Analysis of the Consolidation Phase of Immunological Memory within the IgG Response to a B Cell Epitope Displayed on a Filamentous Bacteriophage
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Model Vaccine
2.3. Immunization and Bleeding Schedules
2.4. Antibody Titer Measures
2.5. Statistical Analysis and Data Availability
3. Results
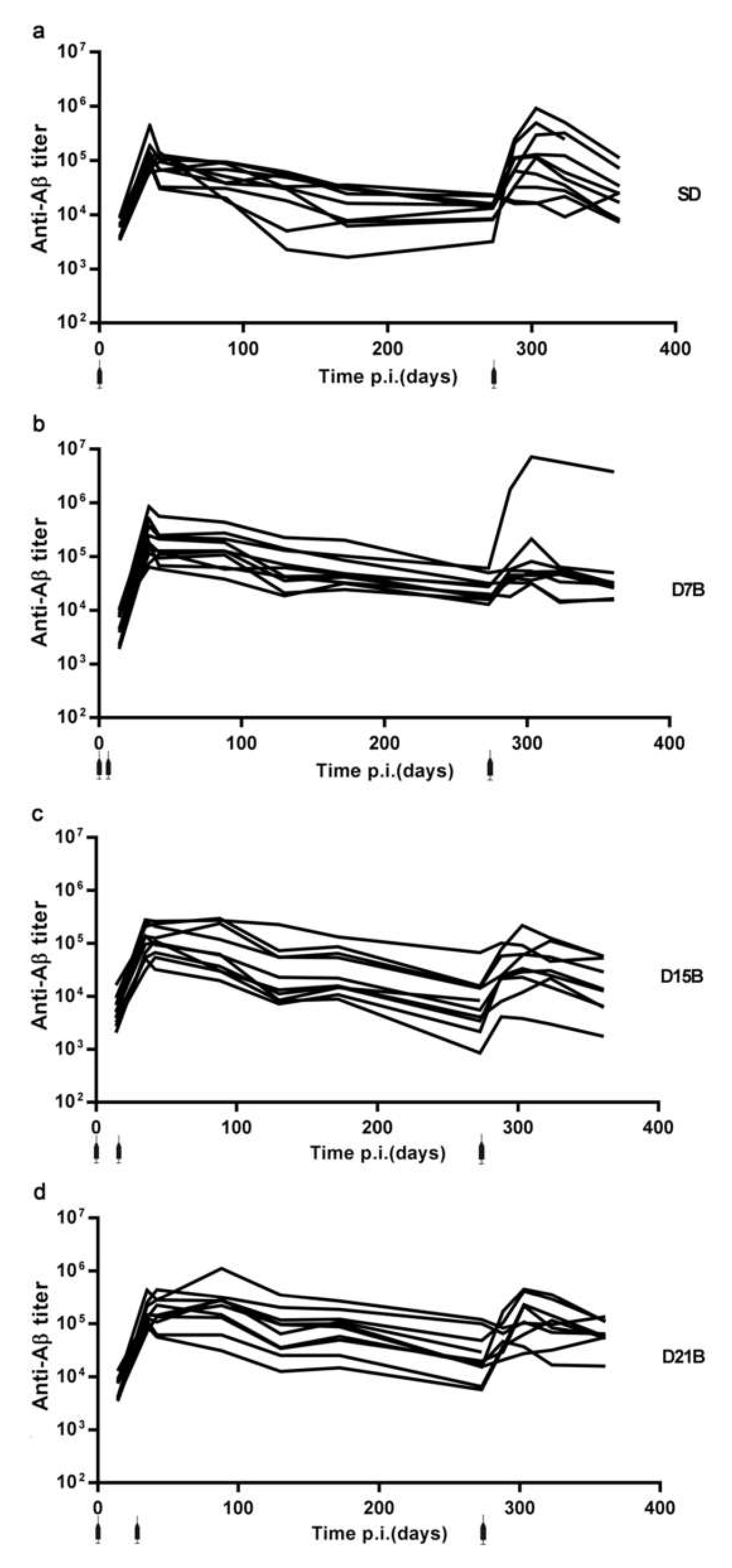
3.1. Generation of a Dataset of Antibody Titers Aimed at Testing the Model of a Consolidation Phase of Immunological Memory in the Context of the IgG Response to a Phage-Displayed B Cell Epitope
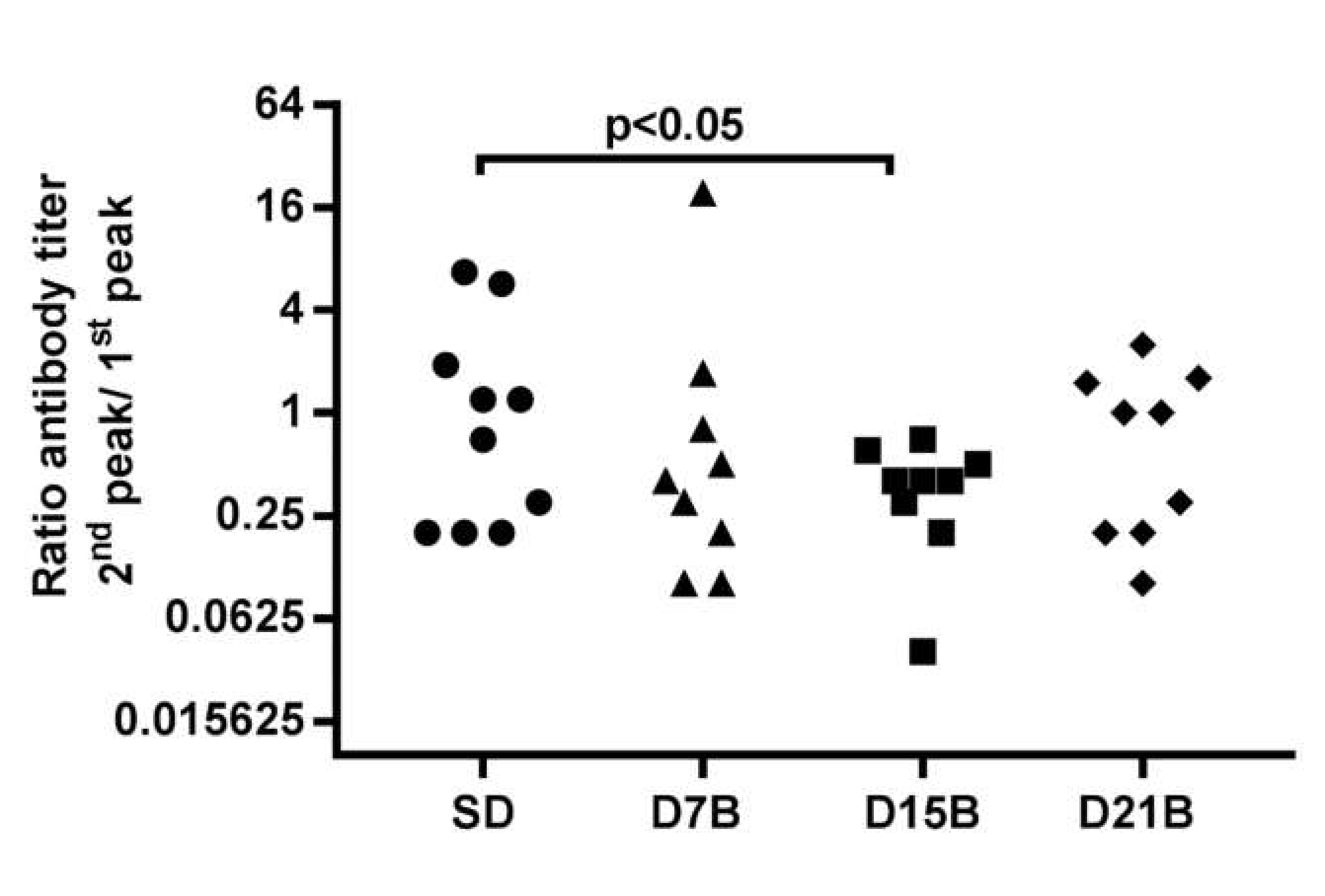
3.1.1. Analysis of the Consolidation Phase of Immunological Memory in the IgG Response to a Phage-Displayed B Cell Epitope
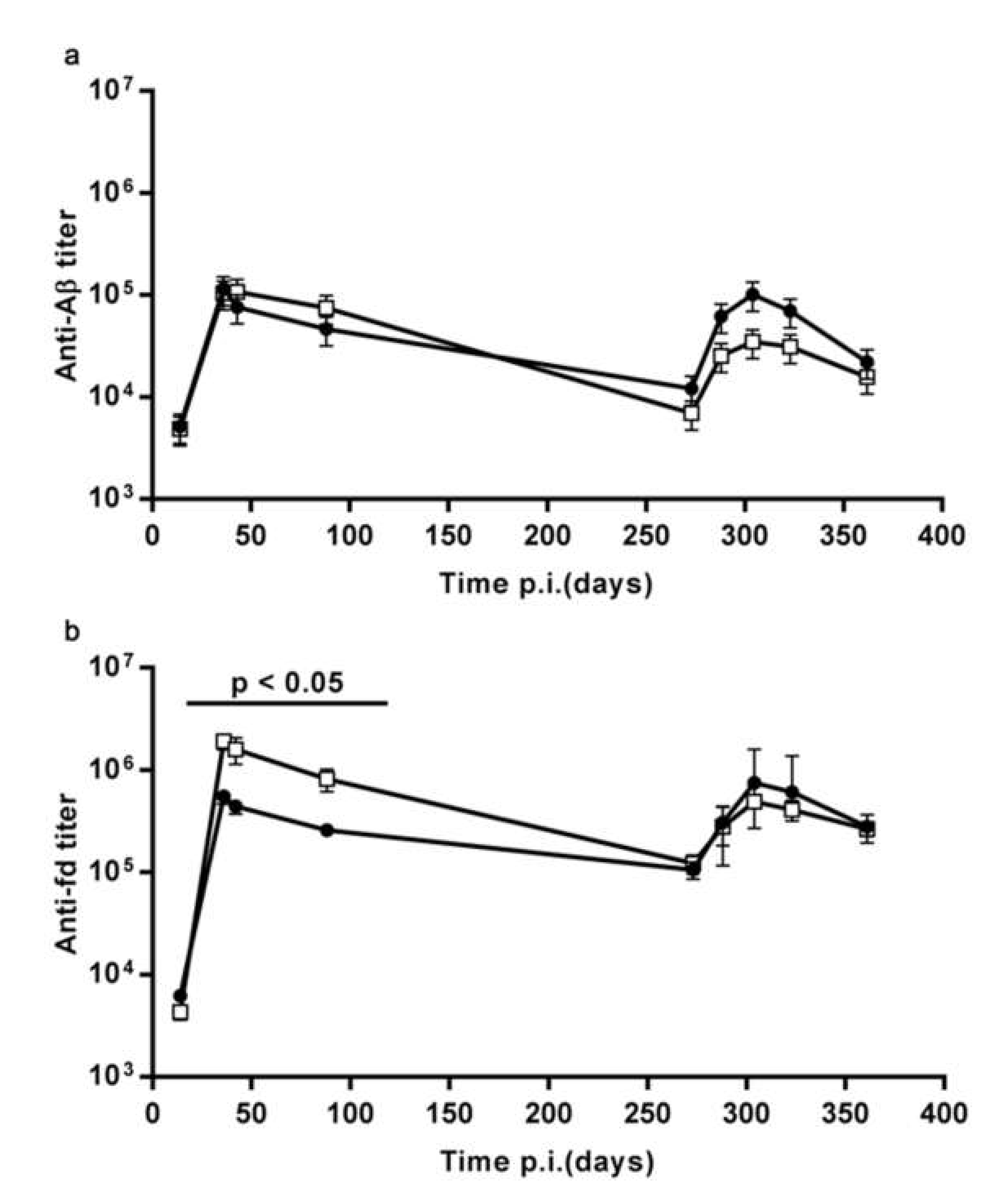
3.1.2. The Pre-Existing Antibody Titers Against Aβ and Against the fd Phage do not Affect the Ability to Display an Enhanced Response to Recall
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Plotkin, S.A. Correlates of protection induced by vaccination. Clin. Vaccine Immunol. 2010, 17, 1055–1065. [Google Scholar] [CrossRef] [PubMed]
- Pulendran, B.; Ahmed, R. Immunological mechanisms of vaccination. Nat. Immunol. 2011, 12, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Koff, W.C.; Burton, D.R.; Johnson, P.R.; Walker, B.D.; King, C.R.; Nabel, G.J.; Ahmed, R.; Bhan, M.K.; Plotkin, S.A. Accelerating next-generation vaccine development for global disease prevention. Science 2013, 340, 1232910. [Google Scholar] [CrossRef] [PubMed]
- Sallusto, F.; Lanzavecchia, A.; Araki, K.; Ahmed, R. From vaccines to memory and back. Immunity 2010, 33, 451–463. [Google Scholar] [CrossRef]
