Ascorbic Acid Changes Growth of Food-Borne Pathogens in the Early Stage of Biofilm Formation
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains
2.2. Biofilm Formation
2.3. The Assessment of Ascorbic Acid Addition on Bacterial Biofilm Elimination
- OD K(+)—optical density of the positive control;
- OD—optical density of the biofilm treated with vitamin C.
2.4. Statistical Analysis
- ODc—optical density cut-off value;
- x—average optical density of negative control;
- SD—standard deviation of optical density of negative control.
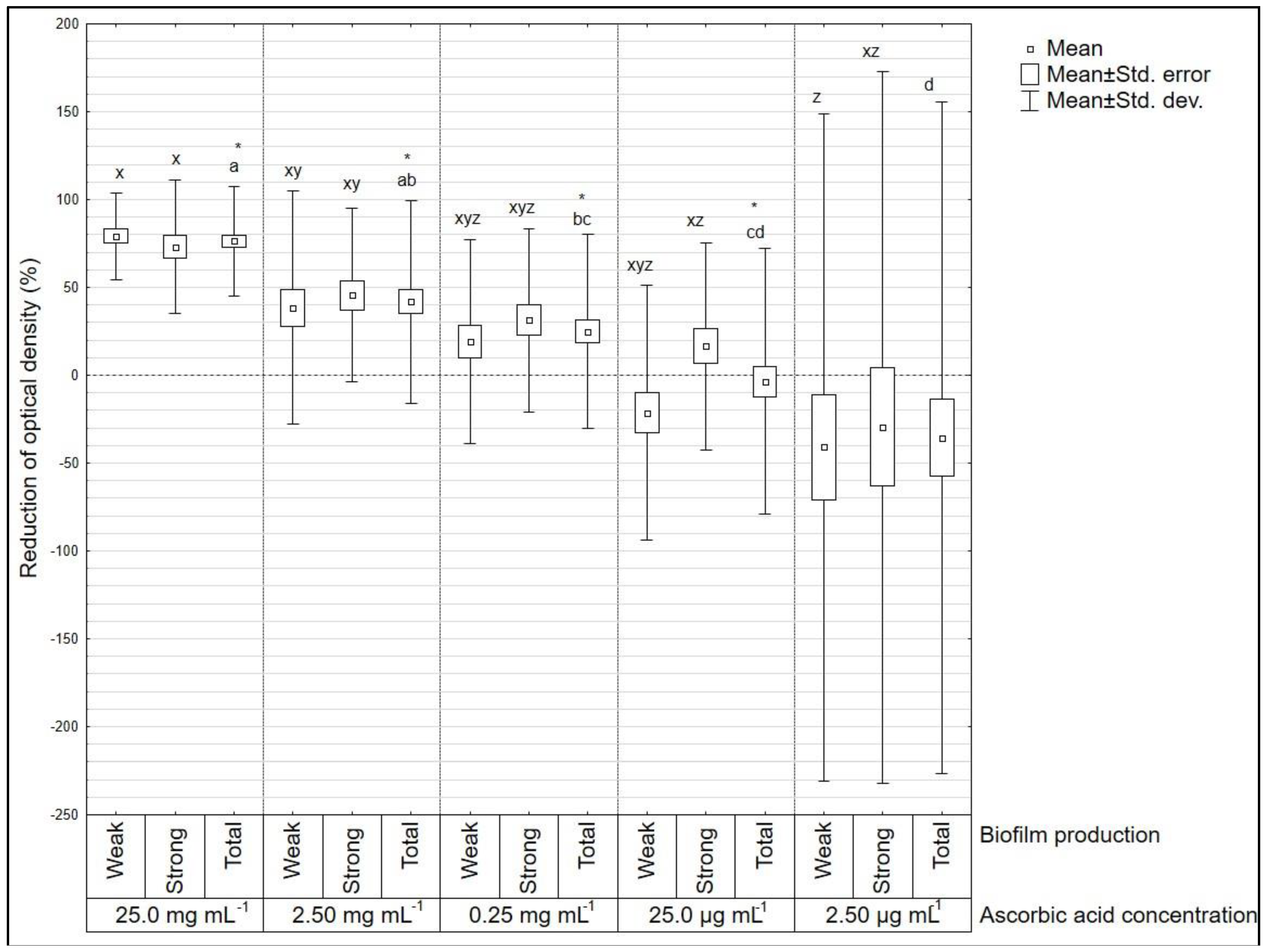
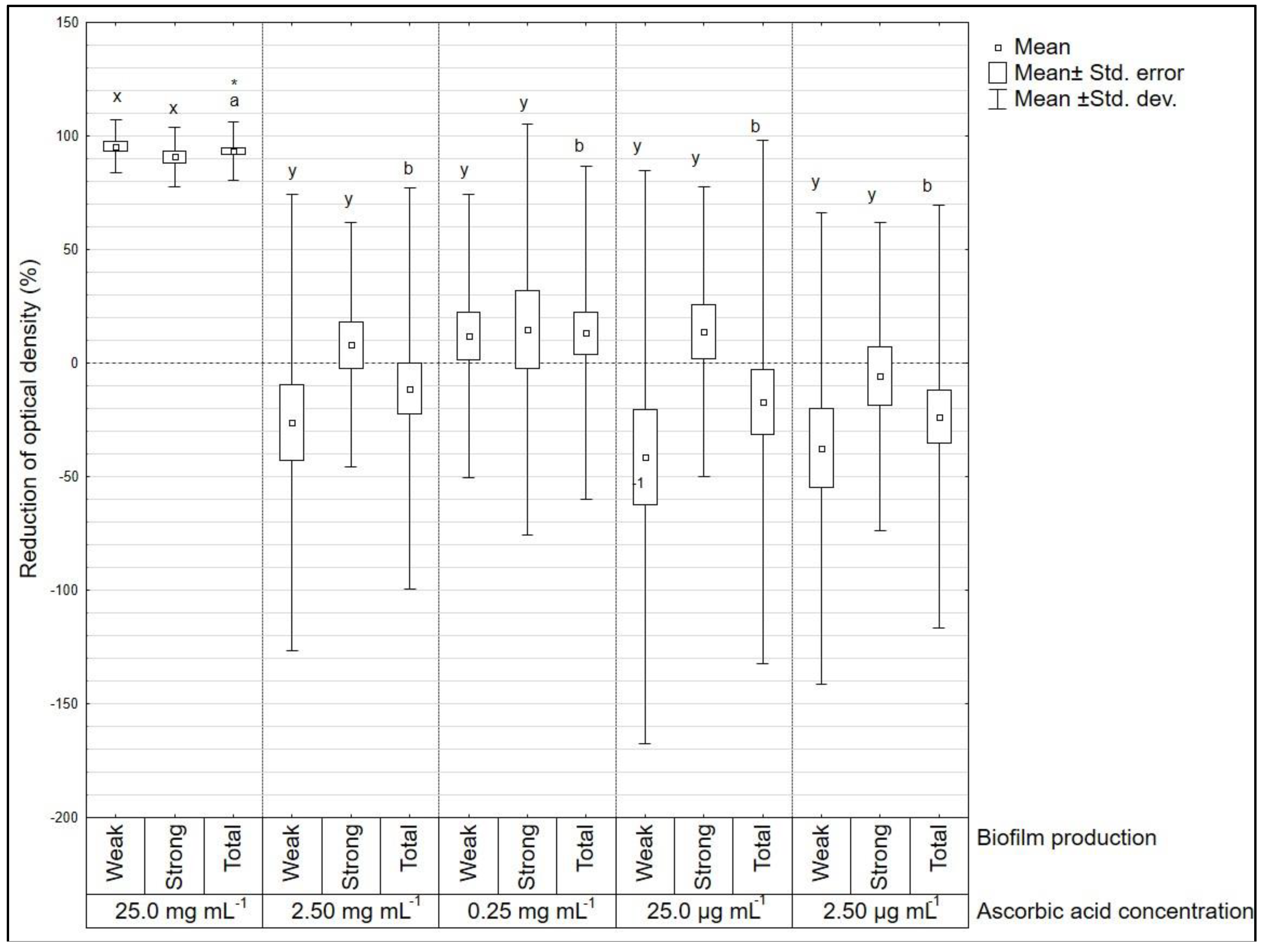
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- WHO Fact Sheet N_237, Food Safety and Foodborne Illness. Available online: https://www.who.int/news-room/fact-sheets/detail/food-safety (accessed on 25 February 2020).
