Lipocalin2 Induced by Bacterial Flagellin Protects Mice against Cyclophosphamide Mediated Neutropenic Sepsis
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents, Flagellin, and Recombinant Lcn2 Peptide
2.2. Mouse Model
2.3. Blood Leukocyte Count
2.4. Histopathological Analysis of the Intestines
2.5. Measurement of the Bacterial Burden in the Mouse Model
2.6. Sample Collection, DNA Isolation, and Sequencing
2.7. Bioinformatics Analysis
2.8. Measurement of Serum Lcn2 and Liver lcn2 Levels
2.9. Western Blot Analysis of Liver Lcn2
2.10. Immunofluorescence Staining and Confocal Microscopy
2.11. Statistical Analysis
3. Results
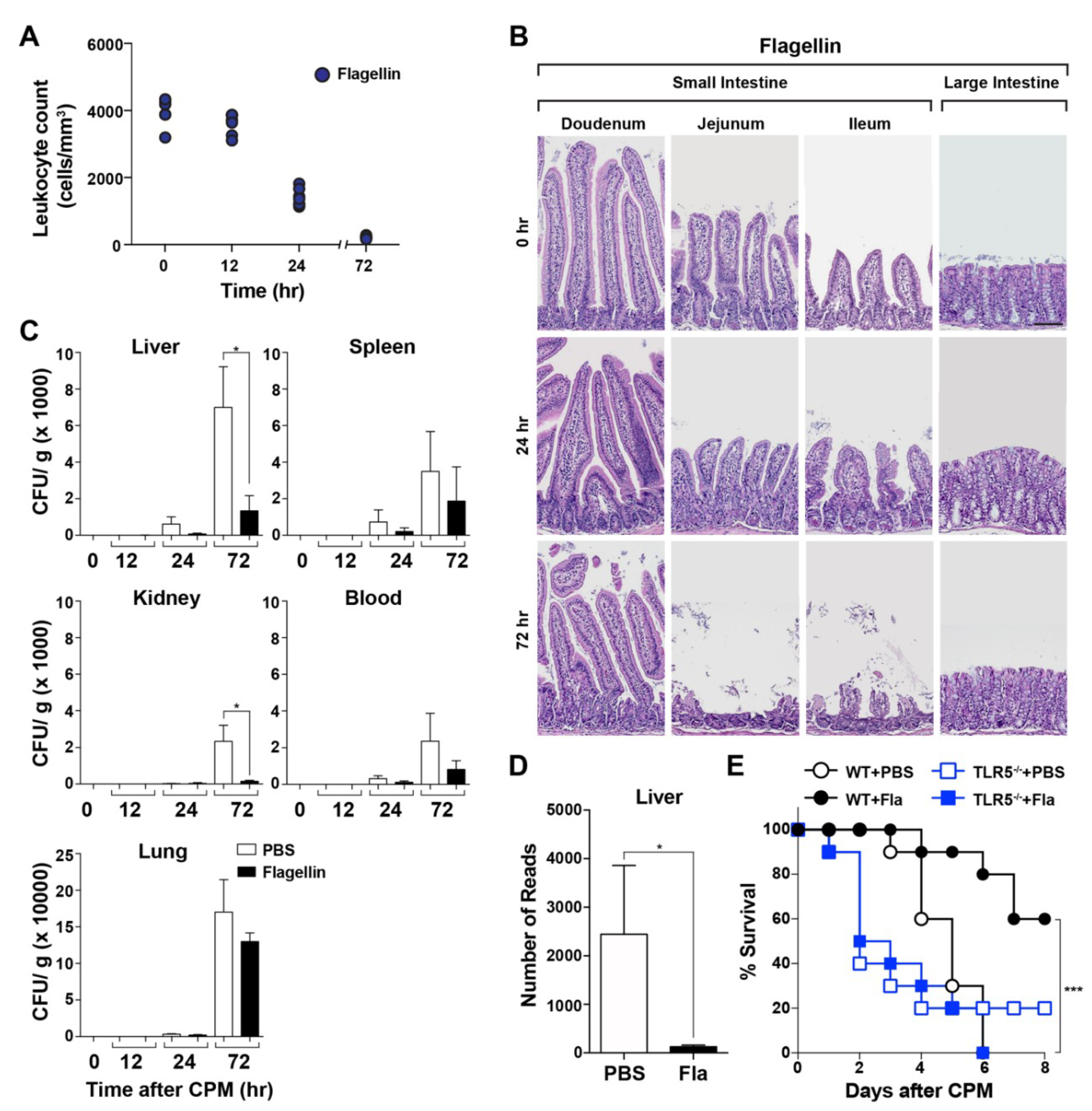
3.1. Protective Effect of a TLR5 Agonist (Bacterial Flagellin) on CPM-Treated Mice
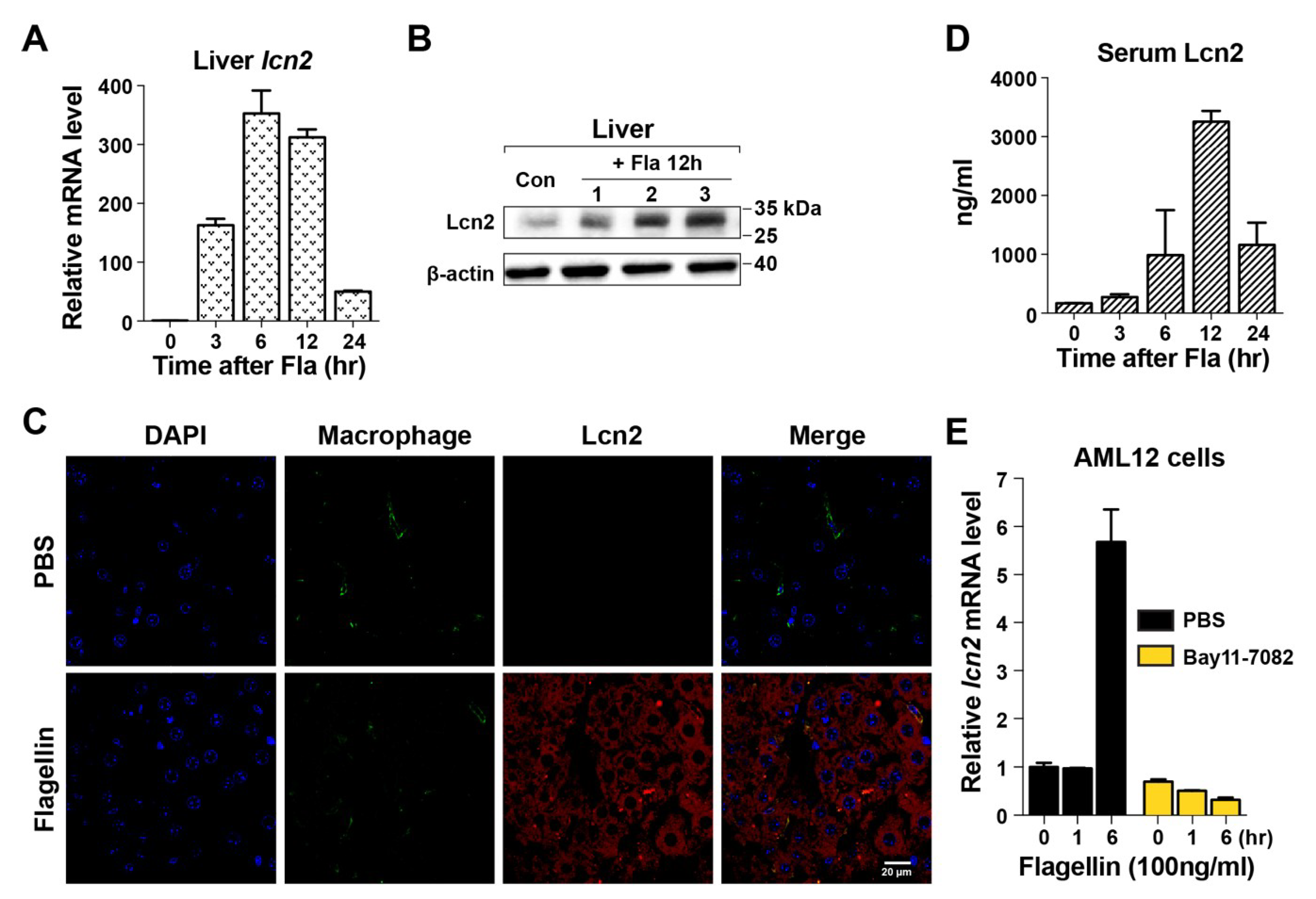
3.2. Induction of Lcn2 by Flagellin in the Liver
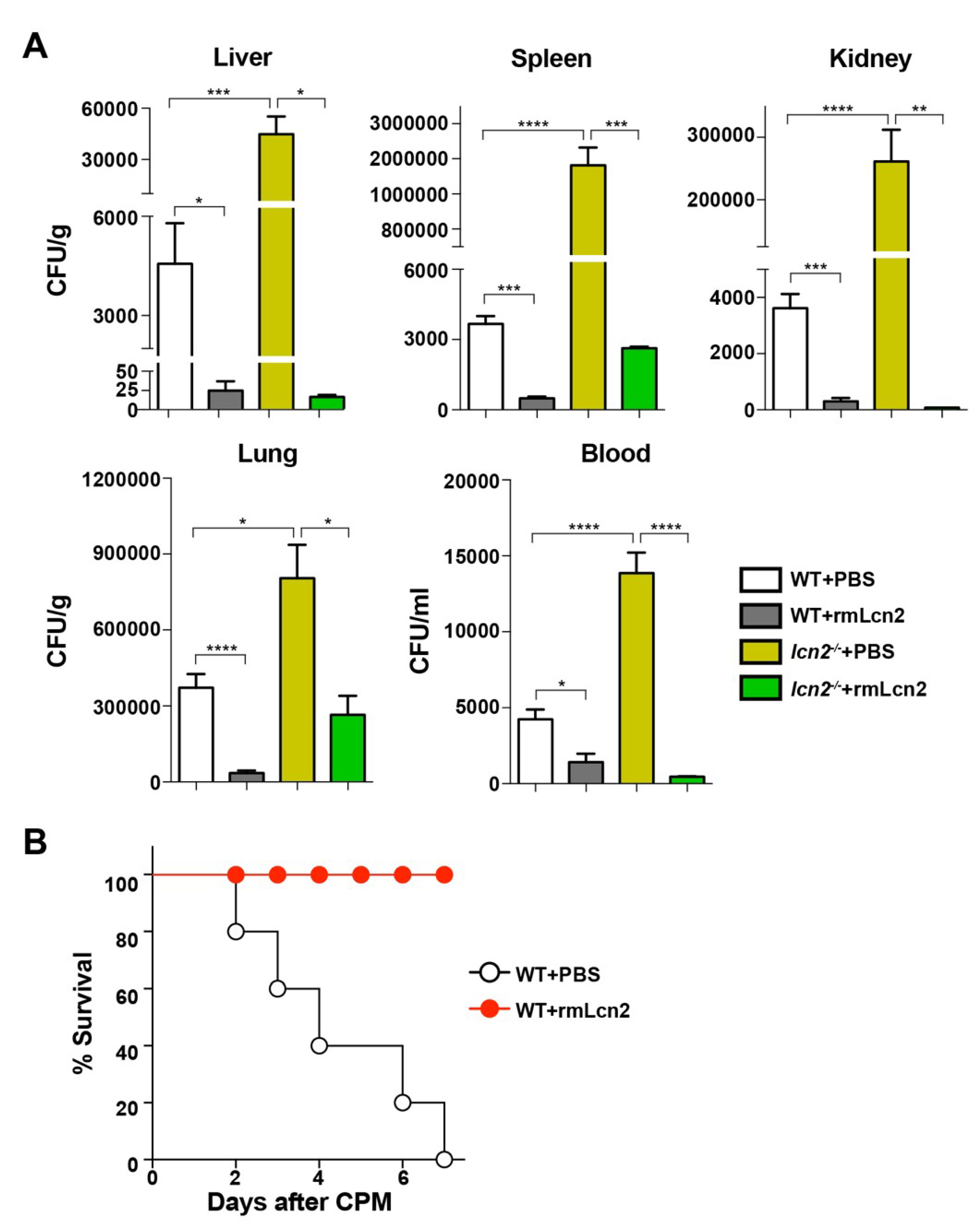
3.3. Antimicrobial Effect of Lcn2 on CPM-Treated Mice
3.4. Effect of Lcn2 on the Enterobacteriaceae Family in a Mouse Model of Sepsis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Informed consent
References
- Orazi, A.; Du, X.; Yang, Z.; Kashai, M.; Williams, D.A. Interleukin-11 prevents apoptosis and accelerates recovery of small intestinal mucosa in mice treated with combined chemotherapy and radiation. Lab. Investig. J. Tech. Methods Pathol. 1996, 75, 33–42. [Google Scholar]
- Van Meir, H.; Nout, R.A.; Welters, M.J.P.; Loof, N.M.; de Kam, M.L.; van Ham, J.J.; Samuels, S.; Kenter, G.G.; Cohen, A.F.; Melief, C.J.M.; et al. Impact of (chemo)radiotherapy on immune cell composition and function in cervical cancer patients. Oncoimmunology 2017, 6, e1267095. [Google Scholar] [CrossRef] [PubMed]
- Keefe, D.M.; Cummins, A.G.; Dale, B.M.; Kotasek, D.; Robb, T.A.; Sage, R.E. Effect of high-dose chemotherapy on intestinal permeability in humans. Clin. Sci. Lond. Engl. 1997, 92, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.; Lee, S.S.; Oh, S.J.; Lim, S.Y.; Lim, S.Y.; Jeon, W.K.; Oh, T.Y.; Kim, J.W. The effect of oral glutamine on 5-fluorouracil/leucovorin-induced mucositis/stomatitis assessed by intestinal permeability test. Clin. Nutr. Edinb. Scotl. 2007, 26, 57–62. [Google Scholar] [CrossRef]
