Microbial Screening Based on the Mizoroki–Heck Reaction Permits Exploration of Hydroxyhexylitaconic-Acid-Producing Fungi in Soils
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation of Fungi from Soils
2.2. Identification of S17-5 Strain
2.3. Purification of Vinyl Compounds Produced by S17-5 Strain Culture
2.4. Structural Characterization of the Two Vinyl Compounds Produced by S17-5
2.5. Antibacterial Test
2.6. Anti-Inflammatory Test
2.7. Cytotoxicity Test
3. Results and Discussion
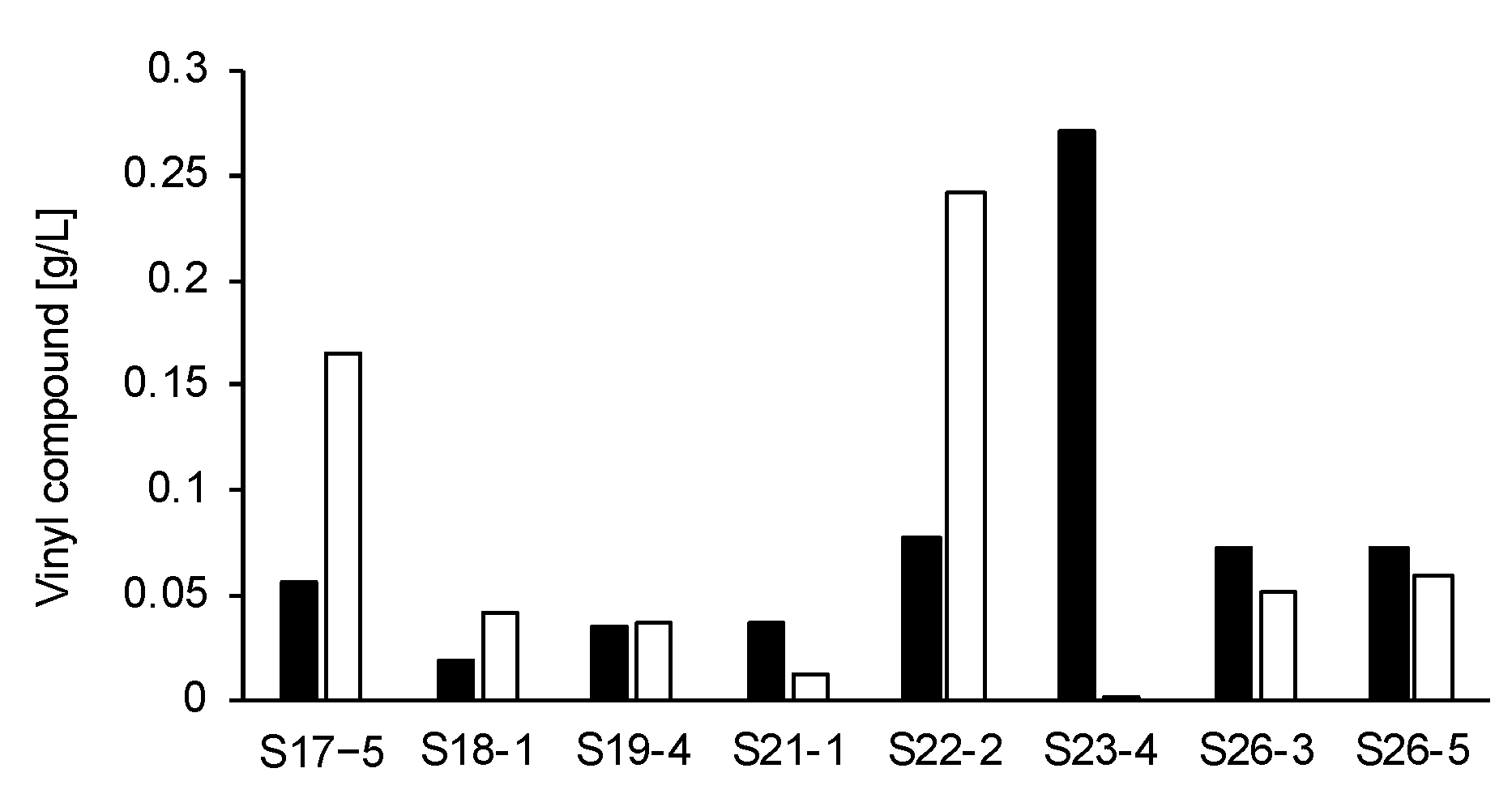
3.1. Characterization of Vinyl Compounds Produced by 37 Fungi Isolated from Soils
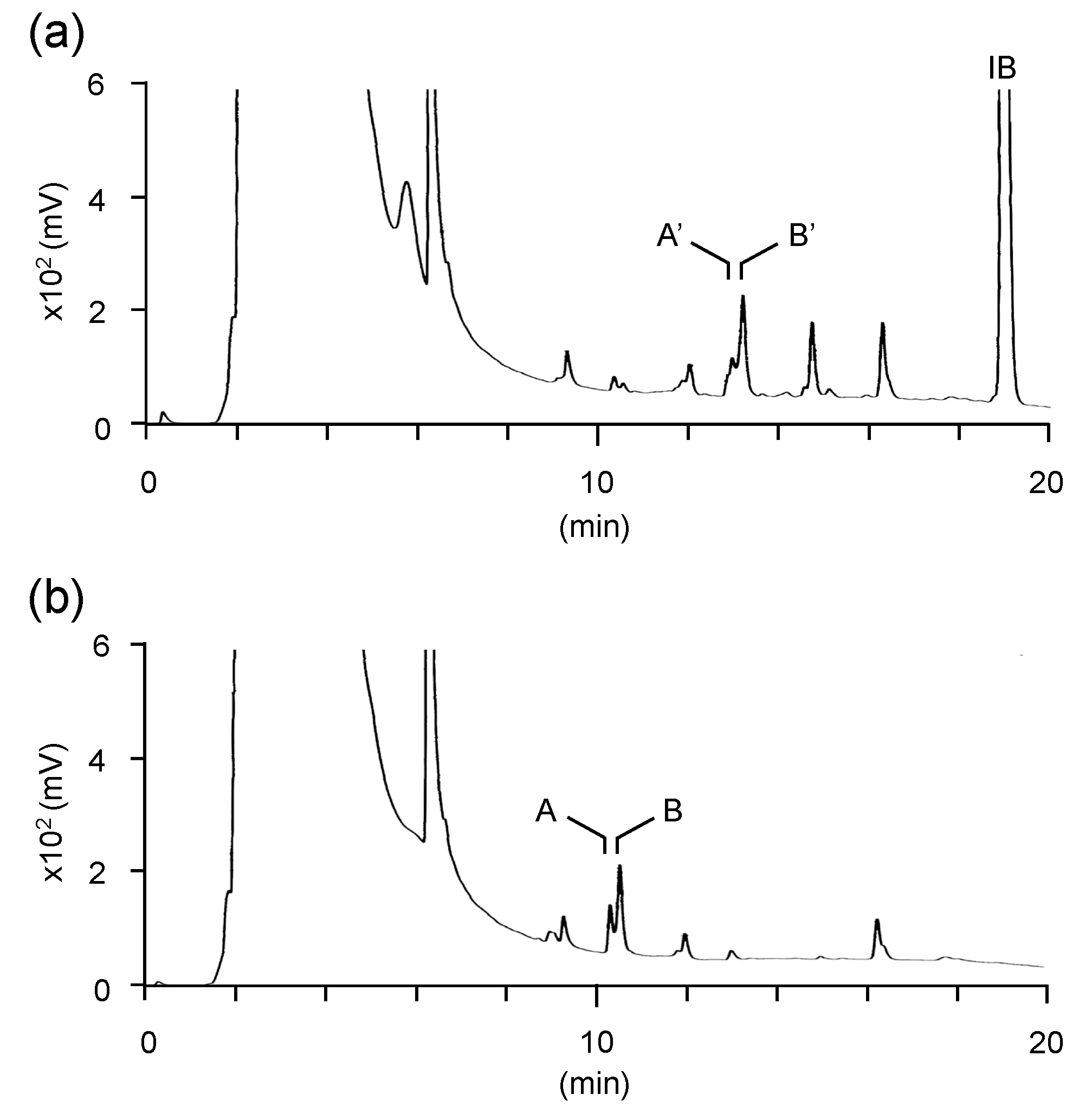
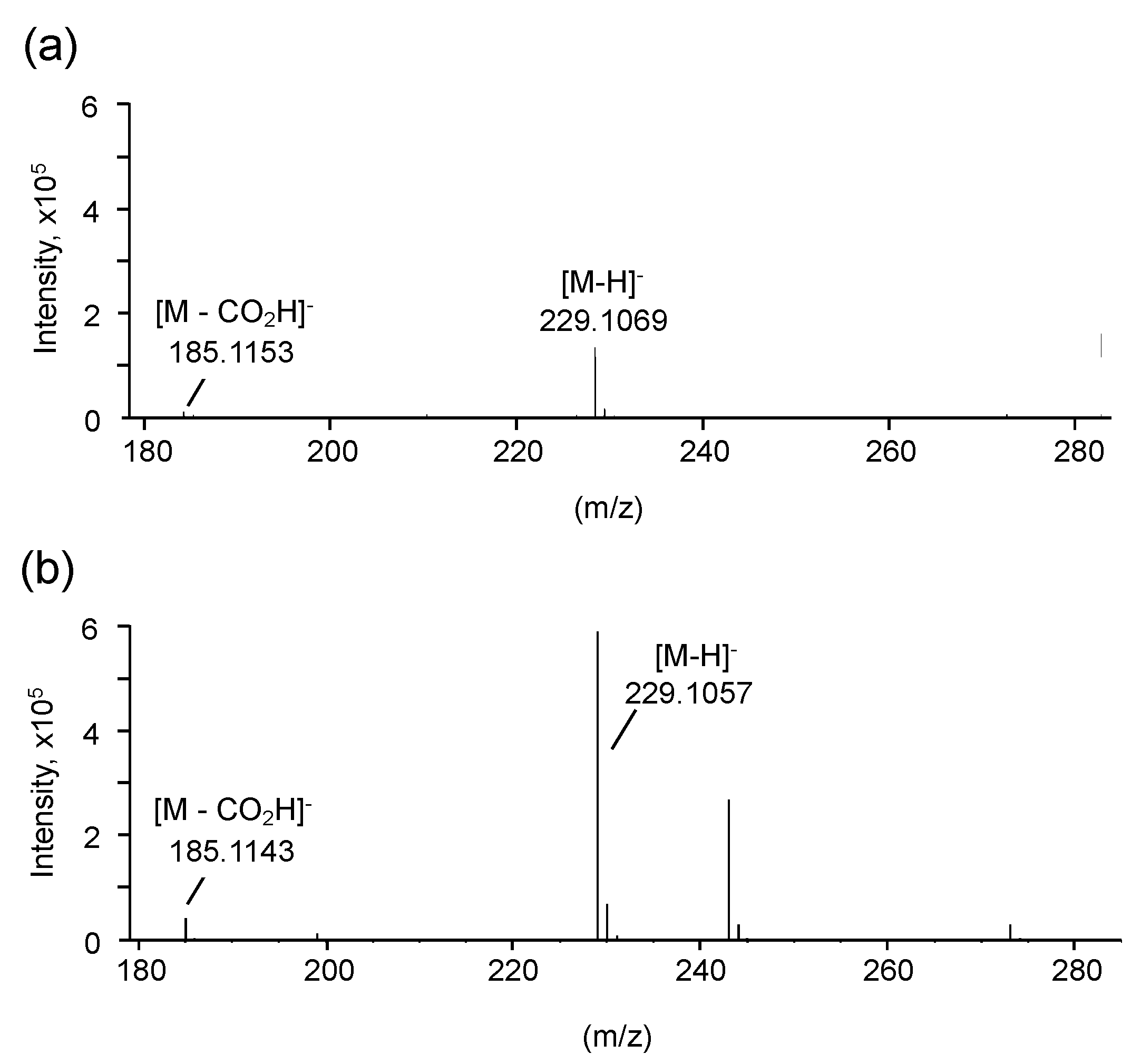
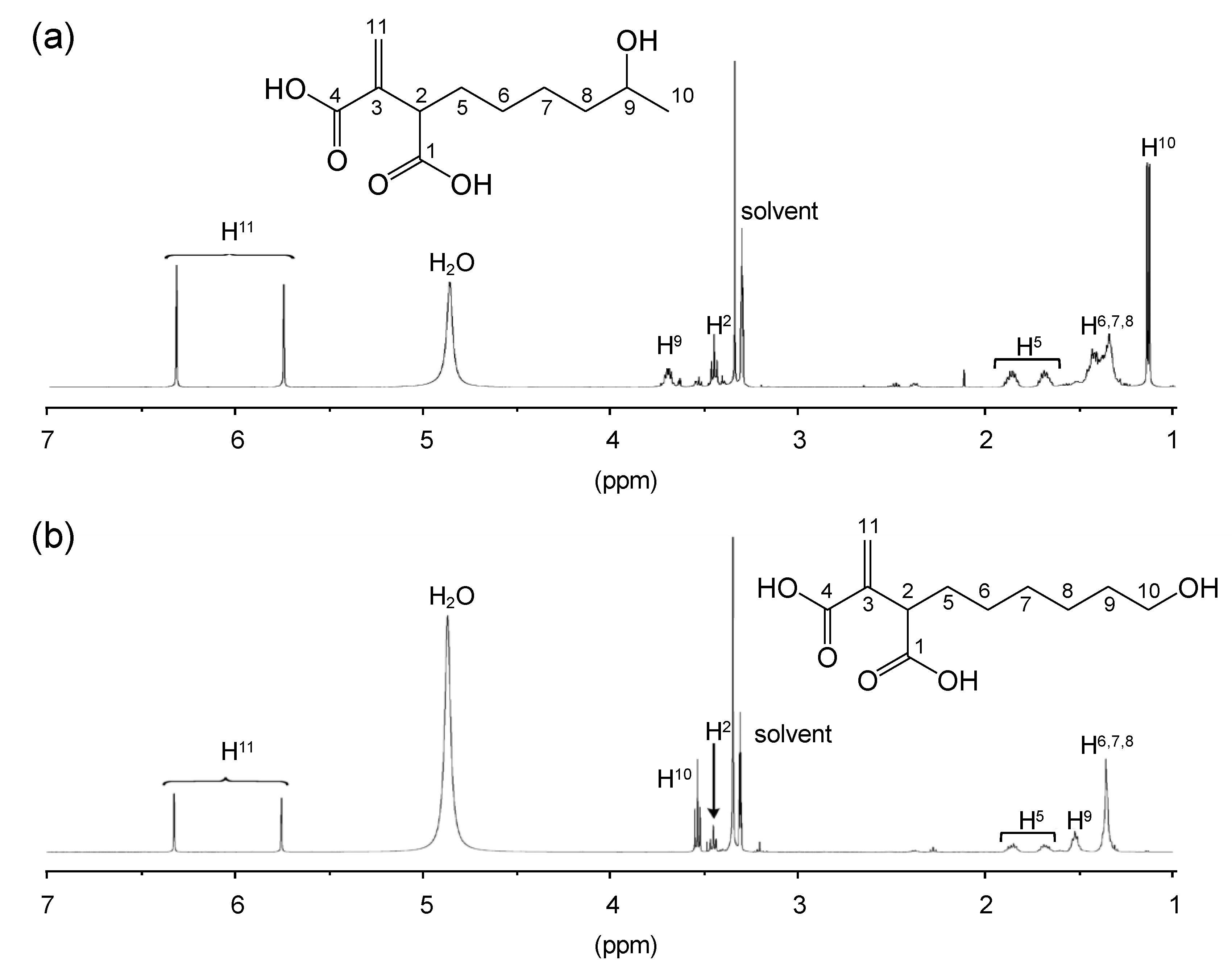
3.2. Structural Characterization of Vinyl Compounds A and B Produced by S17-5
3.3. Bioactive Characterization of 9-HHIA and 10-HHIA
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Teleky, B.-E.; Vodnar, D.C. Biomass-Derived Production of Itaconic Acid as a Building Block in Specialty Polymers. Polymers 2019, 11, 1035. [Google Scholar] [CrossRef] [PubMed]
- Hegde, K.; Prabhu, A.; Sarma, S.; Brar, S.; Dasu, V.V. Potential Applications of Renewable Itaconic Acid for the Synthesis of 3-Methyltetrahydrofuran. In Platform Chemical Biorefinery; Elsevier BV: Amsterdam, The Netherlands, 2016; pp. 181–200. [Google Scholar]
- Saha, B. Emerging biotechnologies for production of itaconic acid and its applications as a platform chemical. J. Ind. Microbiol. Biotechnol. 2016, 44, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Hajian, H.; Yusoff, W.M.W. Itaconic Acid Production by Microorganisms: A Review. Curr. Res. J. Boil. Sci. 2015, 7, 37–42. [Google Scholar] [CrossRef]
- Magalhães, A.I.; De Carvalho, J.C.; Medina, J.D.C.; Soccol, C.R. Downstream process development in biotechnological itaconic acid manufacturing. Appl. Microbiol. Biotechnol. 2016, 101, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Da Cruz, J.C.; Sérvulo, E.F.C.; De Castro, A.M. Microbial Production of Itaconic Acid. In Microbial Production of Food Ingredients and Additives; Elsevier BV: Amsterdam, The Netherlands, 2017; pp. 291–316. [Google Scholar]
