Biofilm Development in C. tropicalis
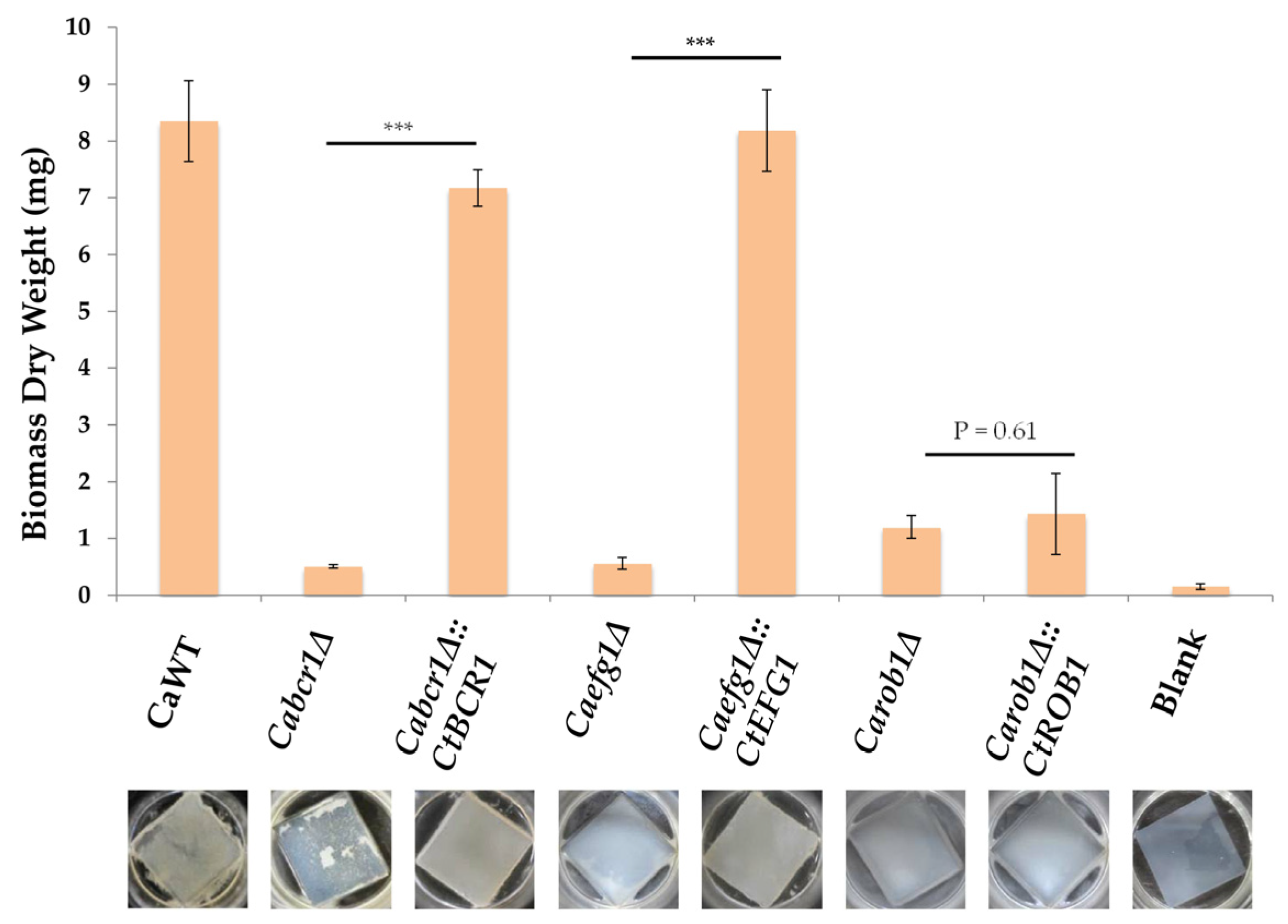
3.5. Introduction of the C. tropicalis ROB1 into C. albicans Rob1δ Was Not Able to Restore Biofilm Growth in C. albicans
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Achkar, J.M.; Fries, B.C. Candida Infections of the genitourinary tract. Clin. Microbiol. Rev. 2010, 23, 253–273. [Google Scholar] [CrossRef] [PubMed]
- Kullberg, B.-J.; Arendrup, M.C. Invasive candidiasis. New Engl. J. Med. 2015, 373, 1445–1456. [Google Scholar] [CrossRef] [PubMed]
- Delaloye, J.; Calandra, T. Invasive candidiasis as a cause of sepsis in the critically ill patient. Virulence 2013, 5, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Diekema, D.; Arbefeville, S.; Boyken, L.; Kroeger, J.; Pfaller, M. The changing epidemiology of healthcare-associated candidemia over three decades. Diagn. Microbiol. Infect. Dis. 2012, 73, 45–48. [Google Scholar] [CrossRef]
- Chang, T.-P.; Lo, P.-C.; Wang, A.-H.; Lo, H.-J.; Ho, M.-W.; Yang, Y.-L.; Lin, P.-S. Distribution and drug susceptibilities of Candida species causing candidemia from a medical center in central Taiwan. J. Infect. Chemother. 2013, 19, 1065–1071. [Google Scholar] [CrossRef]
- Guinea, J.; Ortega, J.V.G. Global trends in the distribution of Candida species causing candidemia. Clin. Microbiol. Infect. 2014, 20, 5–10. [Google Scholar] [CrossRef]
- Falagas, M.E.; Roussos, N.; Vardakas, K. Relative frequency of albicans and the various non-albicans Candida spp among candidemia isolates from inpatients in various parts of the world: A systematic review. Int. J. Infect. Dis. 2010, 14, e954–e966. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.-Y.; Chuang, Y.-C.; Wang, J.-T.; Sheng, W.-H.; Yu, C.-J.; Chu, C.-C.; Hsueh, P.-R.; Chang, S.-C.; Chen, Y.-C. Comparison of epidemiology and treatment outcome of patients with candidemia at a teaching hospital in Northern Taiwan, in 2002 and 2010. J. Microbiol. Immunol. Infect. 2014, 47, 95–103. [Google Scholar] [CrossRef]
- Barchiesi, F.; Calabrese, D.; Sanglard, D.; Di Francesco, L.F.; Caselli, F.; Giannini, D.; Giacometti, A.; Gavaudan, S.; Scalise, G. Experimental induction of fluconazole resistance in Candida tropicalis ATCC 750. Antimicrob. Agents Chemother. 2000, 44, 1578–1584. [Google Scholar] [CrossRef]
- Yang, Y.-L.; Wang, A.-H.; Wang, C.-W.; Cheng, W.-T.; Li, S.-Y.; Lo, H.-J. Susceptibilities to amphotericin B and fluconazole of Candida species in Taiwan Surveillance of Antimicrobial Resistance of Yeasts 2006. Diagn. Microbiol. Infect. Dis. 2008, 61, 175–180. [Google Scholar] [CrossRef]