- Farber, D.L.; Netea, M.G.; Radbruch, A.; Rajewsky, K.; Zinkernagel, R.M. Immunological memory: Lessons from the past and a look to the future. Nat. Rev. Immunol. 2016, 16, 124–128. [Google Scholar] [CrossRef]
- Prisco, A.; De Berardinis, P. Memory immune response: A major challenge in vaccination. Biomol. Concepts 2012, 3, 479–486. [Google Scholar] [CrossRef]
- Chang, J.T.; Wherry, E.J.; Goldrath, A.W. Molecular regulation of effector and memory T cell differentiation. Nat. Immunol. 2014, 15, 1104–1115. [Google Scholar] [CrossRef]
- Kunz, D.J.; Gomes, T.; James, K.R. Immune Cell Dynamics Unfolded by Single-Cell Technologies. Front. Immunol. 2018, 9, 1435. [Google Scholar] [CrossRef]
- Mantile, F.; Basile, C.; Cicatiello, V.; De Falco, D.; Caivano, A.; De Berardinis, P.; Prisco, A. A multimeric immunogen for the induction of immune memory to beta-amyloid. Immunol. Cell Biol. 2011, 89, 604–609. [Google Scholar] [CrossRef]
- Mantile, F.; Capasso, A.; De Berardinis, P.; Prisco, A. Identification of a consolidation phase in immunological memory. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef]
- Esposito, M.; Luccarini, I.; Cicatiello, V.; De Falco, D.; Fiorentini, A.; Barba, P.; Casamenti, F.; Prisco, A. Immunogenicity and therapeutic efficacy of phage-displayed beta-amyloid epitopes. Mol. Immunol. 2008, 45, 1056–1062. [Google Scholar] [CrossRef] [PubMed]
- Prisco, A.; De Berardinis, P. Filamentous bacteriophage fd as an antigen delivery system in vaccination. Int. J. Mol. Sci. 2012, 13, 5179–5194. [Google Scholar] [CrossRef] [PubMed]
- Mantile, F.; Trovato, M.; Santoni, A.; Barba, P.; Ottonello, S.; De Berardinis, P.; Prisco, A. Alum and squalene-oil-in-water emulsion enhance the titer and avidity of anti-Abeta antibodies induced by multimeric protein antigen (1-11)E2, preserving the Igg1-skewed isotype distribution. PLoS ONE 2014, 9, e101474. [Google Scholar] [CrossRef] [PubMed]