- Abruzzo, A.; Armenise, N.; Bigucci, F.; Cerchiara, T.; Gösser, M.B.; Samori’, C.; Galletti, P.; Tagliavini, E.; Brown, D.M.; Johnston, H.; et al. Surfactants from itaconic acid: Toxicity to HaCaT keratinocytes in vitro, micellar solubilization, and skin permeation enhancement of hydrocortisone. Int. J. Pharm. 2017, 524, 9–15. [Google Scholar] [CrossRef]
- Malferrari, D.; Armenise, N.; Decesari, S.; Galletti, P.; Tagliavini, E. Surfactants from Itaconic Acid: Physicochemical Properties and Assessment of the Synthetic Strategies. ACS Sustain. Chem. Eng. 2015, 3, 1579–1588. [Google Scholar] [CrossRef]
- Calam, C.T.; Oxford, A.E.; Raistrick, H. Studies in the biochemistry of micro-organisms. Biochem. J. 1939, 33, 1488–1495. [Google Scholar] [CrossRef]
- Kuenz, A.; Gallenmüller, Y.; Willke, T.; Vorlop, K.-D. Microbial production of itaconic acid: Developing a stable platform for high product concentrations. Appl. Microbiol. Biotechnol. 2012, 96, 1209–1216. [Google Scholar] [CrossRef]
- Kinoshita, K. Über eine neue Aspergillus-Art, Asp. itaconicus nov. spec. J. Plant Res. 1931, 45, 45–60. [Google Scholar]
- Araki, T.; Yamazaki, Y.; Suzuki, N. Production of itaconic acid by Helicobasidium mompa TANAKA. Jpn. J. Phytopathol. 1957, 22, 83–87. [Google Scholar] [CrossRef]
- Guevarra, E.D.; Tabuchi, T. Accumulation of Itaconic, 2-Hydroxyparaconic, Itatartaric, and Malic Acids by Strains of the Genus Ustilago. J. Agric. Biol. Chem. 1990, 54, 2353–2358. [Google Scholar] [CrossRef][Green Version]
- Haskins, R.H.; Thorn, J.A.; Boothroyd, B. Biochemistry of the ustilaginales: Xi. Metabolic products of ustilago zeae in submerged culture. Can. J. Microbiol. 1955, 1, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Tabuchi, T.; Sugisawa, T.; Ishidori, T.; Nakahara, T.; Sugiyama, J. Itaconic Acid Fermentation by a Yeast Belonging to the Genus Candida. J. Agric. Biol. Chem. 1981, 45, 475–479. [Google Scholar] [CrossRef]