- Melichar, B.; Hyspler, R.; Dragounová, E.; Dvorák, J.; Kalábová, H.; Tichá, A. Gastrointestinal permeability in ovarian cancer and breast cancer patients treated with paclitaxel and platinum. BMC Cancer 2007, 7, 155. [Google Scholar] [CrossRef]
- Russo, F.; Linsalata, M.; Clemente, C.; D’Attoma, B.; Orlando, A.; Campanella, G.; Giotta, F.; Riezzo, G. The effects of fluorouracil, epirubicin, and cyclophosphamide (FEC60) on the intestinal barrier function and gut peptides in breast cancer patients: An observational study. BMC Cancer 2013, 13, 56. [Google Scholar] [CrossRef]
- Green, S.I.; Ajami, N.J.; Ma, L.; Poole, N.M.; Price, R.E.; Petrosino, J.F.; Maresso, A.W. Murine model of chemotherapy-induced extraintestinal pathogenic Escherichia coli translocation. Infect. Immun. 2015, 83, 3243–3256. [Google Scholar] [CrossRef]
- Hayashi, F.; Smith, K.D.; Ozinsky, A.; Hawn, T.R.; Yi, E.C.; Goodlett, D.R.; Eng, J.K.; Akira, S.; Underhill, D.M.; Aderem, A. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 2001, 410, 1099–1103. [Google Scholar] [CrossRef]
- Takeda, K.; Kaisho, T.; Akira, S. Toll-like receptors. Annu. Rev. Immunol. 2003, 21, 335–376. [Google Scholar] [CrossRef]
- Kofoed, E.M.; Vance, R.E. Innate immune recognition of bacterial ligands by NAIPs determines inflammasome specificity. Nature 2011, 477, 592–595. [Google Scholar] [CrossRef]
- Franchi, L.; Muñoz-Planillo, R.; Núñez, G. Sensing and reacting to microbes through the inflammasomes. Nat. Immunol. 2012, 13, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Burdelya, L.G.; Krivokrysenko, V.I.; Tallant, T.C.; Strom, E.; Gleiberman, A.S.; Gupta, D.; Kurnasov, O.V.; Fort, F.L.; Osterman, A.L.; DiDonato, J.A.; et al. An agonist of toll-like receptor 5 has radioprotective activity in mouse and primate models. Science 2008, 320, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Dodd, M.J. Managing the Side Effects of Chemotherapy and Radiation Therapy; UCSF Nursing Press: San Francisco, CA, USA, 2001; ISBN 978-0-943671-20-8. [Google Scholar]
