Human Herpesvirus 8 and Host-Cell Interaction: Long-Lasting Physiological Modifications, Inflammation and Related Chronic Diseases
Abstract
1. Introduction
2. Virology and Molecular Biology of HHV8
2.1. Lytic HHV8 Infection
2.2. Latent HHV8 Infection
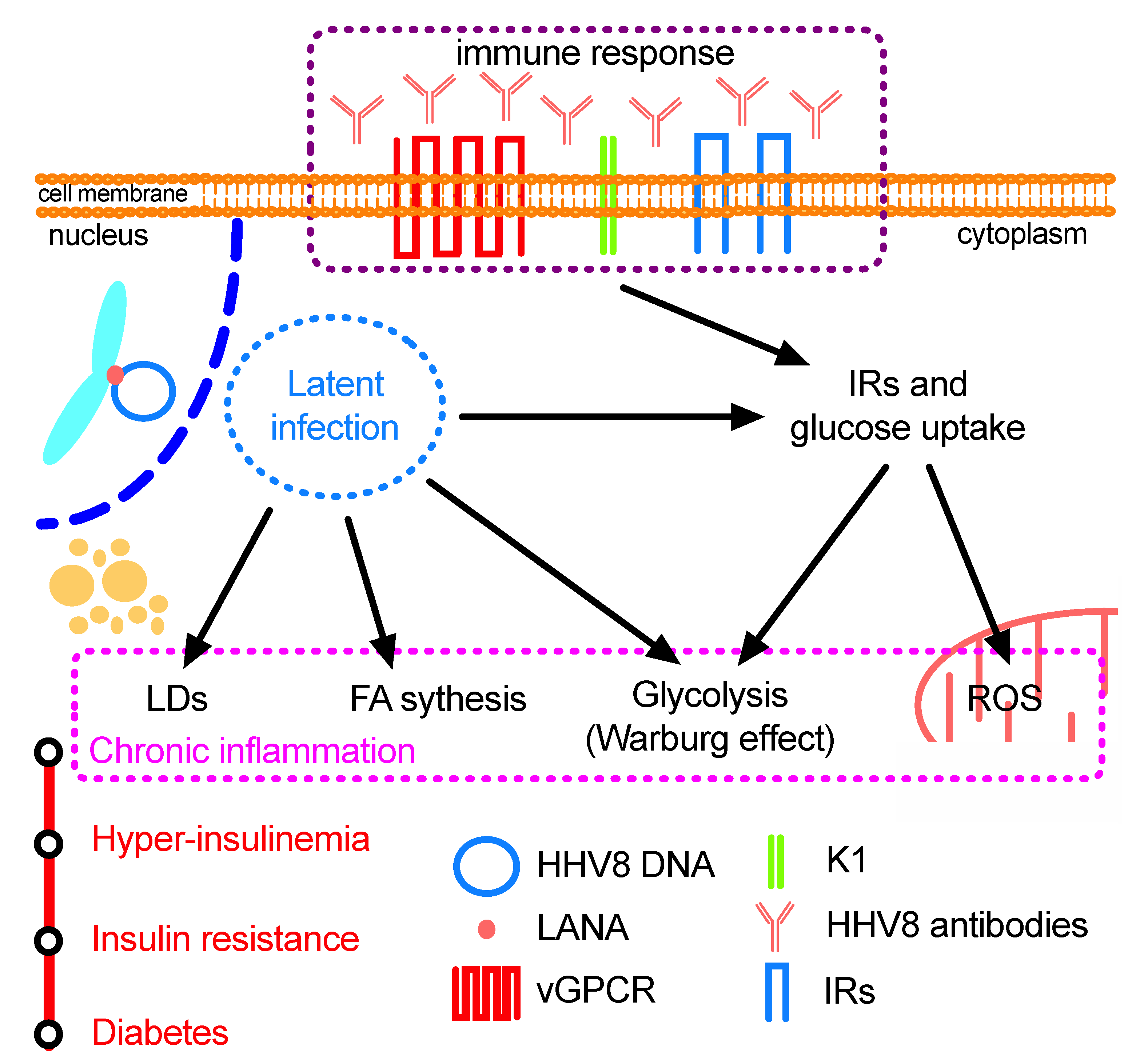
3. HHV8 Infection, Physiological Alterations and Pathogenesis
3.1. Endothelial Cells: Glycolysis, Warburg Effect and Oncogenesis
3.2. HHV8 and Cyclooxygenase: Induction and Suppression of Immune Reaction
3.3. Immune Response and Cell Metabolism
3.4. HHV8 Prevalence in Chronic Diseases
3.5. HHV8-Infection and Oxidative Stress
4. Concluding Remarks and Future Scenarios
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chang, Y.; Cesarman, E.; Pessin, M.S.; Lee, F.; Culpepper, J.; Knowles, D.M.; Moore, P.S. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcoma. Science 1994, 266, 1865–1869. [Google Scholar] [CrossRef] [PubMed]
- Wen, K.W.; Damania, B. Kaposi sarcoma-associated herpesvirus (KSHV): Molecular biology and oncogenesis. Cancer Lett. 2010, 289, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Cousins, E.; Nicholas, J. Molecular biology of human herpesvirus 8: Novel functions and virus-host interactions implicated in viral pathogenesis and replication. Recent Results Cancer Res. 2014, 193, 227–268. [Google Scholar] [CrossRef] [PubMed]
- Vastag, L.; Koyuncu, E.; Grady, S.L.; Shenk, T.E.; Rabinowitz, J.D. Divergent effects of human cytomegalovirus and herpes simplex virus-1 on cellular metabolism. PLoS Pathog. 2011, 7, e1002124. [Google Scholar] [CrossRef] [PubMed]
- Cesarman, E.; Damania, B.; Krown, S.E.; Martin, J.; Bower, M.; Whitby, D. Kaposi sarcoma. Nat. Rev. Dis. Primers 2019, 5, 9. [Google Scholar] [CrossRef]
- Ganem, D. KSHV and the pathogenesis of Kaposi sarcoma: Listening to human biology and medicine. J. Clin. Investig. 2010, 120, 939–949. [Google Scholar] [CrossRef]
- Ojala, P.M.; Schulz, T.F. Manipulation of endothelial cells by KSHV: Implications for angiogenesis and aberrant vascular differentiation. Semin. Cancer Biol. 2014, 26, 69–77. [Google Scholar] [CrossRef]
- Cai, X.; Cullen, B.R. Transcriptional origin of Kaposi’s sarcoma-associated herpesvirus microRNAs. J. Virol. 2006, 80, 2234–2242. [Google Scholar] [CrossRef]
- Cai, X.; Lu, S.; Zhang, Z.; Gonzalez, C.M.; Damania, B.; Cullen, B.R. Kaposi’s sarcoma-associated herpesvirus expresses an array of viral microRNAs in latently infected cells. Proc. Natl. Acad. Sci. USA 2005, 102, 5570–5575. [Google Scholar] [CrossRef]
- Murphy, E.; Vanicek, J.; Robins, H.; Shenk, T.; Levine, A.J. Suppression of immediate-early viral gene expression by herpesvirus-coded microRNAs: Implications for latency. Proc. Natl. Acad. Sci. USA 2008, 105, 5453–5458. [Google Scholar] [CrossRef]
- Pfeffer, S.; Sewer, A.; Lagos-Quintana, M.; Sheridan, R.; Sander, C.; Grasser, F.A.; van Dyk, L.F.; Ho, C.K.; Shuman, S.; Chien, M.; et al. Identification of microRNAs of the herpesvirus family. Nat. Methods 2005, 2, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Samols, M.A.; Hu, J.; Skalsky, R.L.; Renne, R. Cloning and identification of a microRNA cluster within the latency-associated region of Kaposi’s sarcoma-associated herpesvirus. J. Virol. 2005, 79, 9301–9305. [Google Scholar] [CrossRef] [PubMed]
- Samols, M.A.; Skalsky, R.L.; Maldonado, A.M.; Riva, A.; Lopez, M.C.; Baker, H.V.; Renne, R. Identification of cellular genes targeted by KSHV-encoded microRNAs. PLoS Pathog. 2007, 3, e65. [Google Scholar] [CrossRef] [PubMed]