- Levinson, W.E.; Kurtzman, C.P.; Kuo, T.M. Production of itaconic acid by Pseudozyma antarctica NRRL Y-7808 under nitrogen-limited growth conditions. Enzym. Microb. Technol. 2006, 39, 824–827. [Google Scholar] [CrossRef]
- Li, G.; Kusari, S.; Lamshöft, M.; Schüffler, A.; Laatsch, H.; Spiteller, M.; Schüffler, A. Antibacterial Secondary Metabolites from an Endophytic Fungus, Eupenicillium sp. LG41. J. Nat. Prod. 2014, 77, 2335–2341. [Google Scholar] [CrossRef]
- Cai, J.; Zhou, X.-M.; Yang, X.; Tang, M.-M.; Liao, Q.-Y.; Meng, B.-Z.; Liao, S.; Chen, G. Three new bioactive natural products from the fungus Talaromyces assiutensis JTY2. Bioorganic Chem. 2020, 94, 103362. [Google Scholar] [CrossRef]
- Tsukamoto, S.; Yoshida, T.; Hosono, H.; Ohta, T.; Yokosawa, H. Hexylitaconic acid: A new inhibitor of p53–HDM2 interaction isolated from a marine-derived fungus, Arthrinium sp. Bioorganic Med. Chem. Lett. 2006, 16, 69–71. [Google Scholar] [CrossRef]
- Li, J.L.; Zhang, P.; Lee, Y.M.; Hong, J.; Yoo, E.S.; Bae, K.S.; Jung, J.H. Oxygenated hexylitaconates from a marine sponge-derived fungus Penicillium sp. Chem. Pharm. Bull. 2011, 59, 120–123. [Google Scholar] [CrossRef]
- Almassi, F.; Ghisalberti, E.L.; Rowland, C.Y. Alkylcitrate-Derived Metabolites from Aspergillus niger. J. Nat. Prod. 1994, 57, 833–836. [Google Scholar] [CrossRef]
- Klemke, C.; Kehraus, S.; Wright, A.D.; König, G.M. New Secondary Metabolites from the Marine Endophytic Fungus Apiospora montagnei †. J. Nat. Prod. 2004, 67, 1058–1063. [Google Scholar] [CrossRef]
- Kaaniche, F.; Hamed, A.; Abdel-Razek, A.S.; Wibberg, D.; Abdissa, N.; El Euch, I.Z.; Allouche, N.; Mellouli, L.; Shaaban, M.; Sewald, N. Bioactive secondary metabolites from new endophytic fungus Curvularia. sp isolated from Rauwolfia macrophylla. PLoS ONE 2019, 14, e0217627. [Google Scholar] [CrossRef] [PubMed]
- Antia, B.S.; Aree, T.; Kasettrathat, C.; Wiyakrutta, S.; Ekpa, O.D.; Ekpe, U.J.; Mahidol, C.; Ruchirawat, S.; Kittakoop, P. Itaconic acid derivatives and diketopiperazine from the marine-derived fungus Aspergillus aculeatus CRI322-03. Phytochemistry 2011, 72, 816–820. [Google Scholar] [CrossRef] [PubMed]