- Fleer, R.; Brendel, M. Toxicity, interstrand cross-links and DNA fragmentation induced by “activated” cyclophosphamide in yeast. Chem. Biol. Interact. 1981, 37, 123–140. [Google Scholar] [CrossRef]
- Wang, J.Y.; Prorok, G.; Vaughan, W.P. Cytotoxicity, DNA cross-linking, and DNA single-strand breaks induced by cyclophosphamide in a rat leukemia in vivo. Cancer Chemother. Pharm. 1993, 31, 381–386. [Google Scholar] [CrossRef]
- Emadi, A.; Jones, R.J.; Brodsky, R.A. Cyclophosphamide and cancer: Golden anniversary. Nat. Rev. Clin. Oncol. 2009, 6, 638–647. [Google Scholar] [CrossRef]
- Lee, S.E.; Kim, S.Y.; Jeong, B.C.; Kim, Y.R.; Bae, S.J.; Ahn, O.S.; Lee, J.-J.; Song, H.-C.; Kim, J.M.; Choy, H.E.; et al. A bacterial flagellin, Vibrio vulnificus FlaB, has a strong mucosal adjuvant activity to induce protective immunity. Infect. Immun. 2006, 74, 694–702. [Google Scholar] [CrossRef]
- Nguyen, C.T.; Kim, S.Y.; Kim, M.S.; Lee, S.E.; Rhee, J.H. Intranasal immunization with recombinant PspA fused with a flagellin enhances cross-protective immunity against Streptococcus pneumoniae infection in mice. Vaccine 2011, 29, 5731–5739. [Google Scholar] [CrossRef]
- Lim, J.S.; Nguyen, K.C.T.; Nguyen, C.T.; Jang, I.-S.; Han, J.M.; Fabian, C.; Lee, S.E.; Rhee, J.H.; Cho, K.A. Flagellin-dependent TLR5/caveolin-1 as a promising immune activator in immunosenescence. Aging Cell 2015, 14, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.H.; Nguyen, V.H.; Jiang, S.-N.; Park, S.-H.; Tan, W.; Hong, S.H.; Shin, M.G.; Chung, I.-J.; Hong, Y.; Bom, H.-S.; et al. Two-step enhanced cancer immunotherapy with engineered Salmonella typhimurium secreting heterologous flagellin. Sci. Transl. Med. 2017, 9. [Google Scholar] [CrossRef]
- Kjeldsen, L.; Bainton, D.F.; Sengeløv, H.; Borregaard, N. Identification of neutrophil gelatinase-associated lipocalin as a novel matrix protein of specific granules in human neutrophils. Blood 1994, 83, 799–807. [Google Scholar] [CrossRef]
