Identification of Rhizospheric Actinomycete Streptomyces lavendulae SPS-33 and the Inhibitory Effect of its Volatile Organic Compounds against Ceratocystis fimbriata in Postharvest Sweet Potato (Ipomoea batatas (L.) Lam.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganism, Plant Materials, and Chemicals
2.2. Identification of Strain SPS-33
2.3. Antagonistic Test of VOCs Emitted by SPS-33
2.4. Pathogenicity Assay
2.5. Analysis of VOCs Emitted by SPS-33
2.6. Inhibitory Effect of Selected Volatile Compounds on C. fimbriata
2.7. Statistical Analysis
3. Results
3.1. Identification of Strain SPS-33
3.2. Antagonistic Test of VOCs Produced by SPS-33
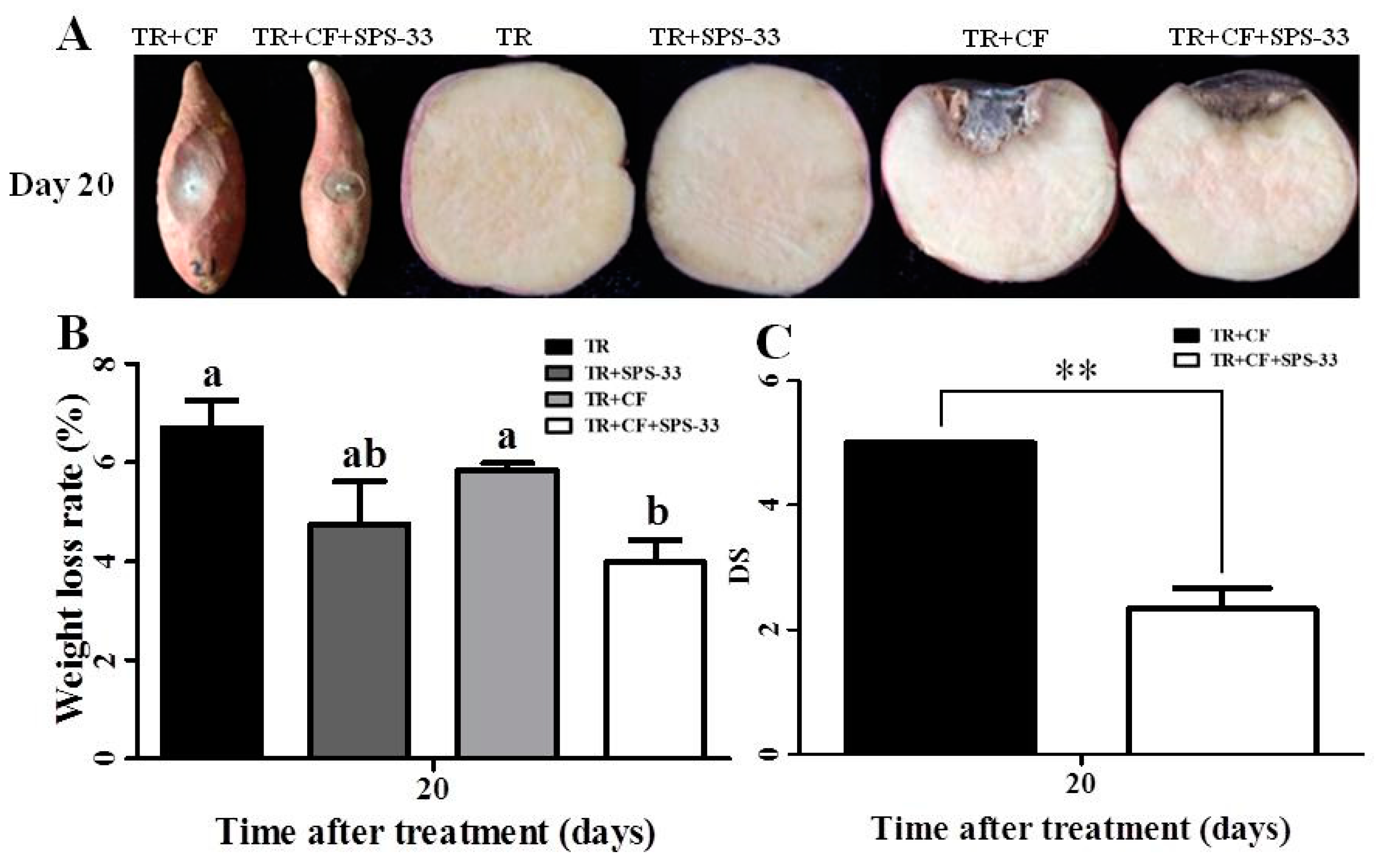
3.3. Pathogenicity Assay
3.4. Analysis of VOCs Emitted by SPS-33
3.5. Inhibitory Effect of Selected Volatile Compounds on C. fimbriata
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| TRs | tuberous roots, |
| VOC | volatile organic compound, |
| PDA | potato dextrose agar, |
| POD | peroxidase, |
| CAT | catalase, |
| SOD | superoxide dismutase, |
| MDA | malondialdehyde, |
| TSS | total soluble sugar, |
| HS-SPME | headspace solid-phase microextraction, |
| GC-MS | gas chromatography-mass spectrometry, |
| ROS | reactive oxygen species, |
| CF | C. fimbriata, |
| DS | disease severity. |
References
- Mohanraj, R.; Sivasankar, S. Chemical constituents and health effects of sweet potato. Food Res. Int. 2016, 89, 90–116. [Google Scholar]
- Mohanraj, R.; Sivasankar, S. Sweet potato (Ipomoea batatas [L.] Lam)—A valuable medicinal food: A review. J. Med. Food 2014, 17, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Sun, H.; Zhang, C.; Xu, Z.; Zhao, Y.; Xie, Y. Biological control of Bacillus amyloliquefaciens strain XZ-1 against black rot on sweet potato. Southwest China J. Agric. Sci. 2016, 31, 736–741. [Google Scholar]
- Yang, D.; Sun, H.; Zhao, Y.; Xu, Z.; Zhang, C.; Xie, Y. Biological characteristics of Ceratocystis fimbriata and selection of fungicides in laboratory. Southwest China J. Agric. Sci. 2013, 26, 2336–2339. [Google Scholar]
- Zhang, D.-S.; Zhang, Y.-C.; Qiao, Q.; Qin, Y.-H.; Tian, Y.-T.; Wang, S.; Zhang, Z.-C. Toxicity and co-toxicity of ten fungicides to Ceratocystis fimbriata. Agrochemicals 2012, 51, 452–454. [Google Scholar]
- Xing, K.; Li, T.J.; Liu, Y.F.; Zhang, J.; Zhang, Y.; Shen, X.Q.; Li, X.Y.; Miao, X.M.; Feng, Z.Z.; Peng, X.; et al. Antifungal and eliciting properties of chitosan against Ceratocystis fimbriata in sweet potato. Food Chem. 2018, 268, 188–195. [Google Scholar] [CrossRef]
- Kanchiswamy, C.N.; Malnoy, M.; Maffei, M.E. Bioprospecting bacterial and fungal volatiles for sustainable agriculture. Trends Plant Sci. 2015, 20, 206–211. [Google Scholar] [CrossRef]
- Gong, A.D.; Wu, N.N.; Kong, X.W.; Zhang, Y.M.; Hu, M.J.; Gong, S.J.; Dong, F.Y.; Wang, J.H.; Zhao, Z.Y.; Liao, Y.C. Inhibitory effect of volatiles emitted from Alcaligenes faecalis N1-4 on Aspergillus flavus and aflatoxins in storage. Front. Microbiol. 2019, 10, 1419. [Google Scholar] [CrossRef]