- Enoki, M.; Honda, Y.; Watanabe, T.; Kuwahara, M. A novel dicarboxylic acid produced by white-rot fungus Ceriporiopsis subvermispora. In Proceedings of the 44th Lignin Symp, Gifu, Japan, 7–8 October 1999; pp. 69–72. [Google Scholar]
- Mondal, G.; Dureja, P.; Sen, B. Fungal metabolites from Aspergillus niger AN27 related to plant growth promotion. Indian J. Exp. Boil. 2000, 38, 84–87. [Google Scholar]
- Varoglu, M.; Crews, P. Biosynthetically diverse compounds from a saltwater culture of sponge-derived Aspergillus niger. J. Nat. Prod. 2000, 63, 41–43. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Yan, W.; Gu, C.; Wang, Z.; Zhao, S.; Kang, S.; Khan, B.; Zhu, H.; Li, J.; Ye, Y. New Alkylitaconic Acid Derivatives from Nodulisporium sp. A21 and Their Auxin Herbicidal Activities on Weed Seeds. J. Agric. Food Chem. 2019, 67, 2811–2817. [Google Scholar] [CrossRef]
- Liu, L.; Han, Y.; Xiao, J.; Li, L.; Guo, L.; Jiang, X.; Kong, L.; Che, Y. Chlorotheolides A and B, Spiroketals Generated via Diels–Alder Reactions in the Endophytic Fungus Pestalotiopsis theae. J. Nat. Prod. 2016, 79, 2616–2623. [Google Scholar] [CrossRef]
- Enoki, M.; Watanabe, T.; Nakagame, S.; Koller, K.; Messner, K.; Honda, Y.; Kuwahara, M. Extracellular lipid peroxidation of selective white-rot fungus, Ceriporiopsis subvermispora. FEMS Microbiol. Lett. 1999, 180, 205–211. [Google Scholar] [CrossRef]
- Amirta, R.; Fujimori, K.; Shirai, N.; Honda, Y.; Watanabe, T. Ceriporic acid C, a hexadecenylitaconate produced by a lignin-degrading fungus, Ceriporiopsis subvermispora. Chem. Phys. Lipids 2003, 126, 121–131. [Google Scholar] [CrossRef]
- Nishimura, H.; Tsuda, S.; Shimizu, H.; Ohashi, Y.; Watanabe, T.; Honda, Y.; Watanabe, T. De novo synthesis of (Z)- and (E)-7-hexadecenylitaconic acids by a selective lignin-degrading fungus, Ceriporiopsis subvermispora. Phytochemistry 2008, 69, 2593–2602. [Google Scholar] [CrossRef]