- Kjeldsen, L.; Cowland, J.B.; Borregaard, N. Human neutrophil gelatinase-associated lipocalin and homologous proteins in rat and mouse. Biochim. Biophys. Acta 2000, 1482, 272–283. [Google Scholar] [CrossRef]
- Flo, T.H.; Smith, K.D.; Sato, S.; Rodriguez, D.J.; Holmes, M.A.; Strong, R.K.; Akira, S.; Aderem, A. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature 2004, 432, 917–921. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Eddy, S.R. Accelerated Profile HMM Searches. PLoS Comput. Biol. 2011, 7, e1002195. [Google Scholar] [CrossRef]
- Lee, B.; Moon, T.; Yoon, S.; Weissman, T. DUDE-Seq: Fast, flexible, and robust denoising for targeted amplicon sequencing. PLoS ONE 2017, 12, e0181463. [Google Scholar] [CrossRef]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef]
- Yoon, S.-H.; Ha, S.-M.; Kwon, S.; Lim, J.; Kim, Y.; Seo, H.; Chun, J. Introducing EzBioCloud: A taxonomically united database of 16S rRNA gene sequences and whole-genome assemblies. Int. J. Syst. Evol. Microbiol. 2017, 67, 1613–1617. [Google Scholar] [CrossRef]
- Yarza, P.; Yilmaz, P.; Pruesse, E.; Glöckner, F.O.; Ludwig, W.; Schleifer, K.-H.; Whitman, W.B.; Euzéby, J.; Amann, R.; Rosselló-Móra, R. Uniting the classification of cultured and uncultured bacteria and archaea using 16S rRNA gene sequences. Nat. Rev. Microbiol. 2014, 12, 635–645. [Google Scholar] [CrossRef]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef]
- Forsgård, R.A.; Korpela, R.; Holma, R.; Lindén, J.; Frias, R.; Spillmann, T.; Österlund, P. Intestinal permeability to iohexol as an in vivo marker of chemotherapy-induced gastrointestinal toxicity in Sprague-Dawley rats. Cancer Chemother. Pharm. 2016, 78, 863–874. [Google Scholar] [CrossRef]
- Berg, R.D. Bacterial translocation from the gastrointestinal tracts of mice receiving immunosuppressive chemotherapeutic agents. Curr. Microbiol. 1983, 8, 285–292. [Google Scholar] [CrossRef]
- Nakayama, M.; Itoh, K.; Takahashi, E. Cyclophosphamide-induced bacterial translocation in Escherichia coli C25-monoassociated specific pathogen-free mice. Microbiol. Immunol. 1997, 41, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Collins, H.L. Withholding iron as a cellular defence mechanism-friend or foe? Eur. J. Immunol. 2008, 38, 1803–1806. [Google Scholar] [CrossRef] [PubMed]
