High Throughput Sediment DNA Sequencing Reveals Azo Dye Degrading Bacteria Inhabit Nearshore Sediments
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Chemical Analysis
2.2. DNA Extraction and High-Throughput Sequencing
2.3. PICRUSt Approach
2.4. Data Analysis
2.5. Data Availability
3. Results and Discussion
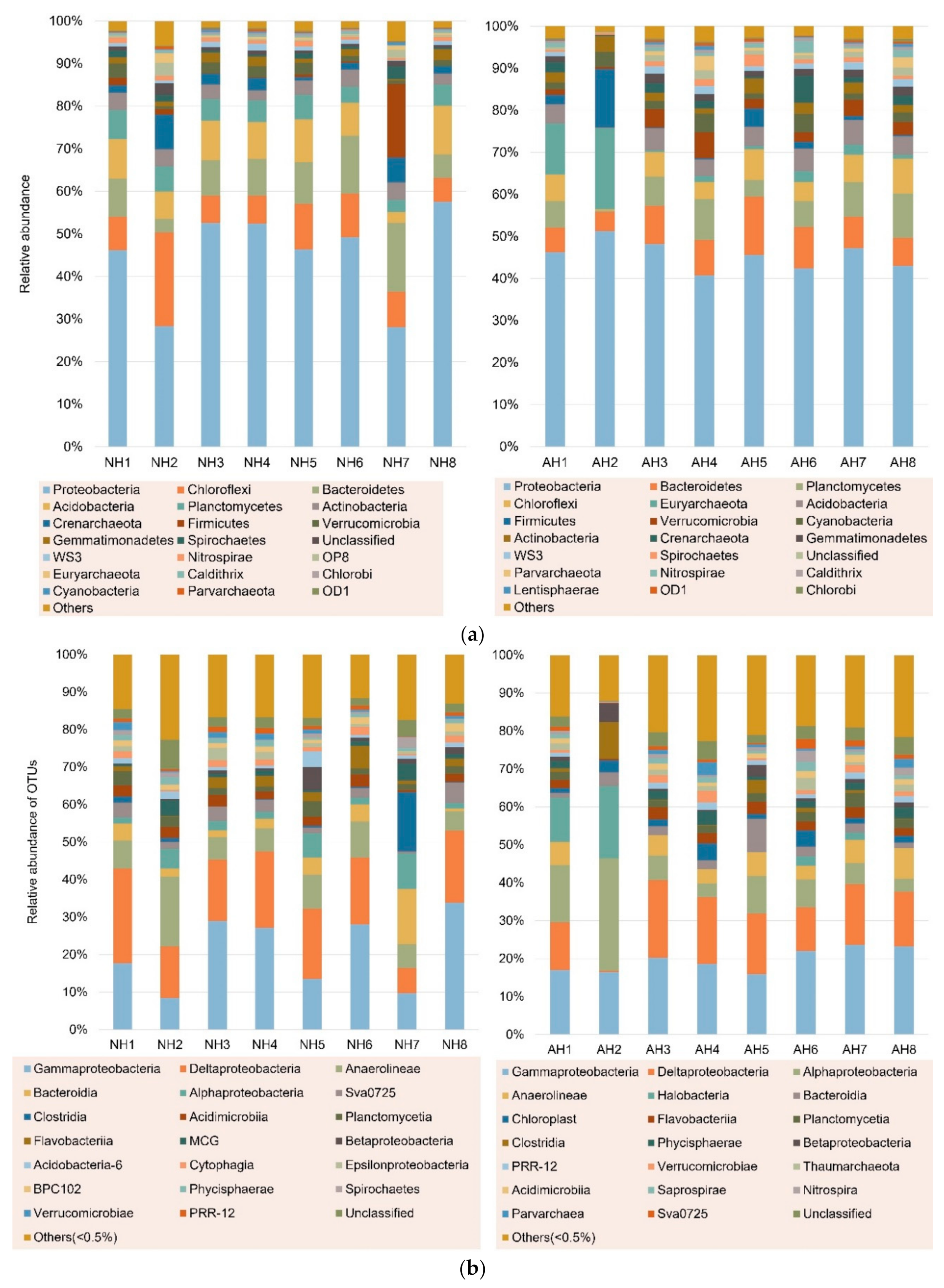
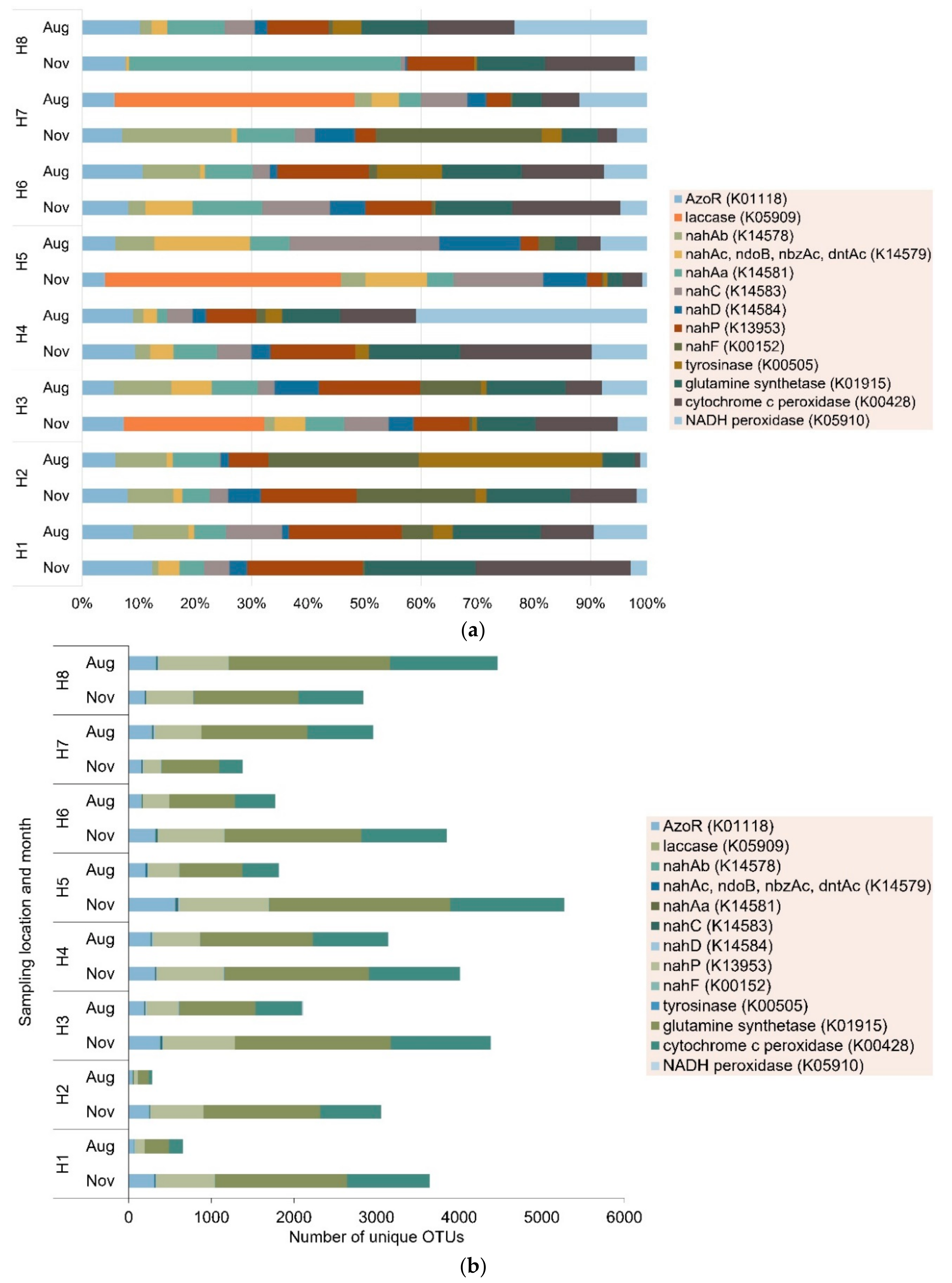
3.1. Spatial Distribution of Azo Dye-Related Degradation Genes
3.2. Seasonal Variations in Azo Dye-Related Degradation Genes
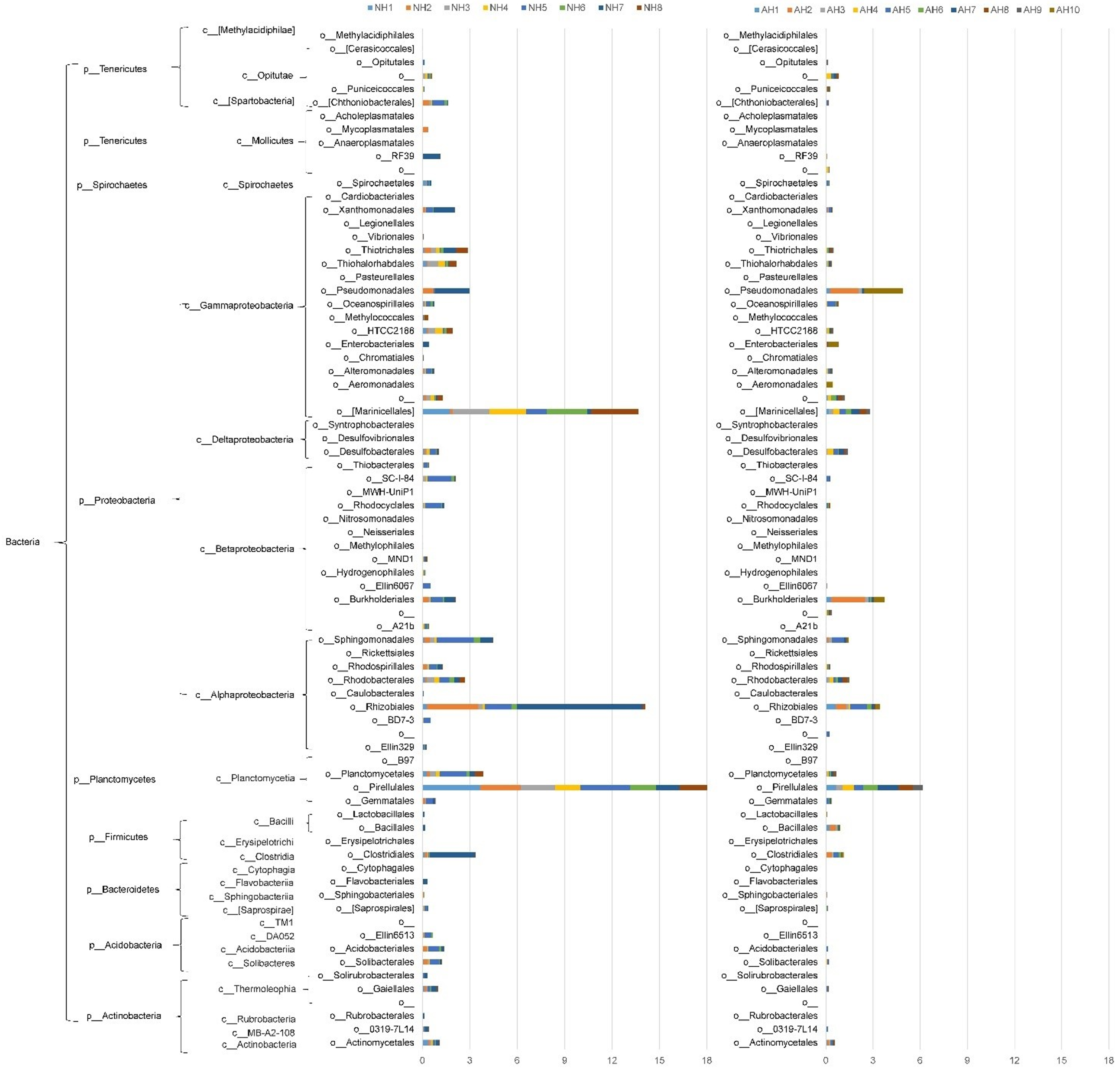
3.3. Distribution of Azo Reductase Genes in Bacteria
3.4. Effect of Local Environmental Factors on Distribution of Azo Dye-Related Genes
3.5. Environmental Implications
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sathishkumar, K.; AlSalhi, M.S.; Sanganyado, E.; Devanesan, S.; Arulprakash, A.; Rajasekar, A. Sequential electrochemical oxidation and bio-treatment of the azo dye congo red and textile effluent. J. Photochem. Photobiol. B Biol. 2019, 200, 111655. [Google Scholar] [CrossRef] [PubMed]
- Shanker, U.; Rani, M.; Jassal, V. Degradation of hazardous organic dyes in water by nanomaterials. Environ. Chem. Lett. 2017, 15, 623–642. [Google Scholar] [CrossRef]
