Draft Genome Sequence of the Urinary Catheter Isolate Enterobacter ludwigii CEB04 with High Biofilm Forming Capacity
Abstract
1. Introduction
2. Materials and Methods
2.1. Growth Conditions and DNA Isolation
2.2. Genome Sequencing, Assembly and Annotation
2.3. Phenotypic Analysis
2.4. Genome and Protein Analysis
3. Results
3.1. Biofilm Characteristics
3.2. Genome Assembly and Annotation
3.3. Antimicrobial Resistance Genes
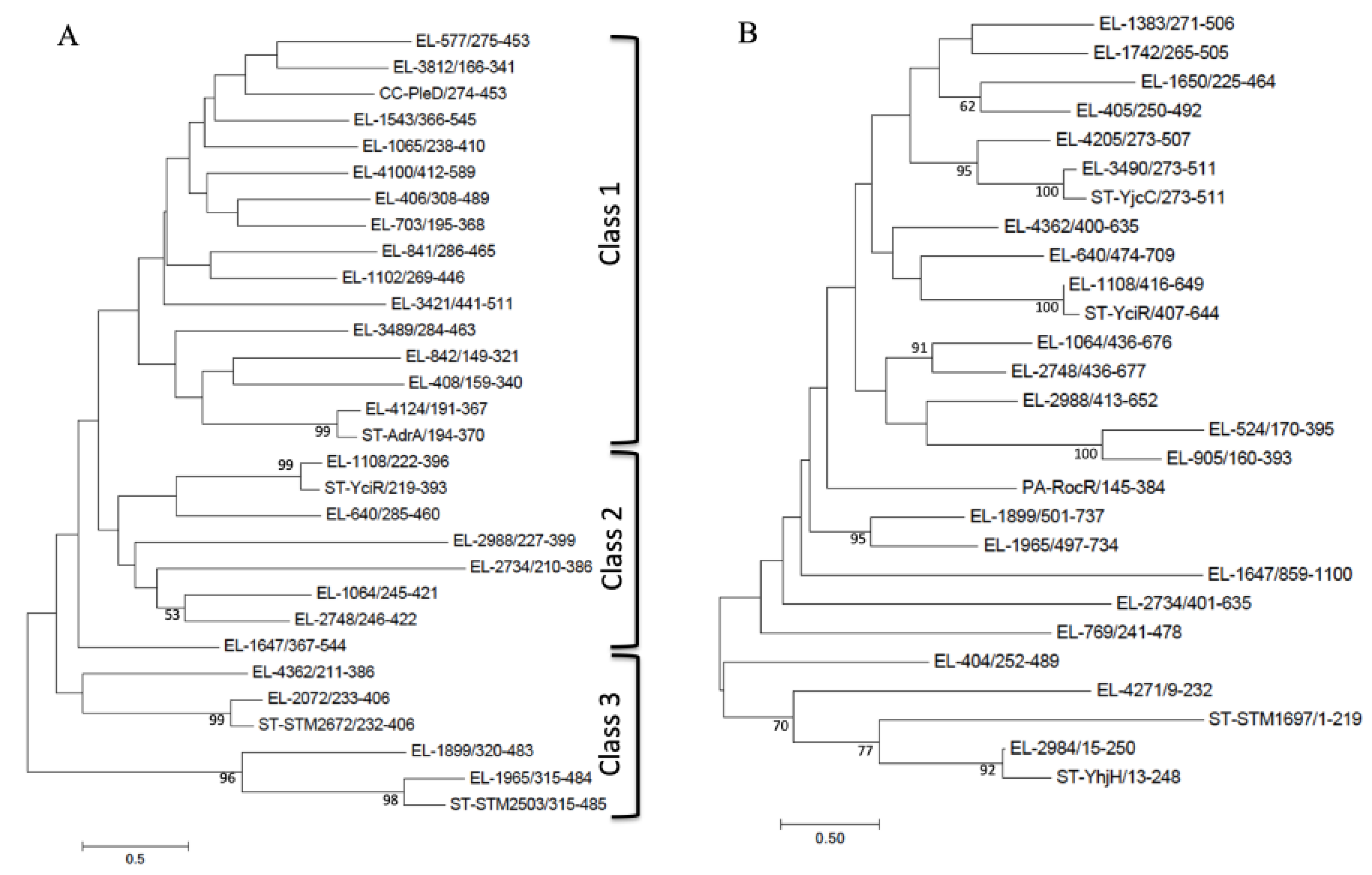
3.4. GGDEF/EAL/HD-GYP Domain Proteins
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
Data
References
- Wagenlehner, F.M.; Naber, K.G. Hospital-acquired urinary tract infections. J. Hosp. Infect. 2000, 46, 171–181. [Google Scholar] [CrossRef]
- Saint, S.; Lipsky, B.A. Preventing catheter-related bacteriuria: Should we? Can we? How? Arch. Intern. Med. 1999, 159, 800–808. [Google Scholar] [CrossRef]
- Ha, U.-S.; Cho, Y.-H. Catheter-associated urinary tract infections: New aspects of novel urinary catheters. Int. J. Antimicrob. Agents 2006, 28, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, S.M.; Stickler, D.J.; Mobley, H.L.T.; Shirtliff, M.E. Complicated catheter-associated urinary tract infections due to Escherichia coli and Proteus mirabilis. Clin. Microbiol. Rev. 2008, 21, 26–59. [Google Scholar] [CrossRef]
- Donlan, R.M.; Costerton, J.W. Biofilms: Survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193. [Google Scholar] [CrossRef] [PubMed]
- Hatt, J.K.; Rather, P.N. Role of bacterial biofilms in urinary tract infections. Curr. Top. Microbiol. Immunol. 2008, 322, 163–192. [Google Scholar]
- Madsen, J.S.; Burmölle, M.; Hansen, L.H.; Sorensen, S.J. The interconnection between biofilm formation and horizontal gene transfer. FEMS Immunol. Med. Microbiol. 2012, 65, 183–195. [Google Scholar] [CrossRef]
- Bagshaw, S.M.; Laupland, K.B. Epidemiology of intensive care unit-acquired urinary tract infections. Curr. Opin. Infect. Dis. 2006, 19, 67–71. [Google Scholar] [CrossRef]