- Renne, R.; Zhong, W.; Herndier, B.; McGrath, M.; Abbey, N.; Kedes, D.; Ganem, D. Lytic growth of Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) in culture. Nat. Med. 1996, 2, 342–346. [Google Scholar] [CrossRef]
- Gao, S.J.; Deng, J.H.; Zhou, F.C. Productive lytic replication of a recombinant Kaposi’s sarcoma-associated herpesvirus in efficient primary infection of primary human endothelial cells. J. Virol. 2003, 77, 9738–9749. [Google Scholar] [CrossRef]
- Moore, P.S.; Chang, Y. Kaposi’s sarcoma (KS), KS-associated herpesvirus, and the criteria for causality in the age of molecular biology. Am. J. Epidemiol. 1998, 147, 217–221. [Google Scholar] [CrossRef]
- Delgado, T.; Carroll, P.A.; Punjabi, A.S.; Margineantu, D.; Hockenbery, D.M.; Lagunoff, M. Induction of the Warburg effect by Kaposi’s sarcoma herpesvirus is required for the maintenance of latently infected endothelial cells. Proc. Natl. Acad. Sci. USA 2010, 107, 10696–10701. [Google Scholar] [CrossRef]
- Rose, P.P.; Bogyo, M.; Moses, A.V.; Fruh, K. Insulin-like growth factor II receptor-mediated intracellular retention of cathepsin B is essential for transformation of endothelial cells by Kaposi’s sarcoma-associated herpesvirus. J. Virol. 2007, 81, 8050–8062. [Google Scholar] [CrossRef][Green Version]
- Wang, L.; Damania, B. Kaposi’s sarcoma-associated herpesvirus confers a survival advantage to endothelial cells. Cancer Res. 2008, 68, 4640–4648. [Google Scholar] [CrossRef]
- Ablashi, D.V.; Chatlynne, L.G.; Whitman, J.E., Jr.; Cesarman, E. Spectrum of Kaposi’s sarcoma-associated herpesvirus, or human herpesvirus 8, diseases. Clin. Microbiol. Rev. 2002, 15, 439–464. [Google Scholar] [CrossRef]
- Li, N.; Thompson, S.; Schultz, D.C.; Zhu, W.; Jiang, H.; Luo, C.; Lieberman, P.M. Discovery of selective inhibitors against EBNA1 via high throughput in silico virtual screening. PLoS ONE 2010, 5, e10126. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.G.; Sharma-Walia, N.; Chandran, B. Targeting KSHV/HHV-8 latency with COX-2 selective inhibitor nimesulide: A potential chemotherapeutic modality for primary effusion lymphoma. PLoS ONE 2011, 6, e24379. [Google Scholar] [CrossRef]
- Angius, F.; Piras, E.; Uda, S.; Madeddu, C.; Serpe, R.; Bigi, R.; Chen, W.; Dittmer, D.P.; Pompei, R.; Ingianni, A. Antimicrobial sulfonamides clear latent Kaposi sarcoma herpesvirus infection and impair MDM2-p53 complex formation. J. Antibiot. 2017, 70, 962–966. [Google Scholar] [CrossRef] [PubMed]
- Angius, F.; Piras, E.; Spolitu, S.; Marras, L.; Armas, S.F.; Ingianni, A.; Contini, P.; Pompei, R. Anti-human herpesvirus 8 antibodies affect both insulin and glucose uptake by virus-infected human endothelial cells. J. Infect. Dev. Ctries. 2018, 12, 485–491. [Google Scholar] [CrossRef]
- Delgado, T.; Sanchez, E.L.; Camarda, R.; Lagunoff, M. Global metabolic profiling of infection by an oncogenic virus: KSHV induces and requires lipogenesis for survival of latent infection. PLoS Pathog. 2012, 8, e1002866. [Google Scholar] [CrossRef]
- Fontaine, K.A.; Sanchez, E.L.; Camarda, R.; Lagunoff, M. Dengue virus induces and requires glycolysis for optimal replication. J. Virol. 2015, 89, 2358–2366. [Google Scholar] [CrossRef]
- Sanchez, E.L.; Lagunoff, M. Viral activation of cellular metabolism. Virology 2015, 479–480, 609–618. [Google Scholar] [CrossRef]