- Havelaar, A.H.; Kirk, M.D.; Torgerson, P.R.; Gibb, H.J.; Hald, T.; Lake, R.J.; Praet, N.; Bellinger, D.C.; de Silva, N.R.; Gargouri, N.; et al. World Health Organization global estimates and regional comparisons of the burden of foodborne disease in 2010. PLoS Med. 2010, 12, e1001923. [Google Scholar] [CrossRef]
- European Food Safety Authority and European Centre for Disease Prevention and Control. The European Union One Health 2018 Zoonoses Report. EFSA J. 2019. [Google Scholar] [CrossRef]
- Ölmez, H.; Temur, S.D. Effects of different sanitizing treatments on biofilms and attachment of Escherichia coli and Listeria monocytogenes on green leaf lettuce. LWT Food Sci. Technol. 2010, 43, 964–970. [Google Scholar] [CrossRef]
- Annous, B.A.; Solomon, E.B.; Cooke, P.H.; Burke, A. Biofilm formation by Salmonella spp. on cantaloupe melons. J. Food Saf. 2005, 25, 276–287. [Google Scholar] [CrossRef]
- Lapidot, A.; Romling, U.; Yaron, S. Biofilm formation and the survival of Salmonella typhimurium on parsley. Int. J. Food Microbiol. 2006, 109, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Stepanovic, S.; Cirkovic, I.; Ranin, L.; Svabic-Vlahovic, M. Biofilm formation by Salmonella spp. and Listeria monocytogenes on plastic surfaces. Lett. Appl. Microbiol. 2004, 38, 428–432. [Google Scholar] [CrossRef]
- Mohamed, J.A.; Huang, D.B. Biofilm formation by enterococci. J. Med. Microbiol. 2007, 56, 1581–1588. [Google Scholar] [CrossRef]
- Tienungoon, S.; Ratkowsky, D.A.; McMeekin, T.A.; Ross, T. Growth Limits of Listeria monocytogenes as a Function of Temperature, pH, NaCl, and Lactic Acid. Appl. Environ. Microbiol. 2000, 66, 4979–4987. [Google Scholar] [CrossRef]
- Wałecka-Zacharska, E.; Gmyrek, R.; Skowron, K.; Kosek-Paszkowska, K.; Bania, J. Duration of Heat Stress Effect on Invasiveness of L. monocytogenes Strains. BioMed Res. Int. 2018, 1457480. [Google Scholar] [CrossRef]
- Santos, T.; Viala, D.; Chambon, C.; Esbelin, J.; Hébraud, M. Listeria monocytogenes Biofilm Adaptation to Different Temperatures Seen Through Shotgun Proteomics. Front. Nutr. 2019, 6, 89. [Google Scholar] [CrossRef]
- Galié, S.; García-Gutiérrez, C.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Biofilms in the Food Industry: Health Aspects and Control Methods. Front. Microbiol. 2018, 9, 898. [Google Scholar] [CrossRef] [PubMed]
- Habimana, O.; Heir, E.; Langsrud, S.; Asli, A.W.; Møretrø, T. Enhanced surface colonization by Escherichia coli O157:H7 in biofilms formed by an Acinetobacter calcoaceticus isolate from meat-processing environments. Appl. Environ. Microbiol. 2010, 6, 4557–4559. [Google Scholar] [CrossRef] [PubMed]
- Bridier, A.; Sanchez-Vizuete, P.; Guilbaud, M.; Piard, J.-C.; Naïtali, M.; Briandet, R. Biofilm-associated persistence of food-borne pathogens. Food Microbiol. 2015, 45, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Isela, S.; Sergio, N.; Jose, M.; Rene, H.; Claudio, C. Ascorbic acid on oral microbial growth and biofilm formation. Pharma Innov. 2013, 2, 104–109. [Google Scholar]
- Vilchèze, C.; Hartman, T.; Weinrick, B.; Jacobs, W.R., Jr. Mycobacterium tuberculosis is extraordinarily sensitive to killing by a vitamin C-induced Fenton reaction. Nat. Commun. 2013, 4, 1881. [Google Scholar] [CrossRef] [PubMed]