- Pena, L.C.; Jungklaus, G.H.; Savi, D.C.; Ferreira-Maba, L.; Servienski, A.; Maia, B.H.L.N.S.; Annies, V.; Galli-Terasawa, L.V.; Glienke, C.; Kava, V. Muscodor brasiliensis sp. nov. produces volatile organic compounds with activity against Penicillium digitatum. Microbiol. Res. 2019, 221, 28–35. [Google Scholar] [CrossRef]
- Tahir, H.A.S.; Gu, Q.; Wu, H.; Raza, W.; Safdar, A.; Huang, Z.; Rajer, F.U.; Gao, X. Effect of volatile compounds produced by Ralstonia solanacearum on plant growth promoting and systemic resistance inducing potential of Bacillus volatiles. BMC Plant Biol. 2017, 17, 133. [Google Scholar] [CrossRef]
- Kaddes, A.; Fauconnier, M.L.; Sassi, K.; Nasraoui, B.; Jijakli, M.H. Endophytic fungal volatile compounds as solution for sustainable agriculture. Molecules 2019, 24, 1065. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Feng, W.W.; Wang, T.T.; Ding, P.; Xing, K.; Jiang, J.H. Plant growth-promoting effect and genomic analysis of the beneficial endophyte Streptomyces sp. KLBMP 5084 isolated from halophyte Limonium sinense. Plant Soil 2017, 416, 117–132. [Google Scholar] [CrossRef]
- Qin, S.; Feng, W.W.; Zhang, Y.J.; Wang, T.T.; Xiong, Y.W.; Xing, K. Diversity of bacterial microbiota of coastal halophyte Limonium sinense and amelioration of salinity stress damage by symbiotic plant growth-promoting actinobacterium Glutamicibacter halophytocola KLBMP 5180. Appl. Environ. Microbiol. 2018, 84, e01533-18. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Miao, Q.; Feng, W.W.; Wang, Y.; Zhu, X.; Xing, K.; Jiang, J.H. Biodiversity and plant growth promoting traits of culturable endophytic actinobacteria associated with Jatropha curcas L. growing in Panxi dry-hot valley soil. Appl. Soil Ecol. 2015, 93, 47–55. [Google Scholar] [CrossRef]
- Viaene, T.; Langendries, S.; Beirinckx, S.; Maes, M.; Goormachtig, S. Streptomyces as a plant’s best friend? FEMS Microbiol. Ecol. 2016, 92, fiw119. [Google Scholar] [CrossRef]
- Olanrewaju, O.S.; Babalola, O.O. Streptomyces: Implications and interactions in plant growth promotion. Appl. Microbiol. Biotechnol. 2019, 103, 1179–1188. [Google Scholar] [CrossRef]
- D’Alessandro, M.; Erb, M.; Ton, J.; Brandenburg, A.; Karlen, D.; Zopfi, J.; Turlings, T.C.J. Volatiles produced by soil-borne endophytic bacteria increase plant pathogen resistance and affect tritrophic interactions. Plant Cell Environ. 2014, 37, 813–826. [Google Scholar] [CrossRef]
- Cordovez, V.; Carrion, V.J.; Etalo, D.W.; Mumm, R.; Zhu, H.; van Wezel, G.P.; Raaijmakers, J.M. Diversity and functions of volatile organic compounds produced by Streptomyces from a disease-suppressive soil. Front. Microbiol. 2015, 6, 1081. [Google Scholar] [CrossRef]