- Thai, M.; Graham, N.A.; Braas, D.; Nehil, M.; Komisopoulou, E.; Kurdistani, S.K.; McCormick, F.; Graeber, T.G.; Christofk, H.R. Adenovirus E4ORF1-induced MYC activation promotes host cell anabolic glucose metabolism and virus replication. Cell Metab. 2014, 19, 694–701. [Google Scholar] [CrossRef]
- Bilz, N.C.; Jahn, K.; Lorenz, M.; Ludtke, A.; Hubschen, J.M.; Geyer, H.; Mankertz, A.; Hubner, D.; Liebert, U.G.; Claus, C. Rubella viruses shift cellular bioenergetics to a more oxidative and glycolytic phenotype with a strain-specific requirement for glutamine. J. Virol. 2018, 92. [Google Scholar] [CrossRef]
- Burysek, L.; Yeow, W.S.; Lubyova, B.; Kellum, M.; Schafer, S.L.; Huang, Y.Q.; Pitha, P.M. Functional analysis of human herpesvirus 8-encoded viral interferon regulatory factor 1 and its association with cellular interferon regulatory factors and p300. J. Virol. 1999, 73, 7334–7342. [Google Scholar] [CrossRef]
- Gao, S.J.; Boshoff, C.; Jayachandra, S.; Weiss, R.A.; Chang, Y.; Moore, P.S. KSHV ORF K9 (vIRF) is an oncogene which inhibits the interferon signaling pathway. Oncogene 1997, 15, 1979–1985. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Lee, H.; Guo, J.; Neipel, F.; Fleckenstein, B.; Ozato, K.; Jung, J.U. Kaposi’s sarcoma-associated herpesvirus viral interferon regulatory factor. J. Virol. 1998, 72, 5433–5440. [Google Scholar] [CrossRef] [PubMed]
- Pozharskaya, V.P.; Weakland, L.L.; Zimring, J.C.; Krug, L.T.; Unger, E.R.; Neisch, A.; Joshi, H.; Inoue, N.; Offermann, M.K. Short duration of elevated vIRF-1 expression during lytic replication of human herpesvirus 8 limits its ability to block antiviral responses induced by alpha interferon in BCBL-1 cells. J. Virol. 2004, 78, 6621–6635. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Russo, J.J.; Bohenzky, R.A.; Chien, M.C.; Chen, J.; Yan, M.; Maddalena, D.; Parry, J.P.; Peruzzi, D.; Edelman, I.S.; Chang, Y.; et al. Nucleotide sequence of the Kaposi sarcoma-associated herpesvirus (HHV8). Proc. Natl. Acad. Sci. USA 1996, 93, 14862–14867. [Google Scholar] [CrossRef]
- Seo, T.; Park, J.; Lee, D.; Hwang, S.G.; Choe, J. Viral interferon regulatory factor 1 of Kaposi’s sarcoma-associated herpesvirus binds to p53 and represses p53-dependent transcription and apoptosis. J. Virol. 2001, 75, 6193–6198. [Google Scholar] [CrossRef]
- Arvanitakis, L.; Geras-Raaka, E.; Varma, A.; Gershengorn, M.C.; Cesarman, E. Human herpesvirus KSHV encodes a constitutively active G-protein-coupled receptor linked to cell proliferation. Nature 1997, 385, 347–350. [Google Scholar] [CrossRef]
- Bais, C.; van Geelen, A.; Eroles, P.; Mutlu, A.; Chiozzini, C.; Dias, S.; Silverstein, R.L.; Rafii, S.; Mesri, E.A. Kaposi’s sarcoma associated herpesvirus G protein-coupled receptor immortalizes human endothelial cells by activation of the VEGF receptor-2/KDR. Cancer Cell 2003, 3, 131–143. [Google Scholar] [CrossRef]
- Cesarman, E.; Nador, R.G.; Bai, F.; Bohenzky, R.A.; Russo, J.J.; Moore, P.S.; Chang, Y.; Knowles, D.M. Kaposi’s sarcoma-associated herpesvirus contains G protein-coupled receptor and cyclin D homologs which are expressed in Kaposi’s sarcoma and malignant lymphoma. J. Virol. 1996, 70, 8218–8223. [Google Scholar] [CrossRef]
- Guo, H.G.; Browning, P.; Nicholas, J.; Hayward, G.S.; Tschachler, E.; Jiang, Y.W.; Sadowska, M.; Raffeld, M.; Colombini, S.; Gallo, R.C.; et al. Characterization of a chemokine receptor-related gene in human herpesvirus 8 and its expression in Kaposi’s sarcoma. Virology 1997, 228, 371–378. [Google Scholar] [CrossRef]