- Trovato, M.; Maurano, F.; D’Apice, L.; Costa, V.; Sartorius, R.; Cuccaro, F.; McBurney, S.P.; Krebs, S.J.; Prisco, A.; Ciccodicola, A.; et al. E2 multimeric scaffold for vaccine formulation: Immune response by intranasal delivery and transcriptome profile of E2-pulsed dendritic cells. BMC Microbiol. 2016, 16, 152. [Google Scholar] [CrossRef] [PubMed]
- Granoff, D.M.; Pollard, A.J. Reconsideration of the use of meningococcal polysaccharide vaccine. Pediatr. Infect. Dis. J. 2007, 26, 716–722. [Google Scholar] [CrossRef] [PubMed]
- Bjarnarson, S.P.; Benonisson, H.; Del Giudice, G.; Jonsdottir, I. Pneumococcal polysaccharide abrogates conjugate-induced germinal center reaction and depletes antibody secreting cell pool, causing hyporesponsiveness. PLoS ONE 2013, 8, e72588. [Google Scholar] [CrossRef][Green Version]
- Orgogozo, J.M.; Gilman, S.; Dartigues, J.F.; Laurent, B.; Puel, M.; Kirby, L.C.; Jouanny, P.; Dubois, B.; Eisner, L.; Flitman, S.; et al. Subacute meningoencephalitis in a subset of patients with AD after Abeta42 immunization. Neurology 2003, 61, 46–54. [Google Scholar] [CrossRef]
- Lacosta, A.M.; Pascual-Lucas, M.; Pesini, P.; Casabona, D.; Pérez-Grijalba, V.; Marcos-Campos, I.; Sarasa, L.; Canudas, J.; Badi, H.; Monleón, I.; et al. Safety, tolerability and immunogenicity of an active anti-Abeta40 vaccine (ABvac40) in patients with Alzheimer’s disease: A randomised, double-blind, placebo-controlled, phase I trial. Alzheimer’s Res. Ther. 2018, 10, 12. [Google Scholar] [CrossRef]