- Wang, X.; Lünsdorf, H.; Ehren, I.; Brauner, A.; Römling, U. Characteristics of biofilms from urinary tract catheters and presence of biofilm-related components in Escherichia coli. Curr. Microbiol. 2010, 60, 446–453. [Google Scholar] [CrossRef]
- Niveditha, S.; Pramodhini, S.; Umadevi, S.; Kumar, S.; Stephen, S. The isolation and the biofilm formation of uropathogens in the patients with Catheter Associated Urinary Tract Infections (UTIs). J. Clin. Diagn. Res. 2012, 6, 1478–1482. [Google Scholar] [CrossRef]
- Brady, C.; Cleenwerck, I.; Venter, S.; Coutinho, T.; De Vos, P. Taxonomic evaluation of the genus Enterobacter based on multilocus sequence analysis (MLSA): Proposal to reclassify E. nimipressuralis and E. amnigenus into Lelliottia gen. nov. as Lelliottia nimipressuralis comb. nov. and Lelliottia amnigena comb. nov., respectively, E. gergoviae and E. pyrinus into Pluralibacter gen. nov. as Pluralibacter gergoviae comb. nov. and Pluralibacter pyrinus comb. nov., respectively, E. cowanii, E. radicincitans, E. oryzae and E. arachidis into Kosakonia gen. nov. as Kosakonia cowanii comb. nov., Kosakonia radicincitans comb. nov., Kosakonia oryzae comb. nov. and Kosakonia arachidis comb. nov., respectively, and E. turicensis, E. helveticus and E. pulveris into Cronobacter as Cronobacter zurichensis nom. nov., Cronobacter helveticus comb. nov. and Cronobacter pulveris comb. nov., respectively, and emended description of the genera Enterobacter and Cronobacter. Syst. Appl. Microbiol. 2013, 36, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, H.; Stindl, S.; Stumpf, A.; Mehlen, A.; Monget, D.; Heesemann, J.; Schleifer, K.H.; Roggenkamp, A. Description of Enterobacter ludwigii sp. nov., a novel Enterobacter species of clinical relevance. Syst. Appl. Microbiol. 2005, 28, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Mosharaf, M.K.; Tanvir, M.Z.H.; Haque, M.M.; Haque, M.A.; Khan, M.A.A.; Molla, A.H.; Alam, M.Z.; Islam, M.S.; Talukder, M.R. Metal-adapted bacteria isolated from wastewaters produce biofilms by expressing proteinaceous curli fimbriae and cellulose nanofibers. Front. Microbiol. 