- Montaner, S.; Sodhi, A.; Pece, S.; Mesri, E.A.; Gutkind, J.S. The Kaposi’s sarcoma-associated herpesvirus G protein-coupled receptor promotes endothelial cell survival through the activation of Akt/protein kinase B. Cancer Res. 2001, 61, 2641–2648. [Google Scholar]
- Hussein, H.A.M.; Alfhili, M.A.; Pakala, P.; Simon, S.; Hussain, J.; McCubrey, J.A.; Akula, S.M. miRNAs and their roles in KSHV pathogenesis. Virus Res. 2019, 266, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Dourmishev, L.A.; Dourmishev, A.L.; Palmeri, D.; Schwartz, R.A.; Lukac, D.M. Molecular genetics of Kaposi’s sarcoma-associated herpesvirus (human herpesvirus-8) epidemiology and pathogenesis. Microbiol. Mol. Biol. Rev. 2003, 67, 175–212. [Google Scholar] [CrossRef] [PubMed]
- Friborg, J., Jr.; Kong, W.; Hottiger, M.O.; Nabel, G.J. p53 inhibition by the LANA protein of KSHV protects against cell death. Nature 1999, 402, 889–894. [Google Scholar] [CrossRef] [PubMed]
- Djerbi, M.; Screpanti, V.; Catrina, A.I.; Bogen, B.; Biberfeld, P.; Grandien, A. The inhibitor of death receptor signaling, FLICE-inhibitory protein defines a new class of tumor progression factors. J. Exp. Med. 1999, 190, 1025–1032. [Google Scholar] [CrossRef] [PubMed]
- Efklidou, S.; Bailey, R.; Field, N.; Noursadeghi, M.; Collins, M.K. vFLIP from KSHV inhibits anoikis of primary endothelial cells. J. Cell Sci. 2008, 121, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Fakhari, F.D.; Dittmer, D.P. Charting latency transcripts in Kaposi’s sarcoma-associated herpesvirus by whole-genome real-time quantitative PCR. J. Virol. 2002, 76, 6213–6223. [Google Scholar] [CrossRef]
- Sadler, R.; Wu, L.; Forghani, B.; Renne, R.; Zhong, W.; Herndier, B.; Ganem, D. A complex translational program generates multiple novel proteins from the latently expressed kaposin (K12) locus of Kaposi’s sarcoma-associated herpesvirus. J. Virol. 1999, 73, 5722–5730. [Google Scholar] [CrossRef]
- Lagunoff, M.; Ganem, D. The structure and coding organization of the genomic termini of Kaposi’s sarcoma-associated herpesvirus. Virology 1997, 236, 147–154. [Google Scholar] [CrossRef]
- Lee, H.; Guo, J.; Li, M.; Choi, J.K.; DeMaria, M.; Rosenzweig, M.; Jung, J.U. Identification of an immunoreceptor tyrosine-based activation motif of K1 transforming protein of Kaposi’s sarcoma-associated herpesvirus. Mol. Cell Biol. 1998, 18, 5219–5228. [Google Scholar] [CrossRef]
- Wang, L.; Dittmer, D.P.; Tomlinson, C.C.; Fakhari, F.D.; Damania, B. Immortalization of primary endothelial cells by the K1 protein of Kaposi’s sarcoma-associated herpesvirus. Cancer Res. 2006, 66, 3658–3666. [Google Scholar] [CrossRef]
- Wang, L.; Wakisaka, N.; Tomlinson, C.C.; DeWire, S.M.; Krall, S.; Pagano, J.S.; Damania, B. The Kaposi’s sarcoma-associated herpesvirus (KSHV/HHV-8) K1 protein induces expression of angiogenic and invasion factors. Cancer Res. 2004, 64, 2774–2781. [Google Scholar] [CrossRef] [PubMed]
- Aoki, Y.; Jaffe, E.S.; Chang, Y.; Jones, K.; Teruya-Feldstein, J.; Moore, P.S.; Tosato, G. Angiogenesis and hematopoiesis induced by Kaposi’s sarcoma-associated herpesvirus-encoded interleukin-6. Blood 1999, 93, 4034–4043. [Google Scholar] [CrossRef]