- Nishimura, H.; Setogawa, Y.; Watanabe, T.; Honda, Y.; Watanabe, T. Epoxy ceriporic acid produced by selective lignin-degrading fungus Ceriporiopsis subvermispora. Chem. Phys. Lipids 2011, 164, 707–712. [Google Scholar] [CrossRef]
- Nishimura, H.; Sasaki, M.; Seike, H.; Nakamura, M.; Watanabe, T. Alkadienyl and alkenyl itaconic acids (ceriporic acids G and H) from the selective white-rot fungus Ceriporiopsis subvermispora: A new class of metabolites initiating ligninolytic lipid peroxidation. Org. Biomol. Chem. 2012, 10, 6432. [Google Scholar] [CrossRef]
- Nishimura, H.; Murayama, K.; Watanabe, T.; Honda, Y.; Watanabe, T. Diverse rare lipid-related metabolites including ω-7 and ω-9 alkenylitaconic acids (ceriporic acids) secreted by a selective white rot fungus, Ceriporiopsis subvermispora. Chem. Phys. Lipids 2012, 165, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Furukawa, H.; Tomura, T.; Fudou, R.; Kaida, K.; Choi, B.-K.; Imokawa, G.; Ojika, M. Bioactive Maleic Anhydrides and Related Diacids from the Aquatic Hyphomycete Tricladium castaneicola. J. Nat. Prod. 2015, 78, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, Y.; Fukuda, T.; Hagimori, K.; Tomoda, H.; Omura, S. Tensyuic acids, new antibiotics produced by Aspergillus niger FKI-2342. Chem. Pharm. Bull. 2007, 55, 1338–1341. [Google Scholar] [CrossRef]
- Abate, D.; Abraham, W.-R.; Meyer, H. Cytochalasins and phytotoxins from the fungus Xylaria obovata. Phytochemistry 1997, 44, 1443–1448. [Google Scholar] [CrossRef]
- McCorkindale, N.; Wright, J.; Brian, P.; Clarke, S.; Hutchinson, S. Canadensolide - an antifungal metabolite of penicillium canadense. Tetrahedron Lett. 1968, 9, 727–730. [Google Scholar] [CrossRef]
- Cao, L.-L.; Zhang, Y.-Y.; Liu, Y.-J.; Yang, T.-T.; Zhang, J.-L.; Zhang, Z.-G.; Shen, L.; Liu, J.-Y.; Ye, Y.-H. Anti-phytopathogenic activity of sporothriolide, a metabolite from endophyte Nodulisporium sp. A21 in Ginkgo biloba. Pestic. Biochem. Physiol. 2016, 129, 7–13. [Google Scholar] [CrossRef]