- Yang, Y.-L.; Lin, C.-C.; Chang, T.-P.; Lauderdale, T.-L.; Chen, H.-T.; Lee, C.-F.; Hsieh, C.-W.; Chen, P.-C.; Lo, H.J. Comparison of human and soil Candida tropicalis isolates with reduced susceptibility to fluconazole. PLoS ONE 2012, 7, e34609. [Google Scholar] [CrossRef] [PubMed]
- Finkel, J.S.; Mitchell, A.P. Genetic control of Candida albicans biofilm development. Nat. Rev. Genet. 2010, 9, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Angiolella, L.; Stringaro, A.; De Bernardis, F.; Posteraro, B.; Bonito, M.; Toccacieli, L.; Torosantucci, A.; Colone, M.; Sanguinetti, M.; Cassone, A.; et al. Increase of virulence and its phenotypic traits in drug-resistant strains of Candida albicans. Antimicrob. Agents Chemother. 2008, 52, 927–936. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ford, C.B.; Funt, J.M.; Abbey, D.; Issi, L.; Guiducci, C.; A Martinez, D.; DeLorey, T.; Li, B.Y.; White, T.C.; Cuomo, C.A.; et al. The evolution of drug resistance in clinical isolates of Candida albicans. eLife 2015, 4. [Google Scholar] [CrossRef]
- Douglas, L.J. Candida biofilms and their role in infection. Trends Microbiol. 2003, 11, 30–36. [Google Scholar] [CrossRef]
- Nobile, C.J.; Fox, E.P.; Nett, J.E.; Sorrells, T.R.; Mitrovich, Q.M.; Hernday, A.D.; Tuch, B.B.; Andes, D.R.; Johnson, A.D. A recently evolved transcriptional network controls biofilm development in Candida albicans. Cell 2012, 148, 126–138. [Google Scholar] [CrossRef]
- Fox, E.P.; Bui, C.K.; Nett, J.E.; Hartooni, N.; Mui, M.C.; Andes, D.R.; Nobile, C.J.; Johnson, A.D. An expanded regulatory network temporally controls Candida albicans biofilm formation. Mol. Microbiol. 2015, 96, 1226–1239. [Google Scholar] [CrossRef]
- Lohse, M.B.; Gulati, M.; Johnson, A.D.; Nobile, C.J. Development and regulation of single- and multi-species Candida albicans biofilms. Nat. Rev. Genet. 2017, 16, 19–31. [Google Scholar] [CrossRef]
- Negri, M.; Silva, S.; Henriques, M.; Azeredo, J.; Svidzinski, T.I.E.; Oliveira, R. Candida tropicalis biofilms: Artificial urine, urinary catheters and flow model. Med Mycol. 2011, 49, 1–9. [Google Scholar] [CrossRef]
- Mancera, E.; Porman, A.M.; Cuomo, C.A.; Bennett, R.J.; Johnson, A.D. Finding a Missing Gene: EFG1 regulates morphogenesis in Candida tropicalis. G3 Genes Genomes Genet. 2015, 5, 849–856. [Google Scholar] [CrossRef]