- Chen, D.; Nicholas, J. Structural requirements for gp80 independence of human herpesvirus 8 interleukin-6 (vIL-6) and evidence for gp80 stabilization of gp130 signaling complexes induced by vIL-6. J. Virol. 2006, 80, 9811–9821. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, D.; Sandford, G.; Nicholas, J. Intracellular signaling mechanisms and activities of human herpesvirus 8 interleukin-6. J. Virol. 2009, 83, 722–733. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Majerciak, V.; Zheng, Z.M.; Lan, K. Towards better understanding of KSHV life cycle: From transcription and posttranscriptional regulations to pathogenesis. Virol. Sin. 2019, 34, 135–161. [Google Scholar] [CrossRef] [PubMed]
- De Bock, K.; Georgiadou, M.; Schoors, S.; Kuchnio, A.; Wong, B.W.; Cantelmo, A.R.; Quaegebeur, A.; Ghesquiere, B.; Cauwenberghs, S.; Eelen, G.; et al. Role of PFKFB3-driven glycolysis in vessel sprouting. Cell 2013, 154, 651–663. [Google Scholar] [CrossRef]
- Saka, H.A.; Valdivia, R. Emerging roles for lipid droplets in immunity and host-pathogen interactions. Annu. Rev. Cell Dev. Biol. 2012, 28, 411–437. [Google Scholar] [CrossRef]
- Carroll, P.A.; Kenerson, H.L.; Yeung, R.S.; Lagunoff, M. Latent Kaposi’s sarcoma-associated herpesvirus infection of endothelial cells activates hypoxia-induced factors. J. Virol. 2006, 80, 10802–10812. [Google Scholar] [CrossRef]
- Lagunoff, M. Activation of cellular metabolism during latent Kaposi’s Sarcoma herpesvirus infection. Curr. Opin. Virol. 2016, 19, 45–49. [Google Scholar] [CrossRef]
- Angius, F.; Uda, S.; Piras, E.; Spolitu, S.; Ingianni, A.; Batetta, B.; Pompei, R. Neutral lipid alterations in human herpesvirus 8-infected HUVEC cells and their possible involvement in neo-angiogenesis. BMC Microbiol. 2015, 15, 74. [Google Scholar] [CrossRef]
- Sanchez, E.L.; Pulliam, T.H.; Dimaio, T.A.; Thalhofer, A.B.; Delgado, T.; Lagunoff, M. Glycolysis, glutaminolysis, and fatty acid synthesis are required for distinct stages of Kaposi’s sarcoma-associated herpesvirus lytic replication. J. Virol. 2017, 91. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Dong, Y.; Atefi, M.; Liu, Y.; Elshimali, Y.; Vadgama, J.V. Lactate, a neglected factor for diabetes and cancer interaction. Mediat. Inflamm. 2016, 2016, 6456018. [Google Scholar] [CrossRef] [PubMed]
- Rose, P.P.; Carroll, J.M.; Carroll, P.A.; DeFilippis, V.R.; Lagunoff, M.; Moses, A.V.; Roberts, C.T., Jr.; Fruh, K. The insulin receptor is essential for virus-induced tumorigenesis of Kaposi’s sarcoma. Oncogene 2007, 26, 1995–2005. [Google Scholar] [CrossRef] [PubMed]
- Guilluy, C.; Zhang, Z.; Bhende, P.M.; Sharek, L.; Wang, L.; Burridge, K.; Damania, B. Latent KSHV infection increases the vascular permeability of human endothelial cells. Blood 2011, 118, 5344–5354. [Google Scholar] [CrossRef] [PubMed]
- Ingianni, A.; Piras, E.; Laconi, S.; Angius, F.; Batetta, B.; Pompei, R. Latent herpesvirus 8 infection improves both insulin and glucose uptake in primary endothelial cells. New Microbiol. 2013, 36, 257–265. [Google Scholar] [PubMed]
- Gregory, S.M.; Wang, L.; West, J.A.; Dittmer, D.P.; Damania, B. Latent Kaposi’s sarcoma-associated herpesvirus infection of monocytes downregulates expression of adaptive immune response costimulatory receptors and proinflammatory cytokines. J. Virol. 2012, 86, 3916–3923. [Google Scholar] [CrossRef]