- Kil Park, B.; Nakagawa, M.; Hirota, A.; Nakayama, M. Methylenolactocin, a novel antitumor antibiotic from Penicillium sp. J. Antibiot. 1988, 41, 751–758. [Google Scholar] [CrossRef]
- Ding, L.; Li, T.; Liao, X.; He, S.; Xu, S.-H. Asperitaconic acids A-C, antibacterial itaconic acid derivatives produced by a marine-derived fungus of the genus Aspergillus. J. Antibiot. 2018, 71, 902–904. [Google Scholar] [CrossRef]
- Mills, E.L.; Ryan, D.G.; Prag, H.A.; Dikovskaya, D.; Menon, D.; Zaslona, Z.; Jedrychowski, M.P.; Costa, A.S.H.; Higgins, M.; Hams, E.; et al. Itaconate is an anti-inflammatory metabolite that activates Nrf2 via alkylation of KEAP1. Nature 2018, 556, 113–117. [Google Scholar] [CrossRef]
- McFadden, B.A.; Williams, J.O.; Roche, T.E. Mechanism of action of isocitrate lyase from Pseudomonas indigofera. Biochemistry 1971, 10, 1384–1390. [Google Scholar] [CrossRef] [PubMed]
- McFadden, B.; Purohit, S. Itaconate, an isocitrate lyase-directed inhibitor in Pseudomonas indigofera. J. Bacteriol. 1977, 131, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Hooftman, A.; O’Neill, L.A.J. The Immunomodulatory Potential of the Metabolite Itaconate. Trends Immunol. 2019, 40, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Nakahashi, A.; Miura, N.; Monde, K.; Tsukamoto, S. Stereochemical studies of hexylitaconic acid, an inhibitor of p53–HDM2 interaction. Bioorganic Med. Chem. Lett. 2009, 19, 3027–3030. [Google Scholar] [CrossRef]
- Akira, I.; Washizu, M.; Kondo, K.; Murakoshi, S.; Suzuki, A. Isolation and Identification of (+)-Hexylitaconic Acid as a Plant Growth Regulator. Agric. Boil. Chem. 1984, 48, 2607–2609. [Google Scholar] [CrossRef]
- Bambouskova, M.; Gorvel, L.; Lampropoulou, V.; Sergushichev, A.; Loginicheva, E.; Johnson, K.; Korenfeld, D.; Mathyer, M.E.; Kim, H.; Huang, L.-H.; et al. Electrophilic properties of itaconate and derivatives regulate the IκBζ–ATF3 inflammatory axis. Nature 2018, 556, 501–504. [Google Scholar] [CrossRef]
- Ackermann, W.W.; Potter, V.R. Enzyme Inhibition in Relation to Chemotherapy. Exp. Boil. Med. 1949, 72, 1–9. [Google Scholar] [CrossRef]