- Lin, C.-H.; Kabrawala, S.; Fox, E.P.; Nobile, C.J.; Johnson, A.D.; Bennett, R.J. Genetic control of conventional and pheromone-stimulated biofilm formation in Candida albicans. PLOS Pathog. 2013, 9. [Google Scholar] [CrossRef] [PubMed]
- Soll, D.R.; Daniels, K.J. Plasticity of Candida albicans biofilms. Microbiol. Mol. Boil. Rev. 2016, 80, 565–595. [Google Scholar] [CrossRef] [PubMed]
- Uppuluri, P.; Acosta-Zaldívar, M.; Anderson, M.Z.; Dunn, M.J.; Berman, J.; Ribot, J.L.L.; Köhler, J.R. Candida albicans dispersed cells are developmentally distinct from biofilm and planktonic cells. mBio 2018, 9, e01338-18. [Google Scholar] [CrossRef] [PubMed]
- Uppuluri, P.; Dinakaran, H.; Thomas, D.P.; Chaturvedi, A.K.; Lopez-Ribot, J.L. Characteristics of Candida albicans biofilms grown in a synthetic urine medium. J. Clin. Microbiol. 2009, 47, 4078–4083. [Google Scholar] [CrossRef] [PubMed]
- Weerasekera, M.M.; Wijesinghe, G.K.; A Jayarathna, T.; Gunasekara, C.P.; Fernando, N.; Kottegoda, N.; Samaranayake, L.P. Culture media profoundly affect Candida albicans and Candida tropicalis growth, adhesion and biofilm development. Mem. Inst. Oswaldo Cruz 2016, 111, 697–702. [Google Scholar] [CrossRef]
- Butler, G.; Rasmussen, M.D.; Lin, M.F.; Santos, M.A.S.; Sakthikumar, S.; Munro, C.; Rheinbay, E.; Grabherr, M.; Forche, A.; Reedy, J.L.; et al. Evolution of pathogenicity and sexual reproduction in eight Candida genomes. Nature 2009, 459, 657–662. [Google Scholar] [CrossRef]
- Reuss, O.; Vik, Å.; Kolter, R.; Morschhäuser, J. The SAT1 flipper, an optimized tool for gene disruption in Candida albicans. Gene 2004, 341, 119–127. [Google Scholar] [CrossRef]
- Bennett, R.J.; Johnson, A.D. The role of nutrient regulation and the Gpa2 protein in the mating pheromone response of C. albicans. Mol. Microbiol. 2006, 62, 100–119. [Google Scholar] [CrossRef]
- Galan-Ladero, M.A.; Blanco-Blanco, M.T.; Hurtado, C.; Pérez-Giraldo, C.; Blanco, M.T.; Garcia, A.C.G. Determination of biofilm production by Candida tropicalis isolated from hospitalized patients and its relation to cellular surface hydrophobicity, plastic adherence and filamentation ability. Yeast 2013, 30, 331–339. [Google Scholar] [CrossRef]
- Jones, S.; Hirakawa, M.; Bennett, R.J. Sexual biofilm formation in Candida tropicalis opaque cells. Mol. Microbiol. 2014, 92, 383–398. [Google Scholar] [CrossRef]
- Zhang, Q.; Tao, L.; Guan, G.; Yue, H.; Liang, W.; Cao, C.; Dai, Y.; Huang, G. Regulation of filamentation in the human fungal pathogen Candida tropicalis. Mol. Microbiol. 2015, 99, 528–545. [Google Scholar] [CrossRef] [PubMed]