- Caselli, E.; Fiorentini, S.; Amici, C.; Di Luca, D.; Caruso, A.; Santoro, M.G. Human herpesvirus 8 acute infection of endothelial cells induces monocyte chemoattractant protein 1-dependent capillary-like structure formation: Role of the IKK/NF-kappaB pathway. Blood 2007, 109, 2718–2726. [Google Scholar] [CrossRef]
- Spagnuolo, I.; Patti, A.; Sebastiani, G.; Nigi, L.; Dotta, F. The case for virus-induced type 1 diabetes. Curr. Opin. Endocrinol. Diabetes Obes. 2013, 20, 292–298. [Google Scholar] [CrossRef]
- Wan, X.; Wang, H.; Nicholas, J. Human herpesvirus 8 interleukin-6 (vIL-6) signals through gp130 but has structural and receptor-binding properties distinct from those of human IL-6. J. Virol. 1999, 73, 8268–8278. [Google Scholar] [CrossRef]
- Nolan, C.J.; Damm, P.; Prentki, M. Type 2 diabetes across generations: From pathophysiology to prevention and management. Lancet 2011, 378, 169–181. [Google Scholar] [CrossRef]
- Sharma-Walia, N.; Paul, A.G.; Bottero, V.; Sadagopan, S.; Veettil, M.V.; Kerur, N.; Chandran, B. Kaposi’s sarcoma associated herpes virus (KSHV) induced COX-2: A key factor in latency, inflammation, angiogenesis, cell survival and invasion. PLoS Pathog. 2010, 6, e1000777. [Google Scholar] [CrossRef]
- Wu, T.T.; Qian, J.; Ang, J.; Sun, R. Vaccine prospect of Kaposi sarcoma-associated herpesvirus. Curr. Opin. Virol. 2012, 2, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Chen, D.; Xiang, Q.; Nicholas, J. Insulin-like growth factor 2 receptor expression is promoted by human herpesvirus 8-encoded interleukin-6 and contributes to viral latency and productive replication. J. Virol. 2019, 93. [Google Scholar] [CrossRef]
- Bottero, V.; Chakraborty, S.; Chandran, B. Reactive oxygen species are induced by Kaposi’s sarcoma-associated herpesvirus early during primary infection of endothelial cells to promote virus entry. J. Virol. 2013, 87, 1733–1749. [Google Scholar] [CrossRef] [PubMed]
- Caselli, E.; Rizzo, R.; Ingianni, A.; Contini, P.; Pompei, R.; Di Luca, D. High prevalence of HHV8 infection and specific killer cell immunoglobulin-like receptors allotypes in Sardinian patients with type 2 diabetes mellitus. J. Med. Virol. 2014, 86, 1745–1751. [Google Scholar] [CrossRef] [PubMed]
- Gastaldi, G.; Goossens, N.; Clement, S.; Negro, F. Current level of evidence on causal association between hepatitis C virus and type 2 diabetes: A review. J. Adv. Res. 2017, 8, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Incani, A.; Marras, L.; Serreli, G.; Ingianni, A.; Pompei, R.; Deiana, M.; Angius, F. Human herpesvirus 8 infection may contribute to oxidative stress in diabetes type 2 patients. BMC Res. Notes 2020, 13, 75. [Google Scholar] [CrossRef]
- Pompei, R. The role of human herpesvirus 8 in diabetes mellitus type 2: State of the art and a medical hypothesis. Adv. Exp. Med. Biol. 2016, 901, 37–45. [Google Scholar] [CrossRef]
- Piras, E.; Madeddu, M.A.; Palmieri, G.; Angius, F.; Contini, P.; Pompei, R.; Ingianni, A. High prevalence of human herpesvirus 8 infection in diabetes type 2 patients and detection of a new virus subtype. Adv. Exp. Med. Biol. 2017, 973, 41–51. [Google Scholar] [CrossRef]
- Douglas, J.L.; Gustin, J.K.; Viswanathan, K.; Mansouri, M.; Moses, A.V.; Fruh, K. The great escape: Viral strategies to counter BST-2/tetherin. PLoS Pathog. 2010, 6, e1000913. [Google Scholar] [CrossRef]
- Ingianni, A.; Carta, F.; Reina, A.; Manai, M.; Desogus, A.; Pompei, R. Prevalence of herpesvirus 8 infection in type 2 diabetes mellitus patients. Am. J. Infect. Dis. 2007, 3, 123–127. [Google Scholar] [CrossRef]