- Ganz, T.; Nemeth, E. Iron sequestration and anemia of inflammation. Semin. Hematol. 2009, 46, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, G.; Aitken, J.D.; Zhang, B.; Carvalho, F.A.; Chassaing, B.; Shashidharamurthy, R.; Borregaard, N.; Jones, D.P.; Gewirtz, A.T.; Vijay-Kumar, M. Lipocalin 2 deficiency dysregulates iron homeostasis and exacerbates endotoxin-induced sepsis. J. Immunol. 2012, 189, 1911–1919. [Google Scholar] [CrossRef]
- Pierce, J.W.; Schoenleber, R.; Jesmok, G.; Best, J.; Moore, S.A.; Collins, T.; Gerritsen, M.E. Novel inhibitors of cytokine-induced IkappaBalpha phosphorylation and endothelial cell adhesion molecule expression show anti-inflammatory effects in vivo. J. Biol. Chem. 1997, 272, 21096–21103. [Google Scholar] [CrossRef]
- Goetz, D.H.; Holmes, M.A.; Borregaard, N.; Bluhm, M.E.; Raymond, K.N.; Strong, R.K. The neutrophil lipocalin NGAL is a bacteriostatic agent that interferes with siderophore-mediated iron acquisition. Mol. Cell 2002, 10, 1033–1043. [Google Scholar] [CrossRef]
- MacFie, J.; O’Boyle, C.; Mitchell, C.J.; Buckley, P.M.; Johnstone, D.; Sudworth, P. Gut origin of sepsis: A prospective study investigating associations between bacterial translocation, gastric microflora, and septic morbidity. Gut 1999, 45, 223–228. [Google Scholar] [CrossRef]
- Earley, Z.M.; Akhtar, S.; Green, S.J.; Naqib, A.; Khan, O.; Cannon, A.R.; Hammer, A.M.; Morris, N.L.; Li, X.; Eberhardt, J.M.; et al. Burn injury alters the intestinal microbiome and increases gut permeability and bacterial translocation. PLOS ONE 2015, 10, e0129996. [Google Scholar] [CrossRef]
- Li, H.; Feng, D.; Cai, Y.; Liu, Y.; Xu, M.; Xiang, X.; Zhou, Z.; Xia, Q.; Kaplan, M.J.; Kong, X.; et al. Hepatocytes and neutrophils cooperatively suppress bacterial infection by differentially regulating lipocalin-2 and neutrophil extracellular traps. Hepatology 2018, 68, 1604–1620. [Google Scholar] [CrossRef]