- Cihlar, R.L.; Calderone, R.A. Candida albicans: Methods and Protocols; Humana Press: Totowa, NJ, USA, 2009; 206p. [Google Scholar]
- Fisher, J.F.; Kavanagh, K.; Sobel, J.D.; Kauffman, C.A.; Newman, C.A. Candida urinary tract infection: Pathogenesis. Clin. Infect. Dis. 2011, 52, S437–S451. [Google Scholar] [CrossRef] [PubMed]
- Mackie, J.; Szabo, E.K.; Urgast, D.S.; Ballou, E.R.; Childers, D.S.; Maccallum, D.; Feldmann, J.; Brown, A.J. Host-imposed copper poisoning impacts fungal micronutrient acquisition during systemic Candida albicans infections. PLoS ONE 2016, 11, e0158683. [Google Scholar] [CrossRef] [PubMed]
- Cvetkovic, A.; Menon, A.L.; Thorgersen, M.P.; Scott, J.W.; Poole, F.L., II; Jenney, F.E., Jr.; Lancaster, W.A.; Praissman, J.L.; Shanmukh, S.; Vaccaro, B.J.; et al. Microbial metalloproteomes are largely uncharacterized. Nature 2010, 466, 779–782. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.S.; Dietz, K.-J. The relationship between metal toxicity and cellular redox imbalance. Trends Plant Sci. 2009, 14, 43–50. [Google Scholar] [CrossRef]
- De Kerchove, A.J.; Elimelech, M. Calcium and magnesium cations enhance the adhesion of motile and nonmotile Pseudomonas aeruginosaon alginate films. Langmuir 2008, 24, 3392–3399. [Google Scholar] [CrossRef]
- Song, B.; Leff, L.G. Influence of magnesium ions on biofilm formation by Pseudomonas fluorescens. Microbiol. Res. 2006, 161, 355–361. [Google Scholar] [CrossRef]
- García-Betancur, J.-C.; Goni-Moreno, A.; Horger, T.; Schott, M.; Sharan, M.; Eikmeier, J.; Wohlmuth, B.; Zernecke, A.; Ohlsen, K.; Kuttler, C.; et al. Cell differentiation defines acute and chronic infection cell types in Staphylococcus aureus. eLife 2017, 6. [Google Scholar] [CrossRef]
- Patrauchan, M.A.; Sarkisova, S.; Sauer, K.; Franklin, M.J. Calcium influences cellular and extracellular product formation during biofilm-associated growth of a marine Pseudoalteromonas sp. Microbiology 2005, 151, 2885–2897. [Google Scholar] [CrossRef]
- Oknin, H.; Steinberg, D.; Shemesh, M. Magnesium ions mitigate biofilm formation of Bacillus species via downregulation of matrix genes expression. Front. Microbiol. 2015, 6, 907. [Google Scholar] [CrossRef]
- Dunne, W.M.; Burd, E.M. The effects of magnesium, calcium, EDTA, and pH on the in vitro adhesion of Staphylococcus epidermidis to Plastic. Microbiol. Immunol. 1992, 36, 1019–1027. [Google Scholar] [CrossRef] [PubMed]