- Cho, G.; Kim, J.; Park, C.G.; Nislow, C.; Weller, D.M.; Kwak, Y.-S. Caryolan-1-ol, an antifungal volatile produced by Streptomyces spp., inhibits the endomembrane system of fungi. Open Biol. 2017, 7, 170075. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, T.; Liu, Y.; Li, X.; Zhang, C.; Feng, Z.; Peng, X.; Li, Z.; Qin, S.; Xing, K. Volatile organic compounds produced by Pseudomonas chlororaphis subsp. aureofaciens SPS-41 as biological fumigants to control Ceratocystis fimbriata in postharvest sweet potatoes. J. Agric. Food Chem. 2019, 67, 3702–3710. [Google Scholar] [CrossRef]
- Shirling, E.B.; Gottlieb, D. Methods for characterization of Streptomyces species. Int. J. Syst. Bacteriol. 1966, 16, 313–340. [Google Scholar] [CrossRef]
- Qin, S.; Zhang, Y.J.; Yuan, B.; Xu, P.Y.; Xing, K.; Wang, J.; Jiang, J.H. Isolation of ACC deaminase-producing habitat-adapted symbiotic bacteria associated with halophyte Limonium sinense (Girard) Kuntze and evaluating their plant growth-promoting activity under salt stress. Plant Soil 2014, 374, 753–766. [Google Scholar] [CrossRef]
- Felsenstein, J. Evolutionary trees from DNA sequences: A maximum likelihood approach. J. Mol. Evol. 1981, 17, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Fitch, W.M. Toward defining the course of evolution: Minimum change for a specific tree topology. Syst. Zool. 1971, 20, 406–416. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic tree. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar]
- Tamura, K.; Stecker, C.; Peterson, D.; Filipsk, A.; Kumar, S. MEGA 6: Molecular evolutionary genetics analysis version 6. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–789. [Google Scholar] [CrossRef]
- Beauchamp, C.O.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Kar, M.; Mishra, D. Catalase, peroxidase, and polyphenol oxidase activities during rice leaf senescence. Plant Physiol. 1976, 57, 315–319. [Google Scholar] [CrossRef]
- Singh, R.P.; Jha, P.N. The PGPR Stenotrophomonas maltophilia SBP-9 augments resistance against biotic and abiotic stress in wheat plants. Front. Microbiol. 2017, 8, 1945. [Google Scholar] [CrossRef]
- Dhindsa, R.S.; Plumb-Dhindsa, P.; Thorpe, T.A. Leaf senescence: Correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J. Exp. Bot. 1981, 32, 93–101. [Google Scholar] [CrossRef]
- Hansen, J.; Moller, I. Percolation of starch and soluble carbohydrates from plant tissue for quantitative determination with anthrone. Anal. Biochem. 1975, 68, 87–94. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Z.; Qiao, X.; Li, Z.; Li, F.; Chen, M.; Wang, Y.; Huang, Y.; Cui, H. Antifungal activity of volatile organic compounds from Streptomyces alboflavus TD-1. FEMS Microbiol. Lett. 2013, 341, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Ning, P.; Zheng, L.; Huang, J.; Li, G.; Hsiang, T. Fumigant activity of volatiles of Streptomyces globisporus JK-1 against Penicillium italicum on Citrus microcarpa. Postharvest Biol. Technol. 2010, 58, 157–165. [Google Scholar] [CrossRef]