2018, 9, 1334. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Li, J.; Qin, D.; Chen, L.; Zhao, F.; Chen, S.; Hu, H.; Yu, C.P. Characterization of exoelectrogenic bacteria Enterobacter strains isolated from a microbial fuel cell exposed to copper shock load. PLoS ONE 2014, 9, e113379. [Google Scholar] [CrossRef] [PubMed]
- de Almeida Lopes, K.B.; Carpentieri-Pipolo, V.; Oro, T.H.; Stefani Pagliosa, E.; Degrassi, G. Culturable endophytic bacterial communities associated with field-grown soybean. J. Appl. Microbiol. 2016, 120, 740–755. [Google Scholar] [CrossRef]
- Wang, J.; Peiffer, M.; Hoover, K.; Rosa, C.; Zeng, R.; Felton, G.W. Helicoverpa zea gut-associated bacteria indirectly induce defenses in tomato by triggering a salivary elicitor(s). New. Phytol. 2017, 214, 1294–1306. [Google Scholar] [CrossRef]
- Mishra, P.; Samuel, M.K.; Reddy, R.; Tyagi, B.K.; Mukherjee, A.; Chandrasekaran, N. Environmentally benign nanometric neem-laced urea emulsion for controlling mosquito population in environment. Environ. Sci. Pollut. Res. Int. 2018, 25, 2211–2230. [Google Scholar] [CrossRef]
- de Melo Pereira, G.V.; Magalhaes, K.T.; Lorenzetii, E.R.; Souza, T.P.; Schwan, R.F. A multiphasic approach for the identification of endophytic bacterial in strawberry fruit and their potential for plant growth promotion. Microb. Ecol. 2012, 63, 405–417. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, Y.N.; Chen, T.; Ren, C.H.; Li, X.; Liu, G.X. Relationship between intestinal microbial dysbiosis and primary liver cancer. Hepatobiliary Pancreat. Dis. Int. 2019, 18, 149–157. [Google Scholar] [CrossRef]
- Chin, C.-S.; Alexander, D.H.; Marks, P.; Klammer, A.A.; Drake, J.; Heiner, C.; Clum, A.; Copeland, A.; Huddleston, J.; Eichler, E.E.; et al. Nonhybrid, finished microbial genome assemblies from long-read SMRT sequencing data. Nat. Methods 2013, 10, 563–569. [Google Scholar] [CrossRef]
- Tatusova, T.; DiCuccio, M.; Badretdin, A.; Chetvernin, V.; Nawrocki, E.P.; Zaslavsky, L.; Lomsadze, A.; Pruitt, K.D.; Borodovsky, M.; Ostell, J. NCBI prokaryotic genome annotation pipeline. Nucleic Acids Res. 2016, 44, 6614–6624. [Google Scholar] [CrossRef] [PubMed]