- Shafeeq, S.; Pannanusorn, S.; Elsharabasy, Y.; Ramírez-Zavala, B.; Morschhäuser, J.; Römling, U. Impact of manganese on biofilm formation and cell morphology of Candida parapsilosis clinical isolates with different biofilm forming abilities. FEMS Yeast Res. 2019, 19. [Google Scholar] [CrossRef] [PubMed]
- Harrison, J.; Ceri, H.; Yerly, J.; Rabiei, M.; Hu, Y.; Martinuzzi, R.; Turner, R.J. Metal ions may suppress or enhance cellular differentiation in Candida albicans and Candida tropicalis biofilms. Appl. Environ. Microbiol. 2007, 73, 4940–4949. [Google Scholar] [CrossRef] [PubMed]
- Holland, L.M.; Schröder, M.; Turner, S.A.; Taff, H.; Andes, D.; Grózer, Z.; Gacser, A.; Ames, L.; Haynes, K.; Higgins, D.; et al. Comparative phenotypic analysis of the major fungal pathogens Candida parapsilosis and Candida albicans. PLoS Pathog. 2014, 10, e1004365. [Google Scholar] [CrossRef]


| Strain | Species | Genotype | Source |
|---|---|---|---|
| SC5314 | C. albicans | Wild-Type | [26] |
| RBY717 | C. albicans | ura3::imm434/URA3 iro1::imm434/IRO1 | [28] |
| YL477 | C. tropicalis | wild-type sequence strain (MYA3404) | [26] |
| YL1344 | C. albicans | bcr1Δ/bcr1Δ | [21] |
| YL1346 | C. albicans | brg1Δ/brg1Δ | [21] |
| YL1348 | C. albicans | efg1Δ/efg1Δ | [21] |
| YL1350 | C. albicans | ndt80Δ/ndt80Δ | [21] |
| YL1354 | C. albicans | rob1Δ/rob1Δ | [21] |
| YL1356 | C. albicans | tec1Δ/tec1Δ | [21] |
| YL1380 | C. tropicalis | tec1Δ/tec1Δ/-SAT1 | This study |
| YL1381 | C. tropicalis | tec1Δ/tec1Δ/-SAT1 | This study |
| YL1382 | C. tropicalis | ndt80Δ/ndt80Δ/-SAT1 | This study |
| YL1384 | C. tropicalis | ndt80Δ/ndt80Δ/-SAT1 | This study |
| YL1402 | C. tropicalis | brg1Δ/brg1Δ/-SAT1 | This study |
| YL1403 | C. tropicalis | brg1Δ/brg1Δ/-SAT1 | This study |
| YL1404 | C. tropicalis | bcr1Δ/bcr1Δ/-SAT1 | This study |
| YL1406 | C. tropicalis | bcr1Δ/bcr1Δ/-SAT1 | This study |
| YL1410 | C. tropicalis | efg1Δ/efg1/-SAT1 | This study |
| YL1411 | C. tropicalis | efg1Δ/efg1/-SAT1 | This study |
| YL1413 | C. tropicalis | rob1Δ/rob1Δ/-SAT1 | This study |
| YL1414 | C. tropicalis | rob1Δ/rob1Δ/-SAT11 | This study |
| YL1417 | C. albicans | efg1Δ/efg1Δ/::CtEFG1-SAT1 | This study |
| YL1419 | C. albicans | efg1Δ/efg1Δ/::CtEFG1-SAT1 | This study |
| YL1450 | C. albicans | bcr1Δ/bcr1Δ/::CtBCR1-SAT1 | This study |
| YL1451 | C. albicans | bcr1Δ/bcr1Δ/::CtBCR1-SAT1 | This study |
| YL1456 | C. albicans | rob1Δ/rob1Δ/::CtROB1-SAT1 | This study |