- Cordes, T.; Wallace, M.; Michelucci, A.; Divakaruni, A.S.; Sapcariu, S.C.; Sousa, C.; Koseki, H.; Cabrales, P.; Murphy, A.N.; Hiller, K.; et al. Immunoresponsive Gene 1 and Itaconate Inhibit Succinate Dehydrogenase to Modulate Intracellular Succinate Levels. J. Boil. Chem. 2016, 291, 14274–14284. [Google Scholar] [CrossRef]
- Lampropoulou, V.; Sergushichev, A.; Bambouskova, M.; Nair, S.; Vincent, E.; Loginicheva, E.; Cervantes-Barragan, L.; Ma, X.; Huang, S.C.-C.; Griss, T.; et al. Itaconate Links Inhibition of Succinate Dehydrogenase with Macrophage Metabolic Remodeling and Regulation of Inflammation. Cell Metab. 2016, 24, 158–166. [Google Scholar] [CrossRef]
- Lee, Y.-V.; Wahab, H.A.; Choong, Y.S. Potential Inhibitors for Isocitrate Lyase ofMycobacterium tuberculosisand Non-M. tuberculosis: A Summary. BioMed Res. Int. 2015, 2015, 1–20. [Google Scholar] [CrossRef]
- Michelucci, A.; Cordes, T.; Ghelfi, J.; Pailot, A.; Reiling, N.; Goldmann, O.; Binz, T.; Wegner, A.; Tallam, A.; Rausell, A.; et al. Immune-responsive gene 1 protein links metabolism to immunity by catalyzing itaconic acid production. Proc. Natl. Acad. Sci. USA 2013, 110, 7820–7825. [Google Scholar] [CrossRef] [PubMed]
- Van Nguyen, T.; Alfaro, A.C.; Young, T.; Green, S.; Zarate, E.; Merien, F. Itaconic acid inhibits growth of a pathogenic marine Vibrio strain: A metabolomics approach. Sci. Rep. 2019, 9, 5937. [Google Scholar] [CrossRef] [PubMed]
- Hillier, S.; Charnetzky, W.T. Glyoxylate bypass enzymes in Yersinia species and multiple forms of isocitrate lyase in Yersinia pestis. J. Bacteriol. 1981, 145, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Thorne, N.; McKew, J.C. Phenotypic screens as a renewed approach for drug discovery. Drug Discov. Today 2013, 18, 1067–1073. [Google Scholar] [CrossRef] [PubMed]
- Sano, M.; Kuroda, H.; Ohara, H.; Ando, H.; Matsumoto, K.; Aso, Y. A high-throughput screening method based on the Mizoroki-Heck reaction for isolating itaconic acid-producing fungi from soils. Heliyon 2019, 5, e02048. [Google Scholar] [CrossRef]