- Muhs, A.; Hickman, D.T.; Pihlgren, M.; Chuard, N.; Giriens, V.; Meerschman, C.; van der Auwera, I.; van Leuven, F.; Sugawara, M.; Weingertner, M.C.; et al. Liposomal vaccines with conformation-specific amyloid peptide antigens define immune response and efficacy in APP transgenic mice. Proc. Natl. Acad. Sci. USA 2007, 104, 9810–9815. [Google Scholar] [CrossRef]
- Schneeberger, A.; Mandler, M.; Otawa, O.; Zauner, W.; Mattner, F.; Schmidt, W. Development of AFFITOPE vaccines for Alzheimer’s disease (AD)--from concept to clinical testing. J. Nutr. Health Aging 2009, 13, 264–267. [Google Scholar] [CrossRef]
- Wiessner, C.; Wiederhold, K.H.; Tissot, A.C.; Frey, P.; Danner, S.; Jacobson, L.H.; Jennings, G.T.; Lüönd, R.; Ortmann, R.; Reichwald, J.; et al. The second-generation active Abeta immunotherapy CAD106 reduces amyloid accumulation in APP transgenic mice while minimizing potential side effects. J. Neurosci. 2011, 31, 9323–9331. [Google Scholar] [CrossRef] [PubMed]
- Davtyan, H.; Ghochikyan, A.; Petrushina, I.; Hovakimyan, A.; Davtyan, A.; Poghosyan, A.; Marleau, A.M.; Movsesyan, N.; Kiyatkin, A.; Rasool, S.; et al. Immunogenicity, efficacy, safety, and mechanism of action of epitope vaccine (Lu AF20513) for Alzheimer’s disease: Prelude to a clinical trial. J. Neurosci. 2013, 33, 4923–4934. [Google Scholar] [CrossRef] [PubMed]
- Ryan, J.M.; Grundman, M. Anti-amyloid-beta immunotherapy in Alzheimer’s disease: ACC-001 clinical trials are ongoing. J. Alzheimer’s Dis. 2009, 17, 243. [Google Scholar] [CrossRef]
- Mantile, F.; Capasso, A.; Villacampa, N.; Donnini, M.; Liguori, G.L.; Constantin, G.; De Berardinis, P.; Heneka, M.T.; Prisco, A. Vaccination with (1–11) E2 in alum efficiently induces an antibody response to β-amyloid without affecting brain β-amyloid load and microglia activation in 3xTg mice. Aging Clin. Exp. Res. 2019, 1–5. [Google Scholar] [CrossRef] [PubMed]