- Aziz, R.K.; Bartels, D.; Best, A.A.; DeJongh, M.; Disz, T.; Edwards, R.A.; Formsma, K.; Gerdes, S.; Glass, E.M.; Kubal, M.; et al. The RAST Server: Rapid annotations using subsystems technology. BMC Genom. 2008, 9, 75. [Google Scholar] [CrossRef] [PubMed]
- Overbeek, R.; Olson, R.; Pusch, G.D.; Olsen, G.J.; Davis, J.J.; Disz, T.; Edwards, R.A.; Gerdes, S.; Parrello, B.; Shukla, M.; et al. The SEED and the Rapid Annotation of microbial genomes using Subsystems Technology (RAST). Nucleic Acids Res. 2014, 42, D206–D214. [Google Scholar] [CrossRef] [PubMed]
- Brettin, T.; Davis, J.J.; Disz, T.; Edwards, R.A.; Gerdes, S.; Olsen, G.J.; Olson, R.; Overbeek, R.; Parrello, B.; Pusch, G.D.; et al. RASTtk: A modular and extensible implementation of the RAST algorithm for building custom annotation pipelines and annotating batches of genomes. Sci. Rep. 2015, 5, 8365. [Google Scholar] [CrossRef]
- Bokranz, W.; Wang, X.; Tschäpe, H.; Römling, U. Expression of cellulose and curli fimbriae by Escherichia coli isolated from the gastrointestinal tract. J. Med. Microbiol. 2005, 54, 1171–1182. [Google Scholar] [CrossRef]
- Simm, R.; Morr, M.; Kader, A.; Nimtz, M.; Römling, U. GGDEF and EAL domains inversely regulate cyclic di-GMP levels and transition from sessility to motility. Mol. Microbiol. 2004, 53, 1123–1134. [Google Scholar] [CrossRef]
- Yoon, S.-H.; Ha, S.-M.; Lim, J.; Kwon, S.; Chun, J. A large-scale evaluation of algorithms to calculate average nucleotide identity. Antonie Van Leeuwenhoek 2017, 110, 1281–1286. [Google Scholar] [CrossRef]
- Lu, S.; Wang, J.; Chitsaz, F.; Derbyshire, M.K.; Geer, R.C.; Gonzales, N.R.; Gwadz, M.; Hurwitz, D.I.; Marchler, G.H.; Song, J.S.; et al. CDD/SPARCLE: the conserved domain database in 2020. Nucleic Acids Res. 2020, 48, D265–D268. [Google Scholar] [CrossRef]