- Verghese, R.J.; Mathew, S.K.; David, A. Antimicrobial activity of vitamin C demonstrated on uropathogenic Escherichia coli and Klebsiella pneumoniae. J. Curr. Res. Sci. Med. 2017, 3, 88–93. [Google Scholar] [CrossRef]
- Novak, J.S.; Fratamico, P.M. Evaluation of ascorbic acid as a quorum sensing analogue to control growth, sporulation, and enterotoxin production in Clostridium perfringens. J. Food Sci. 2004, 69, FMS72–FMS78. [Google Scholar] [CrossRef]
- Pandit, S.; Mokkapati, V.R.S.S.; Helgadóttir, S.H.; Westerlund, F.; Mijakovic, I. Combination of cold atmospheric plasma and vitamin C effectively disrupts bacterial biofilms. Clin. Microbiol. 2017, 6, 283. [Google Scholar] [CrossRef]
- Han, J.H. Edible Films and Coatings: A Review. In Innovations in Food Packaging, 2nd ed.; Han, J., Ed.; Pepsico Inc.: Plano, TX, USA, 2014; pp. 213–255. [Google Scholar]
- Mlalila, N.; Hilonga, A.; Swai, H.; Devlieghere, F.; Ragaert, P. Antimicrobial packaging based on starch, poly(3-hydroxybutyrate) and poly(lactic-co-glycolide) materials and application challenges. Trends Food Sci. Technol. 2018, 74, 1–11. [Google Scholar] [CrossRef]
- Yam, K.L.; Lee, D.S. Emerging food packaging technologies: An overview. In Emerging Food Packaging Technologies: Principles and Practice, 1st ed.; Yam, K.L., Lee, D.S., Eds.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Sawston, UK, 2012; pp. 1–9. [Google Scholar]
- Meyers, A.; Furtmann, C.; Jose, J. Direct optical density determination of bacterial cultures in microplates for high-throughput screening applications. Enzyme Microb. Technol. 2018, 118, 1–5. [Google Scholar] [CrossRef]
- Zhang, H.M.; Wakisaka, N.; Maeda, O.; Yamamoto, T. Vitamin C inhibits the growth of a bacterial risk factor for gastric carcinoma: Helicobacter Pylori. Cancer 1997, 80, 1897–1903. [Google Scholar] [CrossRef]
- Valero, A.; Carrasco, E.; Pѐrez-Rodriguez, F.; Garcìa-Gimeno, R.M.; Zurera, G. Growth/no growth model of Listeria monocytogenes as a function of temperature, pH, citric acid and ascorbic acid. Eur. Food Res. Technol. 2006, 224, 91–100. [Google Scholar] [CrossRef]
- Mirani, Z.A.; Khan, M.N.; Siddiqui, A.; Khan, F.; Aziz, M.; Naz, S.; Ahmed, A.; Khan, S.I. Ascorbic acid augments colony spreading by reducing biofilm formation of methicillin-resistant Staphylococcus aureus. Iran. J. Basic Med. Sci. 2018, 21, 175–180. [Google Scholar] [CrossRef]
- Diaz De Rienzo, M.A.; Stevenson, P.S.; Marchant, R.; Banat, I.M. Pseudomonas aeruginosa biofilm disruption using microbial surfactants. JAM 2016, 120, 868–876. [Google Scholar] [CrossRef]
- Pandit, S.; Ravikumar, V.; Abdel-Haleem, A.M.; Derouiche, A.; Mokkapati, V.R.S.S.; Sihlbom, C.; Mineta, K.; Gojobori, T.; Gao, X.; Westerlund, F.; et al. Low concentrations of vitamin C reduce the synthesis of extracellular polymers and destabilize bacterial biofilms. Front. Microbiol. 2017, 8. [Google Scholar] [CrossRef]
- Tabak, M.; Armon, R.; Rosenblat, G.; Stermer, E.; Neeman, I. Diverse effect of ascorbic acid and palmitoyl ascorbate on Helicobacter pylori survival and growth. Fed. Eur. Microbiol. Soc. Microbiol. Lett. 2003, 224, 247–253. [Google Scholar] [CrossRef]