| YL1457 | C. albicans | rob1Δ/rob1Δ/::CtROB1-SAT1 | This study |
| YL1493 | C. tropicalis | bcr1Δ/bcr1Δ/::BCR1-SAT1 | This study |
| YL1494 | C. tropicalis | bcr1Δ/bcr1Δ/::BCR1-SAT1 | This study |
| YL1495 | C. tropicalis | brg1Δ/brg1Δ/::BRG1-SAT1 | This study |
| YL1496 | C. tropicalis | brg1Δ/brg1Δ/::BRG1-SAT1 | This study |
| YL1497 | C. tropicalis | rob1Δ/rob1Δ/::ROB1-SAT1 | This study |
| YL1498 | C. tropicalis | tec1Δ/tec1Δ/::TEC1-SAT1 | This study |
| YL1499 | C. tropicalis | tec1Δ tec1Δ/::TEC1-SAT1 | This study |
| YL1504 | C. tropicalis | rob1Δ rob1Δ/::ROB1-SAT1 | This study |
| YL1570 | C. tropicalis | efg1Δ/efg1Δ/::EFG1-SAT1 | This study |
| YL1571 | C. tropicalis | efg1Δ/efg1Δ/::EFG1-SAT1 | This study |
| YL1572 | C. tropicalis | ndt80Δ/ndt80Δ/::NDT80-SAT1 | This study |
| YL1573 | C. tropicalis | ndt80Δ/ndt80Δ/::NDT80-SAT1 | This study |
| Protein in C. tropicalis | Bcr1 | Brg1 | Efg1 | Tec1 | Rob1 | Ndt80 |
|---|---|---|---|---|---|---|
| Identity to respective homolog in C. albicans | 53.4% | 44.0% | 55.4% | 61.4% | 35.9% | 72.1% |
| Similarity to respective homolog in C. albicans | 62.1% | 50.5% | 60.2% | 73.7% | 50% | 75.2% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tseng, Y.-K.; Chen, Y.-C.; Hou, C.-J.; Deng, F.-S.; Liang, S.-H.; Hoo, S.Y.; Hsu, C.-C.; Ke, C.-L.; Lin, C.-H. Evaluation of Biofilm Formation in Candida tropicalis Using a Silicone-Based Platform with Synthetic Urine Medium. Microorganisms 2020, 8, 660. https://doi.org/10.3390/microorganisms8050660
Tseng Y-K, Chen Y-C, Hou C-J, Deng F-S, Liang S-H, Hoo SY, Hsu C-C, Ke C-L, Lin C-H. Evaluation of Biofilm Formation in Candida tropicalis Using a Silicone-Based Platform with Synthetic Urine Medium. Microorganisms. 2020; 8(5):660. https://doi.org/10.3390/microorganisms8050660
Chicago/Turabian StyleTseng, Yi-Kai, Yu-Chia Chen, Chien-Jui Hou, Fu-Sheng Deng, Shen-Huan Liang, Sin Yong Hoo, Chih-Chieh Hsu, Cai-Ling Ke, and Ching-Hsuan Lin. 2020. "Evaluation of Biofilm Formation in Candida tropicalis Using a Silicone-Based Platform with Synthetic Urine Medium" Microorganisms 8, no. 5: 660. https://doi.org/10.3390/microorganisms8050660
APA StyleTseng, Y.-K., Chen, Y.-C., Hou, C.-J., Deng, F.-S., Liang, S.-H., Hoo, S. Y., Hsu, C.-C., Ke, C.-L., & Lin, C.-H. (2020). Evaluation of Biofilm Formation in Candida tropicalis Using a Silicone-Based Platform with Synthetic Urine Medium. Microorganisms, 8(5), 660. https://doi.org/10.3390/microorganisms8050660