- de Castro, E.; Sigrist, C.J.A.; Gattiker, A.; Bulliard, V.; Langendijk-Genevaux, P.S.; Gasteiger, E.; Bairoch, A.; Hulo, N. ScanProsite: Detection of PROSITE signature matches and ProRule-associated functional and structural residues in proteins. Nucleic Acids Res. 2006, 34, W362–W365. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Zankari, E.; Hasman, H.; Cosentino, S.; Vestergaard, M.; Rasmussen, S.; Lund, O.; Aarestrup, F.M.; Larsen, M.V. Identification of acquired antimicrobial resistance genes. J. Antimicrob. Chemother. 2012, 67, 2640–2644. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Miao, V.; Kwong, W.; Xia, R.; Davies, J. Identification of a novel fosfomycin resistance gene (fosA2) in Enterobacter cloacae from the Salmon River, Canada. Lett. Appl. Microbiol. 2011, 52, 427–429. [Google Scholar] [CrossRef] [PubMed]
- Hansen, L.H.; Johannesen, E.; Burmølle, M.; Sørensen, A.H.; Sørensen, S.J. Plasmid-encoded multidrug efflux pump conferring resistance to olaquindox in Escherichia coli. Antimicrob. Agents Chemother. 2004, 48, 3332–3337. [Google Scholar] [CrossRef] [PubMed]
- Römling, U.; Galperin, M.Y.; Gomelsky, M. Cyclic di-GMP: The first 25 years of a universal bacterial second messenger. Microbiol. Mol. Biol. Rev. 2013, 77, 1–52. [Google Scholar] [CrossRef]
- Tischler, A.D.; Camilli, A. Cyclic diguanylate (c-di-GMP) regulates Vibrio cholerae biofilm formation. Mol. Microbiol. 2004, 53, 857–869. [Google Scholar] [CrossRef]
- Chan, C.; Paul, R.; Samoray, D.; Amiot, N.C.; Giese, B.; Jenal, U.; Schirmer, T. Structural basis of activity and allosteric control of diguanylate cyclase. Proc. Natl. Acad. Sci. USA 2004, 101, 17084–17089. [Google Scholar] [CrossRef]
- Römling, U. Characterization of the rdar morphotype, a multicellular behaviour in Enterobacteriaceae. Cell. Mol. Life Sci. 2005, 62, 1234–1246. [Google Scholar] [CrossRef]
- Römling, U.; Liang, Z.X.; Dow, J.M. Progress in understanding the molecular basis underlying functional diversification of cyclic dinucleotide turnover proteins. J Bacteriol. 2017, 199. [Google Scholar] [CrossRef]