- Helgadóttir, S.; Pandit, S.; Mokkapati, V.R.; Westerlund, F.; Apell, P.; Mijakovic, I. Vitamin C pretreatment enhances the antibacterial effect of cold atmospheric plasma. Front. Cell Infect. Microbiol. 2017, 7, 43. [Google Scholar] [CrossRef]
- El-Gebaly, E.; Essam, T.; Hashem, S.; El-Baky, R.A. Effect of levofloxacin and vitamin C on bacterial adherence and preformed biofilm on urethral catheter surfaces. J. Microb. Biochem. Technol. 2012, 4, 131–136. [Google Scholar] [CrossRef]
- Abu-Ghazaleh, B.M. Effects of ascorbic acid, citric acid, lactic acid, NaCl, potassium sorbate and Thymus vulgaris extract on Staphylococcus aureus and Escherichia coli. Afr. J. Microbiol. Res. 2012, 7, 7–12. [Google Scholar] [CrossRef]
- Kallio, J.; Jaakkola, M.; Maki, M.; Kilpelainen, P.; Virtanen, V. Vitamin C inhibits Staphylococcus aureus growth and enhances the inhibitory effect of quercetin on growth of Escherichia coli in vitro. Planta Med. 2012, 78, 1824–1830. [Google Scholar] [CrossRef]
- Khameneh, B.; Fazly Bazzaz, B.S.; Amani, A.; Rostami, J.; Vahdati-Mashhadian, N. Combination of anti-tuberculosis drugs with vitamin C or NAC against different Staphylococcus aureus and Mycobacterium tuberculosis strains. Microb. Pathog. 2016, 93, 83–87. [Google Scholar] [CrossRef]
- McGann, P.; Ivanek, R.; Wiedmann, M.; Boor, K.J. Temperature-dependent expression of Listeria monocytogenes internalin and internalin-like genes suggests functional diversity of these proteins among the listeriae. Appl. Environ. Microbiol. 2007, 73, 2806–2814. [Google Scholar] [CrossRef] [PubMed]
- Mauder, M.; Williams, T.; Fritsch, F.; Kuhn, M.; Beier, D. Response regulator DegU of Listeria monocytogenes controls temperature-responsive flagellar gene expression in its unphosphorylated state. J. Bacteriol. 2008, 13, 4777–4781. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Peel, M.; Donachie, W.; Shaw, A. Temperature-dependent expression of flagella of Listeria manocytogenes studied by electron microscopy, SDS-PAGE and Western blotting. J. Gen. Microbiol. 1988, 134, 2171–2178. [Google Scholar] [CrossRef] [PubMed]
- Way, S.S.; Thompson, L.J.; Lopes, J.E.; Hajjar, A.M.; Kollmann, T.R.; Freitag, N.E.; Wilson, C.B. Characterization of flagellin expression and its role in Listeria monocytogenes infection and immunity. Cell. Microbiol. 2004, 6, 235–242. [Google Scholar] [CrossRef]
- Piercey, M.J.; Hingston, P.A.; Truelstrup Hansen, L. Genes involved in Listeria monocytogenes biofilm formation at a simulated food processing plant temperature of 15 °C. Int. J. Food Microbiol. 2016, 223, 63–74. [Google Scholar] [CrossRef]
- Kadam, S.R.; den Besten, H.M.; van der Veen, S.; Zwietering, M.H.; Moezelaar, R.; Abee, T. Diversity assessment of Listeria monocytogenes biofilm formation: Impact of growth condition, serotype and strain origin. Int. J. Food Microbiol. 2013, 165, 259–264. [Google Scholar] [CrossRef]
- Di Ciccio, P.; Vergara, A.; Festino, A.R.; Paludi, D.; Zanardi, E.; Ghidini, S.; Ianieri, A. Biofilm formation by Staphylococcus aureus on food contact surfaces: Relationship with temperature and cell surface hydrophobicity. Food Control 2015, 50, 930–936. [Google Scholar] [CrossRef]
- Pagedar, A.; Singh, J.; Batish, V.K. Surface hydrophobicity, nutritional contents affect Staphylococcus aureus biofilms and temperature influences its survival in preformed biofilms. J. Basic Microb. 2010, 50, 98–106. [Google Scholar] [CrossRef]