- Sobngwi, E.; Choukem, S.P.; Agbalika, F.; Blondeau, B.; Fetita, L.S.; Lebbe, C.; Thiam, D.; Cattan, P.; Larghero, J.; Foufelle, F.; et al. Ketosis-prone type 2 diabetes mellitus and human herpesvirus 8 infection in sub-saharan africans. JAMA 2008, 299, 2770–2776. [Google Scholar] [CrossRef] [PubMed]
- Ingianni, A.; Madeddu, M.A.; Carta, F.; Reina, A.; Lai, C.; Pompei, R. Epidemiology of human herpesvirus type 8 infection in cardiopathic patients. OnLine J. Biol. Sci. 2009, 9, 36–39. [Google Scholar] [CrossRef]
- Ye, F.; Zeng, Y.; Sha, J.; Jones, T.; Kuhne, K.; Wood, C.; Gao, S.J. High glucose induces reactivation of latent Kaposi’s sarcoma-associated herpesvirus. J. Virol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Angius, F.; Madeddu, M.; Pompei, R. Commentary: High glucose induces reactivation of latent Kaposi’s sarcoma-associated herpesvirus. Front. Microbiol. 2017, 8, 1796. [Google Scholar] [CrossRef] [PubMed]
- Angius, F.; Marras, L.; Ingianni, A.; Pompei, R. Latent-persistent virus infections. Hepatitis C virus and human herpesvirus 8: Immunological response, modification of cell metabolism and association with type 2 diabetes. In Emerging and Reemerging Viral Pathogens; Academic Press: Cambridge, MA, USA, 2020; Volume 1, pp. 169–181. [Google Scholar]
- Abenavoli, L.; Masarone, M.; Peta, V.; Milic, N.; Kobyliak, N.; Rouabhia, S.; Persico, M. Insulin resistance and liver steatosis in chronic hepatitis C infection genotype. World J. Gastroenterol. 2014, 20, 15233–15240. [Google Scholar] [CrossRef]
- Chen, S.; de Craen, A.J.; Raz, Y.; Derhovanessian, E.; Vossen, A.C.; Westendorp, R.G.; Pawelec, G.; Maier, A.B. Cytomegalovirus seropositivity is associated with glucose regulation in the oldest old. Results from the leiden 85-plus study. Immun. Ageing 2012, 9, 18. [Google Scholar] [CrossRef]
- Lontchi-Yimagou, E.; Legoff, J.; Nguewa, J.L.; Boudou, P.; Balti, E.V.; Noubiap, J.J.; Kamwa, V.; Atogho-Tiedeu, B.; Azabji-Kenfack, M.; Djahmeni, E.N.; et al. Human herpesvirus 8 infection DNA positivity is associated with low insulin secretion: A case-control study in a sub-Saharan African population with diabetes. J. Diabetes 2018, 10, 866–873. [Google Scholar] [CrossRef]
- Falkenberg, K.D.; Rohlenova, K.; Luo, Y.L.; Carmeliet, P. The metabolic engine of endothelial cells. Nat. Metab. 2019, 1, 937–946. [Google Scholar] [CrossRef]
- Gimbrone, M.A., Jr.; Garcia-Cardena, G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ. Res. 2016, 118, 620–636. [Google Scholar] [CrossRef]
- Tabit, C.E.; Chung, W.B.; Hamburg, N.M.; Vita, J.A. Endothelial dysfunction in diabetes mellitus: Molecular mechanisms and clinical implications. Rev. Endocr. Metab. Disord. 2010, 11, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Incani, A.; Serra, G.; Atzeri, A.; Melis, M.P.; Serreli, G.; Bandino, G.; Sedda, P.; Campus, M.; Tuberoso, C.I.; Deiana, M. Extra virgin olive oil phenolic extracts counteract the pro-oxidant effect of dietary oxidized lipids in human intestinal cells. Food Chem. Toxicol. 2016, 90, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Corkey, B.E. Banting lecture 2011: Hyperinsulinemia: Cause or consequence? Diabetes 2012, 61, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Erion, K.A.; Corkey, B.E. Hyperinsulinemia: A cause of obesity? Curr. Obes. Rep. 2017, 6, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Vidali, M.; Tripodi, M.F.; Ivaldi, A.; Zampino, R.; Occhino, G.; Restivo, L.; Sutti, S.; Marrone, A.; Ruggiero, G.; Albano, E.; et al. Interplay between oxidative stress and hepatic steatosis in the progression of chronic hepatitis C. J. Hepatol. 2008, 48, 399–406. [Google Scholar] [CrossRef]