- Vacchi, F.I.; Vendemiatti, J.A.S.; da Silva, B.F.; Zanoni, M.V.B.; Umbuzeiro, G.A. Quantifying the contribution of dyes to the mutagenicity of waters under the influence of textile activities. Sci. Total Environ. 2017, 601–602, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.-Y.; Liu, J.; Wang, Y.-X.; He, C.-S.; Zhang, L.-S.; Mu, Y.; Li, W.-H. Bioelectrochemical decolorization of a reactive diazo dye: Kinetics, optimization with a response surface methodology, and proposed degradation pathway. Bioelectrochemistry 2019, 128, 9–16. [Google Scholar] [CrossRef]
- Akansha, K.; Chakraborty, D.; Sachan, S.G. Decolorization and degradation of methyl orange by Bacillus stratosphericus SCA1007. Biocatal. Agric. Biotechnol. 2019, 18, 101044. [Google Scholar] [CrossRef]
- Cao, J.; Sanganyado, E.; Liu, W.; Zhang, W.; Liu, Y. Decolorization and detoxification of Direct Blue 2B by indigenous bacterial consortium. J. Environ. Manag. 2019, 242, 229–237. [Google Scholar] [CrossRef]
- Mathieu-Denoncourt, J.; Martyniuk, C.J.; De Solla, S.R.; Balakrishnan, V.K.; Langlois, V.S. Sediment contaminated with the azo dye disperse yellow 7 alters cellular stress- and androgen-related transcription in Silurana tropicalis larvae. Environ. Sci. Technol. 2014, 48, 2952–2961. [Google Scholar] [CrossRef]
- Parrott, J.L.; Bartlett, A.J.; Balakrishnan, V.K. Chronic toxicity of azo and anthracenedione dyes to embryo-larval fathead minnow. Environ. Pollut. 2016, 210, 40–47. [Google Scholar] [CrossRef]
- Fernandes, F.H.; Botasso-Nasciutti, M.O.; Svio, A.L.V.; Souza, L.C.M.; Fernandes-Cal, J.R.; Cardoso, F.F.; Fontes, M.R.M.; Albuquerque, A.F.; Munari, C.C.; Kummrow, F.; et al. In Vivo genotoxicity of a commercial C.I. Disperse Red 1 dye. Environ. Mol. Mutagen. 2018, 59, 822–828. [Google Scholar] [CrossRef]
- Bilal, M.; Rasheed, T.; Nabeel, F.; Iqbal, H.M.N.; Zhao, Y. Hazardous contaminants in the environment and their laccase-assisted degradation—A review. J. Environ. Manag. 2019, 234, 253–264. [Google Scholar] [CrossRef]
- Rawat, D.; Sharma, R.S.S.; Karmakar, S.; Arora, L.S.S.; Mishra, V. Ecotoxic potential of a presumably non-toxic azo dye. Ecotoxicol. Environ. Saf. 2018, 148, 528–537. [Google Scholar] [CrossRef] [PubMed]