- De Berardinis, P.; D’Apice, L.; Prisco, A.; Ombra, M.N.; Barba, P.; Del Pozzo, G.; Petukhov, S.; Malik, P.; Perham, R.N.; Guardiola, J. Recognition of HIV-derived B and T cell epitopes displayed on filamentous phages. Vaccine 1999, 17, 1434–1441. [Google Scholar] [CrossRef]
- Sartorius, R.; D’Apice, L.; Prisco, A.; De Berardinis, P. Arming Filamentous Bacteriophage, a Nature-Made Nanoparticle, for New Vaccine and Immunotherapeutic Strategies. Pharmaceutics 2019, 11, 437. [Google Scholar] [CrossRef]
- Sartorius, R.; D’Apice, L.; Trovato, M.; Cuccaro, F.; Costa, V.; De Leo, M.G.; Marzullo, V.M.; Biondo, C.; D’Auria, S.; De Matteis, M.A.; et al. Antigen delivery by filamentous bacteriophage fd displaying an anti-DEC-205 single-chain variable fragment confers adjuvanticity by triggering a TLR9-mediated immune response. EMBO Mol. Med. 2015, 7, 973–988. [Google Scholar] [CrossRef]
- Smith, L.L.; Buckley, R.; Lugar, P. Diagnostic immunization with bacteriophage ΦX 174 in patients with common variable Immunodeficiency/Hypogammaglobulinemia. Front. Immunol. 2014, 5, 410. [Google Scholar] [CrossRef]
- Castiglione, F.; Mantile, F.; De Berardinis, P.; Prisco, A. How the interval between prime and boost injection affects the immune response in a computational model of the immune system. Comput. Math. Methods Med. 2012, 2012, 842329. [Google Scholar] [CrossRef]
- Turner, J.S.; Benet, Z.L.; Grigorova, I.L. Antigen acquisition enables newly arriving b cells to enter ongoing immunization-induced germinal centers. J. Immunol. 2017, 199, 1301–1307. [Google Scholar] [CrossRef]