- Wu, Y.; Yuan, J.; E, Y.; Raza, W.; Shen, Q.; Huang, Q. Effects of volatile organic compounds from Streptomyces albulus NJZJSA2 on growth of two fungal pathogens. J. Basic. Microbiol. 2015, 55, 1104–1117. [Google Scholar] [CrossRef]
- Waksman, S.A.; Curtis, R.E. The actinomyces of the soil. Soil Sci. 1916, 1, 99–134. [Google Scholar] [CrossRef]
- Waksman, S.A.; Woodruff, H.B. Streptothricin, a new selective bacteriostatic and bactericidal agent, particularly active against gram-negative bacteria. Proc. Soc. Exptl. Biol. Med. 1942, 49, 207–210. [Google Scholar] [CrossRef]
- Waksman, S.A.; Harris, D.; Lechevalier, M. Studies on Streptomyces lavendulae. J. Bacteriol. 1951, 62, 149–161. [Google Scholar] [CrossRef]
- Groupe, V.; Frankel, J.W.; Leche-Valier, M.P.; Waksman, S.A. Antiviral properties of ehrlichin, an antibiotic produced by Streptomyces lavendulae. J. Immunol. 1951, 67, 471–482. [Google Scholar]
- Akasaki, K.; Abe, H. Yazumycin, a new antibiotic produced by Streptomyces lavendulae. J. Antibiot. 1968, 21, 98–105. [Google Scholar] [CrossRef]
- Hashimoto, K.; Nihira, T.; Sakuda, S.; Yamada, Y. IM-2, a butyrolactone autoregulator, induces production of several nucleoside antibiotics in Streptomyces sp. FRI-5. J. Ferment. Bioeng. 1992, 73, 449–455. [Google Scholar] [CrossRef]
- Saravana Kumar, P.; Al-Dhabi, N.A.; Duraipandiyan, V.; Balachandran, C.; Praveen Kumar, P.; Ignacimuthu, S. In vitro antimicrobial, antioxidant and cytotoxic properties of Streptomyces lavendulae strain SCA5. BMC Microbiol. 2014, 14, 291. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Jamwal, V.; Singh, V.P.; Wazir, P.; Awasthi, P.; Singh, D.; Vishwakarma, R.A.; Gandhi, S.G.; Chaubey, A. Revelation and cloning of valinomycin synthetase genes in Streptomyces lavendulae ACR-DA1 and their expression analysis under different fermentation and elicitation conditions. J. Biotechnol. 2017, 253, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Camejo, D.; Guzmán-Cedeño, Á.; Moreno, A. Reactive oxygen species, essential molecules, during plant-pathogen interactions. Plant Physiol. Biochem. 2016, 103, 10–23. [Google Scholar] [CrossRef]
- Segal, L.M.; Wilson, R.A. Reactive oxygen species metabolism and plant-fungal interactions. Fungal Genet. Biol. 2018, 110, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bailly, C.; Benamar, A.; Corbineau, F.; Come, D. Changes in malondialdehyde content and in superoxide dismutase, catalase and glutathione reductase activities in sunflower seeds as related to deterioration during accelerated aging. Plant Physiol. 1996, 97, 104–110. [Google Scholar] [CrossRef]
- Yang, H.; Li, X.; Lu, G. Effect of carnauba wax-based coating containing glycerol monolaurate on decay and quality of sweet potato roots during storage. J. Food Prot. 2018, 81, 1643–1650. [Google Scholar] [CrossRef]