- Incalza, M.A.; D’Oria, R.; Natalicchio, A.; Perrini, S.; Laviola, L.; Giorgino, F. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vasc. Pharmacol. 2018, 100, 1–19. [Google Scholar] [CrossRef]
- Lo, A.K.; Dawson, C.W.; Young, L.S.; Lo, K.W. The role of metabolic reprogramming in gamma-herpesvirus-associated oncogenesis. Int. J. Cancer 2017, 141, 1512–1521. [Google Scholar] [CrossRef]
- Zecchin, A.; Kalucka, J.; Dubois, C.; Carmeliet, P. How endothelial cells adapt their metabolism to form vessels in tumors. Front. Immunol. 2017, 8, 1750. [Google Scholar] [CrossRef]
- Robson, R.; Kundur, A.R.; Singh, I. Oxidative stress biomarkers in type 2 diabetes mellitus for assessment of cardiovascular disease risk. Diabetes Metab. Syndr. 2018, 12, 455–462. [Google Scholar] [CrossRef]
- Li, X.; Feng, J.; Sun, R. Oxidative stress induces reactivation of Kaposi’s sarcoma-associated herpesvirus and death of primary effusion lymphoma cells. J. Virol. 2011, 85, 715–724. [Google Scholar] [CrossRef]
- Ma, Q.; Cavallin, L.E.; Leung, H.J.; Chiozzini, C.; Goldschmidt-Clermont, P.J.; Mesri, E.A. A role for virally induced reactive oxygen species in Kaposi’s sarcoma herpesvirus tumorigenesis. Antioxid. Redox Signal 2013, 18, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Poli, G.; Sottero, B.; Gargiulo, S.; Leonarduzzi, G. Cholesterol oxidation products in the vascular remodeling due to atherosclerosis. Mol. Asp. Med. 2009, 30, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.D.; Corkey, B.E.; Istfan, N.W.; Apovian, C.M. Hyperinsulinemia: An early indicator of Metabolic dysfunction. J. Endocr. Soc. 2019, 3, 1727–1747. [Google Scholar] [CrossRef] [PubMed]
| Metabolites | HHV8 Lytic Infection | HHV8 Latent Infection |
|---|---|---|
| * Cholesterol esters | − | +++ |
| ** Fatty acids | ++ | − |
| ** Spermidine | − | ++ |
| ** 7-beta-hydroxycholesterol | − | + |
| ** Mannose-6-phosphate | − | ++ |
| * Glucose uptake | − | +++ |
| ** phospho-enol-pyruvate | − | +++ |
| ** 6-phosphogluconate | − | + |
| * Triglycerides | +++ | − |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Angius, F.; Ingianni, A.; Pompei, R. Human Herpesvirus 8 and Host-Cell Interaction: Long-Lasting Physiological Modifications, Inflammation and Related Chronic Diseases. Microorganisms 2020, 8, 388. https://doi.org/10.3390/microorganisms8030388
Angius F, Ingianni A, Pompei R. Human Herpesvirus 8 and Host-Cell Interaction: Long-Lasting Physiological Modifications, Inflammation and Related Chronic Diseases. Microorganisms. 2020; 8(3):388. https://doi.org/10.3390/microorganisms8030388
Chicago/Turabian StyleAngius, Fabrizio, Angela Ingianni, and Raffaello Pompei. 2020. "Human Herpesvirus 8 and Host-Cell Interaction: Long-Lasting Physiological Modifications, Inflammation and Related Chronic Diseases" Microorganisms 8, no. 3: 388. https://doi.org/10.3390/microorganisms8030388
APA StyleAngius, F., Ingianni, A., & Pompei, R. (2020). Human Herpesvirus 8 and Host-Cell Interaction: Long-Lasting Physiological Modifications, Inflammation and Related Chronic Diseases. Microorganisms, 8(3), 388. https://doi.org/10.3390/microorganisms8030388