- Schwickert, T.A.; Alabyev, B.; Manser, T.; Nussenzweig, M.C. Germinal center reutilization by newly activated B cells. J. Exp. Med. 2009, 206, 2907–2914. [Google Scholar] [CrossRef] [PubMed]
- Weisel, F.J.; Zuccarino-Catania, G.V.; Chikina, M.; Shlomchik, M.J. A temporal switch in the germinal center determines differential output of memory B and plasma cells. Immunity 2016, 44, 116–130. [Google Scholar] [CrossRef] [PubMed]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mantile, F.; Capasso, A.; De Berardinis, P.; Prisco, A. Analysis of the Consolidation Phase of Immunological Memory within the IgG Response to a B Cell Epitope Displayed on a Filamentous Bacteriophage. Microorganisms 2020, 8, 564. https://doi.org/10.3390/microorganisms8040564
Mantile F, Capasso A, De Berardinis P, Prisco A. Analysis of the Consolidation Phase of Immunological Memory within the IgG Response to a B Cell Epitope Displayed on a Filamentous Bacteriophage. Microorganisms. 2020; 8(4):564. https://doi.org/10.3390/microorganisms8040564
Chicago/Turabian StyleMantile, Francesca, Angelo Capasso, Piergiuseppe De Berardinis, and Antonella Prisco. 2020. "Analysis of the Consolidation Phase of Immunological Memory within the IgG Response to a B Cell Epitope Displayed on a Filamentous Bacteriophage" Microorganisms 8, no. 4: 564. https://doi.org/10.3390/microorganisms8040564
APA StyleMantile, F., Capasso, A., De Berardinis, P., & Prisco, A. (2020). Analysis of the Consolidation Phase of Immunological Memory within the IgG Response to a B Cell Epitope Displayed on a Filamentous Bacteriophage. Microorganisms, 8(4), 564. https://doi.org/10.3390/microorganisms8040564