- El Mouali, Y.; Kim, H.; Ahmad, I.; Brauner, A.; Liu, Y.; Skurnik, M.; Galperin, M.Y.; Römling, U. Stand-alone EAL domain proteins form a distinct subclass of EAL proteins involved in regulation of cell motility and biofilm formation in Enterobacteria. J. Bacteriol. 2017, 199. [Google Scholar] [CrossRef]
- Herbst, S.; Lorkowski, M.; Sarenko, O.; Nguyen, T.K.L.; Jaenicke, T.; Hengge, R. Transmembrane redox control and proteolysis of PdeC, a novel type of c-di-GMP phosphodiesterase. EMBO J. 2018, 37. [Google Scholar] [CrossRef]
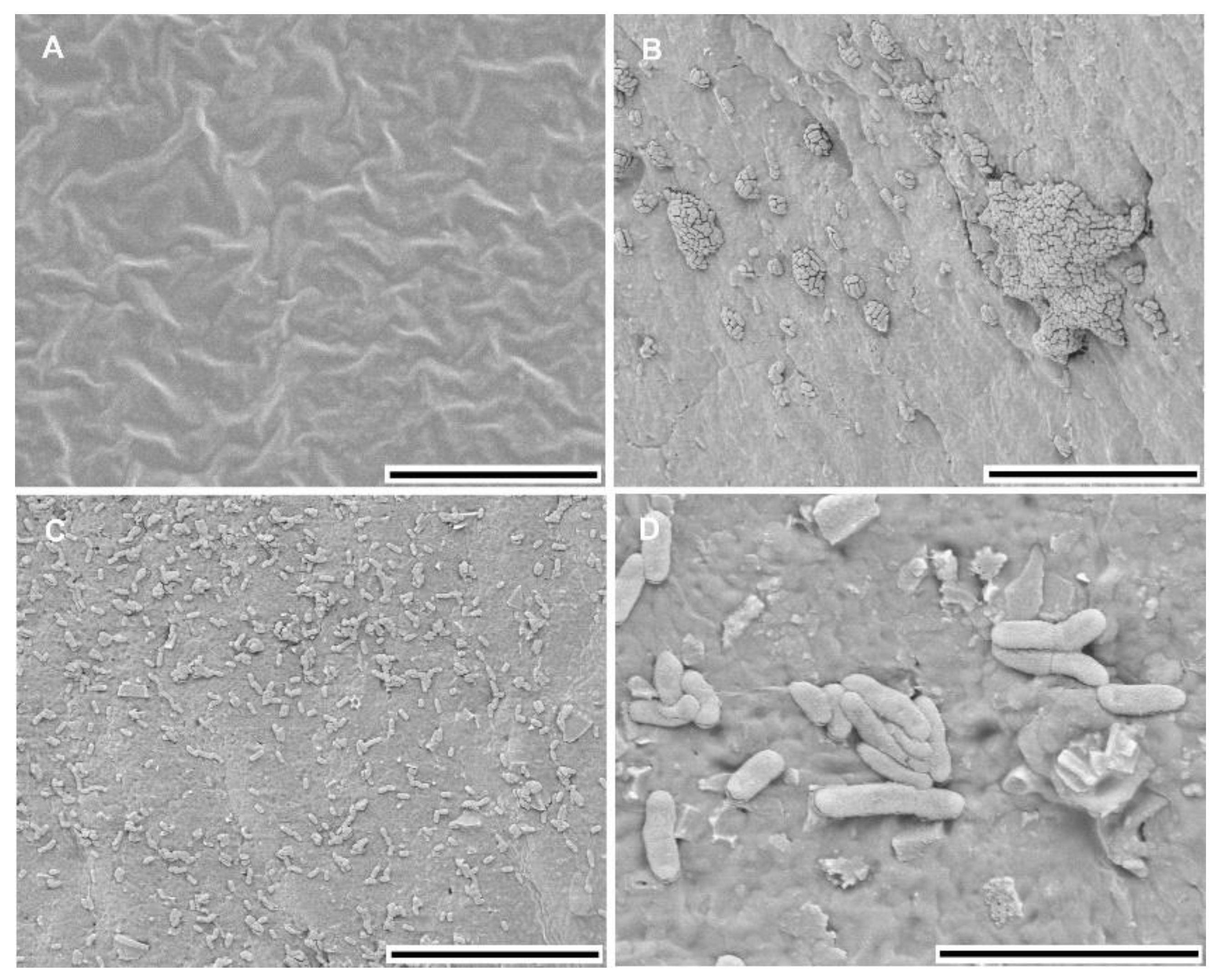
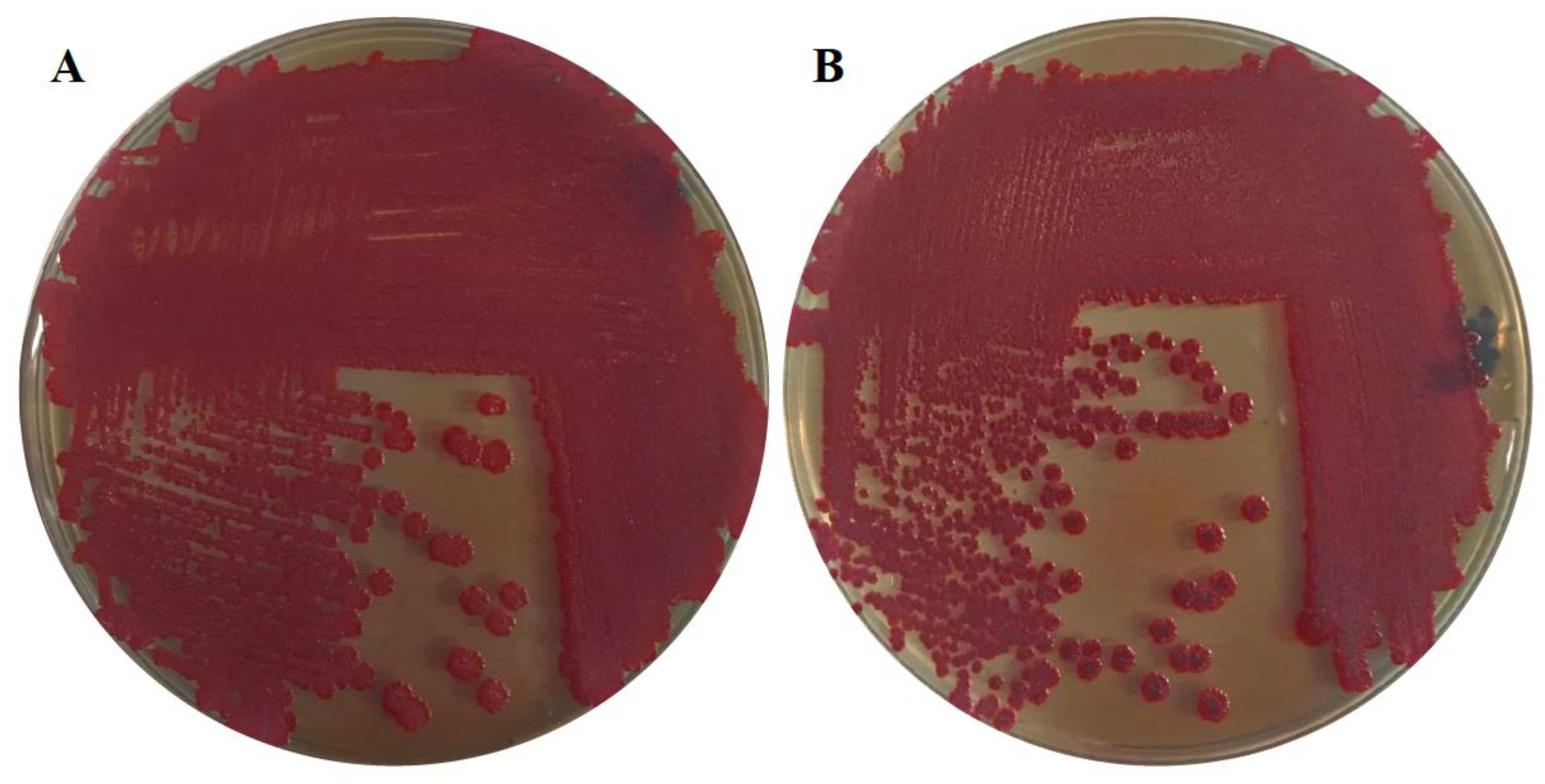
| Pellicle 1 | Cell Aggregation 1 | Adherence 1 | Morphotype 1 | |
|---|---|---|---|---|
| 28 °C | +++ | - | +++ | pdar |
| 37 °C | - | + | + | rdar |
| Resistance Gene | % Gene Identity 1 | Chromosome Position | Predicted Phenotype | Accession Number of Reference |
|---|---|---|---|---|
| blaACT-12 | 99.21 | 3677688–3678833 | Beta-lactam resistance AmpC-type | JX440355 |
| fosA2 | 97.42 | 3879166–3879591 | Fosfomycin resistance | EU487198 |
| oqxA | 86.82 | 4473130–4474305 | Quinolone resistance | EU370913 |
| oqxB | 89.39 | 4474329–4477429 | Quinolone resistance | EU370913 |
| Protein E. ludwigii CEB04 | Locus Tag 1 | Protein E. coli MG1655 2 | % Identity 2,3 | Domain Structure | Highest Identity Outside the Enterobacter Genus |
| GGDEF domain proteins | |||||
| EL-406 | E5283_02015 | - | - | dCache-1-GGDEF | Kosakonia sp.; 50.61%; WP043952574.1 |
| EL-408 | E5283_02035 | YeaP | 67.94 | GAF-GGDEF | Lelliottia nimipressuralis; 86.26%; WP_134350745.1 |
| EL-577 | E5283_02875 | YcdT | 30.04 | MASE4-GGDEF | Leclercia sp.; 73.95%; WP103793192.1 |
| EL-703 | E5283_03510 | - | - | MASE5-GGDEF | Lelliottia sp.; 65.49%; WP103946328.1 |
| EL-841 | E5283_04195 | - | - | Unknown-GGDEF | Lelliottia sp.; 82.52%; WP129034904.1 |
| EL-842 | E5283_04200 | YeaP | 32.52 | GAF-GGDEF | Lelliottia sp.; 70.09%; WP064326108.1 |
| EL-1065 | E5283_05285 | YdaM | 68.54 | PAS-PAS-GGDEF | L. nimipressuralis; 87.07%; WP_134350494.1 |
| EL-1102 | E5283_05465 | YcdT | 43.85 | MASE4-GGDEF | Klebsiella oxytoca; 78.12%; SAP54703.1 |