- Schöller, C.E.; Gürtler, H.; Pedersen, R.; Molin, S.; Wilkins, K. Volatile metabolites from actinomycetes. J. Agric. Food Chem. 2002, 50, 2615–2621. [Google Scholar] [CrossRef]
- Choudoir, M.; Rossabi, S.; Gebert, M.; Helmig, D.; Fierer, N. A phylogenetic and functional perspective on volatile organic compound production by Actinobacteria. MSystems 2019, 4, e00295-18. [Google Scholar] [CrossRef]
- Xing, M.; Zheng, L.; Deng, Y.; Xu, D.; Xi, P.; Li, M.; Kong, G.; Jiang, Z. Antifungal activity of natural volatile organic compounds against litchi downy blight pathogen Peronophythora litchii. Molecules 2018, 23, 358. [Google Scholar] [CrossRef]
- Hu, K.; Jin, G.J.; Mei, W.C.; Li, T.; Tao, Y.S. Increase of medium-chain fatty acid ethyl ester content in mixed H. uvarum/S. cerevisiae fermentation leads to wine fruity aroma enhancement. Food Chem. 2018, 239, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Plyuta, V.; Lipasova, V.; Popova, A.; Koksharova, O.; Kuznetsov, A.; Szegedi, E.; Chernin, L.; Khmel, I. Influence of volatile organic compounds emitted by Pseudomonas and Serratia strains on Agrobacterium tumefaciens biofilms. Apmis 2016, 124, 586–594. [Google Scholar] [CrossRef] [PubMed]


| 20 Days after Treatment | TR | TR+SPS-33 | TR+CF | TR+CF+SPS-33 |
|---|---|---|---|---|
| SOD activity (U/g FW) | 13.98 ± 3.04 b | 15.50 ± 1.79 b | 15.39 ± 1.85 b | 18.86 ± 0.86 a |
| POD activity (U/g FW) | 2.67 ± 0.67 b | 2.89 ± 0.38 b | 2.89 ± 0.38 b | 4.44 ± 0.38 a |
| CAT activity (U/g FW) | 9.82 ± 1.31 b | 8.82 ± 1.04 b | 11.43 ± 1.25 b | 17.51 ± 2.55 a |
| Content of MDA (μmol/L) | 107.90 ± 5.12 a | 77.26 ± 4.52 c | 95.84 ± 4.74 b | 77.43 ± 10.30 c |
| Total Soluble sugar (μg/g) | 7.78 ± 0.46 d | 9.89 ± 0.56 c | 11.09 ± 0.15 b | 13.16 ± 1.11 a |
| Volatiles Name a | Retention Time (min) | Area b (%) | CAS no. | Molecular Formula | Molecular Weight |
|---|---|---|---|---|---|
| Heptadecane | 25.3799 | 16.73 | 629-78-7 | C17H36 | 240.4677 |
| Tetradecane | 17.7886 | 10.84 | 629-59-4 | C14H30 | 198.3900 |
| 3-Methyl-1-butanol | 12.4138 | 9.40 | 123-51-3 | C5H12O | 88.1500 |
| Acetone | 2.50379 | 5.41 | 67-64-1 | C3H6O | 58.0800 |
| Pyridine | 11.4099 | 5.35 | 110-86-1 | C5H5N | 79.1000 |
| Pentadecane | 20.7159 | 2.31 | 629-62-9 | C15H32 | 212.4100 |
| Ethyl decanoate | 23.8919 | 2.27 | 110-38-3 | C12H24O2 | 200.3200 |
| 2-Methyl-1-butanol | 12.3605 | 1.88 | 137-32-6 | C5H12O | 88.1500 |
| Phenylethyl alcohol | 29.7686 | 1.87 | 60-12-8 | C8H10O | 122.1700 |
| Dodecane | 11.5077 | 1.64 | 112-40-3 | C12H26 | 170.3800 |
| 2-Butanone | 3.36553 | 1.63 | 78-93-3 | C4H7O | 72.1100 |
| Tridecane | 15.1679 | 1.57 | 629-50-5 | C13H28 | 184.4100 |
| Ethyl dodecanoate | 28.4849 | 1.48 | 106-33-2 | C14H28O2 | 228.3800 |
| Furan | 2.37497 | 1.37 | 110-00-9 | C4H4O | 68.0740 |
| Hexadecane | 23.0435 | 1.37 | 544-76-3 | C16H34 | 226.4400 |