- Aso, Y.; Sano, M.; Kuroda, H.; Ohara, H.; Ando, H.; Matsumoto, K. DISCOVER: A facile structure-based screening method for vinyl compound producing microbes. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Sano, M.; Chin, T.; Takahashi, T.; Ohara, H.; Aso, Y. A simple TLC-densitometric method for the quantification of acrylic acid in aqueous solutions. J. Planar Chromatogr. Mod. TLC 2015, 28, 12–16. [Google Scholar] [CrossRef]
- Shao, L.; Du, Y.; Zeng, M.; Li, X.; Shen, W.; Zuo, S.; Lu, Y.; Zhang, X.-M.; Qi, C. Ethanol-promoted reductive homocoupling reactions of aryl halides catalyzed by palladium on carbon (Pd/C). Appl. Organomet. Chem. 2010, 24, 421–425. [Google Scholar]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sano, M.; Yada, R.; Nomura, Y.; Kusukawa, T.; Ando, H.; Matsumoto, K.; Wada, K.; Tanaka, T.; Ohara, H.; Aso, Y. Microbial Screening Based on the Mizoroki–Heck Reaction Permits Exploration of Hydroxyhexylitaconic-Acid-Producing Fungi in Soils. Microorganisms 2020, 8, 648. https://doi.org/10.3390/microorganisms8050648
Sano M, Yada R, Nomura Y, Kusukawa T, Ando H, Matsumoto K, Wada K, Tanaka T, Ohara H, Aso Y. Microbial Screening Based on the Mizoroki–Heck Reaction Permits Exploration of Hydroxyhexylitaconic-Acid-Producing Fungi in Soils. Microorganisms. 2020; 8(5):648. https://doi.org/10.3390/microorganisms8050648
Chicago/Turabian StyleSano, Mei, Ryoki Yada, Yusuke Nomura, Takahiro Kusukawa, Hiroshi Ando, Keiji Matsumoto, Kazuhito Wada, Tomonari Tanaka, Hitomi Ohara, and Yuji Aso. 2020. "Microbial Screening Based on the Mizoroki–Heck Reaction Permits Exploration of Hydroxyhexylitaconic-Acid-Producing Fungi in Soils" Microorganisms 8, no. 5: 648. https://doi.org/10.3390/microorganisms8050648
APA StyleSano, M., Yada, R., Nomura, Y., Kusukawa, T., Ando, H., Matsumoto, K., Wada, K., Tanaka, T., Ohara, H., & Aso, Y. (2020). Microbial Screening Based on the Mizoroki–Heck Reaction Permits Exploration of Hydroxyhexylitaconic-Acid-Producing Fungi in Soils. Microorganisms, 8(5), 648. https://doi.org/10.3390/microorganisms8050648