| EL-1543 | E5283_07695 | YdeQ | 62.15 | CHASE7-xCache-GGDEF | K. oxytoca; 78.12%; SAP54703.1 |
| EL-2072 | E5283_10410 | YfiN | 66.17 | CHASE8-HAMP-GGDEF | L. nimipressuralis; 93.84%; WP_134347265.1 |
| EL-3421 | E5283_17410 | - | - | dCache_1-GGDEF | L. nimipressuralis; 85.22%; WP_134348056.1 |
| EL-3489 | E5283_17760 | - | - | Unknown-PAS-GGDEF | Lelliottia sp.; 73.66%; WP123429896.1 |
| EL-3812 | E5283_19415 | - | - | Unknown-GGDEF | L. nimipressuralis; 93.84%; WP_134347265.1 |
| EL-4100 | E5283_20885 | - | - | TMAO_torS-HAMP-NarQ-GGDEF | Citrobacter sp.; 79.97%; MTZ80699.1 |
| EL-4124 | E5283_21005 | AdrA | 61.31 | MASE2-GGDEF | Lelliottia sp.; 76.13%; WP103948306.1 |
| EAL domain proteins | |||||
| EL-404 | E5283_02005 | - | - | CSS-EAL | Klebsiella sp.; 79.66%; SSW82448.1 |
| EL-405 | E5283_02010 | YcgG | 50.40 | CSS-EAL | L. nimipressuralis; 84.39%; P_134349910.1 |
| EL-524 | E5283_02605 | BluR | 62.03 | BLUF-EAL | Leclercia sp.; 90.39%; WP_048027580.1 |
| EL-769 | E5283_03840 | - | - | Unknown-HTH-LuxR-EAL | Leclercia sp.; 90.39%; WP_048027580.1 |
| EL-905 | E5283_04505 | BluR | 44.50 | BLUF-EAL | L. nimipressuralis; 87.87%; WP_134350370.1 |
| EL-1383 | E5283_06890 | YoaD | 68.61 | CSS-EAL | Klebsiella sp.; 64.75%; WP049840643.1 |
| EL-1650 | E5283_08250 | - | - | Unknown-EAL | L. nimipressuralis; 87.87%; WP_134350370.1 |
| EL-1742 | E5283_08705 | Rtn | 60.51 | CSS-EAL | Mesorhizobium sp.; 90%; TIN52519.1 |
| EL-2984 | E5283_15070 | YhjH | 72.73 | EAL | Lelliottila aquatilis; 81.95%; WP_103946061.1 |
| EL-3490 | E5283_17765 | YjcC | 63.13 | CSS-EAL | L. nimipressuralis; 95.59%; WP_134347305.1 |
| EL-4205 | E5283_21415 | YlaB | 59.69 | CSS-EAL | Citrobacter sp.; 61.97%; WP121585463.1 |
| EL-4271 | E5283_21750 | - | - | EAL | Mesorhizobium sp.; 90%; TIN52519.1 |
| GGDEF-EAL domain proteins | |||||
| EL-640 | E5283_03185 | - | - | CHASE4-GGDEF-EAL | Lelliottia sp.; 82.64%; WP059178784.1 |
| EL-1064 | E5283_05280 | - | - | Unknown-GGDEF-EAL | Citrobacter sp.; 58.12%; WP086551269.1 |