- Silva-Meira, Q.G.; Medeiros-Barbosa, I.; Athayde, A.J.A.A.; Siqueira-Júnior, J.P.; Souza, E.L. Influence of temperature and surface kind on biofilm formation by Staphylococcus aureus from food-contact surfaces and sensitivity to sanitizers. Food Control 2012, 25, 469–475. [Google Scholar] [CrossRef]
- White-Ziegler, C.A.; Um, S.; Pérez, N.M.; Berns, A.L.; Malhowski, A.J.; Young, S. Low temperature (23 °C) increases expression of biofilm-, cold-shock- and RpoS-dependent genes in Escherichia coli K-12. Microbiology 2008, 154, 148–166. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, D.; Kazimierczak, W.; Zięba, E.; Mężyńska, M.; Basiura-Cembala, M.; Lisiecki, S.; Karaś, M.; Baraniak, B. Ascorbic acid- and sodium ascorbate-loaded oxidized potato starch films: Comparative evaluation of physicochemical and antioxidant properties. Carbohyd. Polym. 2018, 181, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-S.; Chang, Y.; Lee, E.-S.; Song, H.-G.; Chang, P.-S.; Han, J. Ascorbic Acid-Based Oxygen Scavenger in Active Food Packaging System for Raw Meatloaf. J. Food Sci. 2018, 83, 682–688. [Google Scholar] [CrossRef] [PubMed]
- Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on Food Additives (Text with EEA Relevance). Available online: https://eur-lex.europa.eu/ (accessed on 24 February 2020).
- Stepien, K.; Prinsloo, P.; Hitch, T.; McCulloch, T.; Sims, R. Acute renal failure, microangiopathic haemolytic anaemia, and secondary oxalosis in a young female patient. Int. J. Nephrol. 2011, 679160. [Google Scholar] [CrossRef][Green Version]
- Curhan, G.C.; Willett, W.C.; Rimm, E.B.; Stampfer, M.J. A prospective study of the intake of vitamins C and B6, and the risk of kidney stones in men. J. Urol. 1996, 155, 1847–1851. [Google Scholar] [CrossRef]
- Meyers, D.G.; Maloley, P.A.; Weeks, D. Safety of antioxidant vitamins. Arch. Intern. Med. 1996, 156, 925–935. [Google Scholar] [CrossRef]

| OD K(+)/ODc | Biofilm Production |
|---|---|
| ≤3 | Weak |
| >3 | Strong |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Przekwas, J.; Wiktorczyk, N.; Budzyńska, A.; Wałecka-Zacharska, E.; Gospodarek-Komkowska, E. Ascorbic Acid Changes Growth of Food-Borne Pathogens in the Early Stage of Biofilm Formation. Microorganisms 2020, 8, 553. https://doi.org/10.3390/microorganisms8040553
Przekwas J, Wiktorczyk N, Budzyńska A, Wałecka-Zacharska E, Gospodarek-Komkowska E. Ascorbic Acid Changes Growth of Food-Borne Pathogens in the Early Stage of Biofilm Formation. Microorganisms. 2020; 8(4):553. https://doi.org/10.3390/microorganisms8040553
Chicago/Turabian StylePrzekwas, Jana, Natalia Wiktorczyk, Anna Budzyńska, Ewa Wałecka-Zacharska, and Eugenia Gospodarek-Komkowska. 2020. "Ascorbic Acid Changes Growth of Food-Borne Pathogens in the Early Stage of Biofilm Formation" Microorganisms 8, no. 4: 553. https://doi.org/10.3390/microorganisms8040553
APA StylePrzekwas, J., Wiktorczyk, N., Budzyńska, A., Wałecka-Zacharska, E., & Gospodarek-Komkowska, E. (2020). Ascorbic Acid Changes Growth of Food-Borne Pathogens in the Early Stage of Biofilm Formation. Microorganisms, 8(4), 553. https://doi.org/10.3390/microorganisms8040553