- Li, C.; Chan, Y.R. Lipocalin 2 regulation and its complex role in inflammation and cancer. Cytokine 2011, 56, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Müller, J.M.; Ziegler-Heitbrock, H.W.; Baeuerle, P.A. Nuclear factor kappa B, a mediator of lipopolysaccharide effects. Immunobiology 1993, 187, 233–256. [Google Scholar] [CrossRef]
- Cowland, J.B.; Sørensen, O.E.; Sehested, M.; Borregaard, N. Neutrophil gelatinase-associated lipocalin is up-regulated in human epithelial cells by IL-1 beta, but not by TNF-alpha. J. Immunol. 2003, 171, 6630–6639. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, S.; Yamazaki, S.; Takeshige, K.; Muta, T. Crucial roles of binding sites for NF-kappaB and C/EBPs in IkappaB-zeta-mediated transcriptional activation. Biochem. J. 2007, 405, 605–615. [Google Scholar] [CrossRef]
- Holmes, M.A.; Paulsene, W.; Jide, X.; Ratledge, C.; Strong, R.K. Siderocalin (Lcn 2) also binds carboxymycobactins, potentially defending against mycobacterial infections through iron sequestration. Structure 2005, 13, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Yeoh, B.S.; Chassaing, B.; Zhang, B.; Saha, P.; Xiao, X.; Awasthi, D.; Shashidharamurthy, R.; Dikshit, M.; Gewirtz, A.; et al. Microbiota-inducible innate immune, siderophore binding protein lipocalin 2 is critical for intestinal homeostasis. Cell Mol. Gastroenterol. Hepatol. 2016, 2, 482–498. [Google Scholar] [CrossRef]
- Lupp, C.; Robertson, M.L.; Wickham, M.E.; Sekirov, I.; Champion, O.L.; Gaynor, E.C.; Finlay, B.B. Host-mediated inflammation disrupts the intestinal microbiota and promotes the overgrowth of Enterobacteriaceae. Cell Host Microbe 2007, 2, 119–129. [Google Scholar] [CrossRef]
- Aapro, M.S.; Cameron, D.A.; Pettengell, R.; Bohlius, J.; Crawford, J.; Ellis, M.; Kearney, N.; Lyman, G.H.; Tjan-Heijnen, V.C.; Walewski, J.; et al. EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphomas and solid tumours. Eur. J. Cancer 2006, 42, 2433–2453. [Google Scholar] [CrossRef]