| EL-1108 | E5283_05495 | YciR | 78.78 | PAS-GGDEF-EAL | L. nimipressuralis; 97.44%; TFB20523.1 |
| EL-1647 | E5283_08235 | YegE | 75.32 | MASE1-PAS-PAS_PAS-GGDEF-xEAL | Mesorhizobium sp.; 92.79%; TIM58473.1 |
| EL-1899 | E5283_09475 | YfeA | 58.25 | MASE1-xGGDEF-EAL | L. nimipressuralis; 80.07%; OIR50653.1 |
| EL-1965 | E5283_09845 | YfgF | 62.81 | MASE1-xGGDEF-EAL | L. nimipressuralis; 90.43%; WP_134347661.1 |
| EL-2734 | E5283_13795 | CsrD | 77.55 | GAPES4-xGGDEF-xEAL | L. nimipressuralis; 80.07%; OIR50653.1 |
| EL-2748 | E5283_13865 | - | - | MHYT-GGDEF-EAL | Kosakonia sp.; 79.94%; SEL52885.1 |
| EL-2988 | E5283_15090 | YhjK | 78.15 | GAPES3-HAMP-xGGDEF-EAL | L. nimipressuralis; 90.43%; WP_134347661.1 |
| EL-4362 | E5283_22180 | - | - | Unknown-GGDEF-EAL | Citrobacter sp.; 65.63%; WP003836475.1 |
| HD-GYP domain proteins | |||||
| EL-1478 | E5283_07365 | - | - | SBP_bac_3-SBP_bac_3-HD-GYP | Lelliottia sp.; 72.47%; WP103949684.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shafeeq, S.; Wang, X.; Lünsdorf, H.; Brauner, A.; Römling, U. Draft Genome Sequence of the Urinary Catheter Isolate Enterobacter ludwigii CEB04 with High Biofilm Forming Capacity. Microorganisms 2020, 8, 522. https://doi.org/10.3390/microorganisms8040522
Shafeeq S, Wang X, Lünsdorf H, Brauner A, Römling U. Draft Genome Sequence of the Urinary Catheter Isolate Enterobacter ludwigii CEB04 with High Biofilm Forming Capacity. Microorganisms. 2020; 8(4):522. https://doi.org/10.3390/microorganisms8040522
Chicago/Turabian StyleShafeeq, Sulman, Xiaoda Wang, Heinrich Lünsdorf, Annelie Brauner, and Ute Römling. 2020. "Draft Genome Sequence of the Urinary Catheter Isolate Enterobacter ludwigii CEB04 with High Biofilm Forming Capacity" Microorganisms 8, no. 4: 522. https://doi.org/10.3390/microorganisms8040522
APA StyleShafeeq, S., Wang, X., Lünsdorf, H., Brauner, A., & Römling, U. (2020). Draft Genome Sequence of the Urinary Catheter Isolate Enterobacter ludwigii CEB04 with High Biofilm Forming Capacity. Microorganisms, 8(4), 522. https://doi.org/10.3390/microorganisms8040522