| 2-Pentadecanone | 32.0784 | 1.35 | 2345-28-0 | C15H30O | 226.4000 |
| 2-Octanone | 14.426 | 0.99 | 111-13-7 | C8H16O | 128.2100 |
| Ethyl octanoate | 18.7303 | 0.94 | 106-32-1 | C10H20O2 | 172.2700 |
| 2-Nonanone | 17.4421 | 0.90 | 821-55-6 | C9H18O | 142.2400 |
| Naphthalene | 25.9841 | 0.90 | 624-92-0 | C10H8 | 128.1800 |
| Volatile Compounds | Inhibition Rate (%) of the VOCs at Different Volumes | |||
|---|---|---|---|---|
| 10 μL/plate | 30 μL/plate | 50 μL/plate | 70 μL/plate | |
| 2-Methyl-1-butanol | 38.18 ± 1.55 b | 100.00 ± 0.00 a | 100.00 ± 0.00 a | 100.00 ± 0.00 a |
| 3-Methyl-1-butanol | 33.63 ± 3.38 b | 100.00 ± 0.00 a | 100.00 ± 0.00 a | 100.00 ± 0.00 a |
| Phenylethyl alcohol | 25.59 ± 0.52 c | 44.05 ± 1.11 b | 88.38 ± 1.06 a | 88.72 ± 0.92 a |
| Pyridine | 14.70 ± 1.50 d | 41.75 ± 0.47 c | 86.36 ± 0.29 b | 100.00 ± 0.00 a |
| Ethyl octanoate | 6.06 ± 0.76 c | 10.44 ± 0.38 c | 23.35 ± 0.47 b | 46.41 ± 1.72 a |
| Ethyl decanoate | 4.32 ± 1.61 c | 13.97 ± 0.52 b | 20.87 ± 0.77 a | 24.19 ± 0.61 a |
| 2-Octanone | 1.57 ± 0.08 d | 8.02 ± 0.46 c | 18.74 ± 0.26 b | 23.29 ± 0.70 a |
| 2-Nonanone | 7.74 ± 0.67 c | 16.22 ± 0.21 b | 21.71 ± 3.55 b | 43.77 ± 0.71 a |
| Acetone | 10.66 ± 0.75 c | 15.21 ± 0.82 c | 23.51 ± 0.60 b | 28.68 ± 0.65 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Li, B.; Cai, S.; Zhang, Y.; Xu, M.; Zhang, C.; Yuan, B.; Xing, K.; Qin, S. Identification of Rhizospheric Actinomycete Streptomyces lavendulae SPS-33 and the Inhibitory Effect of its Volatile Organic Compounds against Ceratocystis fimbriata in Postharvest Sweet Potato (Ipomoea batatas (L.) Lam.). Microorganisms 2020, 8, 319. https://doi.org/10.3390/microorganisms8030319
Li X, Li B, Cai S, Zhang Y, Xu M, Zhang C, Yuan B, Xing K, Qin S. Identification of Rhizospheric Actinomycete Streptomyces lavendulae SPS-33 and the Inhibitory Effect of its Volatile Organic Compounds against Ceratocystis fimbriata in Postharvest Sweet Potato (Ipomoea batatas (L.) Lam.). Microorganisms. 2020; 8(3):319. https://doi.org/10.3390/microorganisms8030319
Chicago/Turabian StyleLi, Xuewei, Beibei Li, Shurui Cai, Yu Zhang, Mingjie Xu, Chunmei Zhang, Bo Yuan, Ke Xing, and Sheng Qin. 2020. "Identification of Rhizospheric Actinomycete Streptomyces lavendulae SPS-33 and the Inhibitory Effect of its Volatile Organic Compounds against Ceratocystis fimbriata in Postharvest Sweet Potato (Ipomoea batatas (L.) Lam.)" Microorganisms 8, no. 3: 319. https://doi.org/10.3390/microorganisms8030319
APA StyleLi, X., Li, B., Cai, S., Zhang, Y., Xu, M., Zhang, C., Yuan, B., Xing, K., & Qin, S. (2020). Identification of Rhizospheric Actinomycete Streptomyces lavendulae SPS-33 and the Inhibitory Effect of its Volatile Organic Compounds against Ceratocystis fimbriata in Postharvest Sweet Potato (Ipomoea batatas (L.) Lam.). Microorganisms, 8(3), 319. https://doi.org/10.3390/microorganisms8030319