- Hughes, W.T.; Armstrong, D.; Bodey, G.P.; Feld, R.; Mandell, G.L.; Meyers, J.D.; Pizzo, P.A.; Schimpff, S.C.; Shenep, J.L.; Wade, J.C. From the infectious diseases society of America. Guidelines for the use of antimicrobial agents in neutropenic patients with unexplained fever. J. Infect. Dis. 1990, 161, 381–396. [Google Scholar] [CrossRef]
- Kerr, K.G. The prophylaxis of bacterial infections in neutropenic patients. J. Antimicrob. Chemother. 1999, 44, 587–591. [Google Scholar] [CrossRef]
- Paul, M.; Yahav, D.; Fraser, A.; Leibovici, L. Empirical antibiotic monotherapy for febrile neutropenia: Systematic review and meta-analysis of randomized controlled trials. J. Antimicrob. Chemother. 2006, 57, 176–189. [Google Scholar] [CrossRef] [PubMed]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, D.; Kim, H.K.; Jeong, J.-H.; Jung, Y.S.; Lee, S.E.; Jang, H.-C.; Jung, S.-I.; Choi, H.-S.; Rhee, J.H.; Lee, S.-G.; et al. Lipocalin2 Induced by Bacterial Flagellin Protects Mice against Cyclophosphamide Mediated Neutropenic Sepsis. Microorganisms 2020, 8, 646. https://doi.org/10.3390/microorganisms8050646
Lim D, Kim HK, Jeong J-H, Jung YS, Lee SE, Jang H-C, Jung S-I, Choi H-S, Rhee JH, Lee S-G, et al. Lipocalin2 Induced by Bacterial Flagellin Protects Mice against Cyclophosphamide Mediated Neutropenic Sepsis. Microorganisms. 2020; 8(5):646. https://doi.org/10.3390/microorganisms8050646
Chicago/Turabian StyleLim, Daejin, Hee Kyung Kim, Jae-Ho Jeong, Yoon Seok Jung, Shee Eun Lee, Hee-Chang Jang, Sook-In Jung, Hueng-Sik Choi, Joon Haeng Rhee, Sung-Gwon Lee, and et al. 2020. "Lipocalin2 Induced by Bacterial Flagellin Protects Mice against Cyclophosphamide Mediated Neutropenic Sepsis" Microorganisms 8, no. 5: 646. https://doi.org/10.3390/microorganisms8050646
APA StyleLim, D., Kim, H. K., Jeong, J.-H., Jung, Y. S., Lee, S. E., Jang, H.-C., Jung, S.-I., Choi, H.-S., Rhee, J. H., Lee, S.-G., Park, C., Song, M., & Choy, H. E. (2020). Lipocalin2 Induced by Bacterial Flagellin Protects Mice against Cyclophosphamide Mediated Neutropenic Sepsis. Microorganisms, 8(5), 646. https://doi.org/10.3390/microorganisms8050646