- Chikere, C.B.; Fenibo, E.O. Distribution of PAH-ring hydroxylating dioxygenase genes in bacteria isolated from two illegal oil refining sites in the Niger Delta, Nigeria. Sci. Afr. 2018, 1, e00003. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, J.; Liao, J.; Xie, S.; Huang, Y. Distribution of naphthalene dioxygenase genes in crude oil-contaminated soils. Microb. Ecol. 2014, 68, 785–793. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Hu, H.; Shi, X.; Zhang, L.; He, J. Effects of different agricultural wastes on the dissipation of PAHs and the PAH-degrading genes in a PAH-contaminated soil. Chemosphere 2017, 172, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Winderl, C.; Schaefer, S.; Lueders, T. Detection of anaerobic toluene and hydrocarbon degraders in contaminated aquifers using benzylsuccinate synthase (bssA) genes as a functional marker. Environ. Microbiol. 2007, 9, 1035–1046. [Google Scholar] [CrossRef]
- Zhou, H.W.; Guo, C.L.; Wong, Y.S.; Tam, N.F.Y. Genetic diversity of dioxygenase genes in polycyclic aromatic hydrocarbon-degrading bacteria isolated from mangrove sediments. FEMS Microbiol. Lett. 2006, 262, 148–157. [Google Scholar] [CrossRef]
- Zhang, S.; Hu, Z.; Wang, H. Metagenomic analysis exhibited the co-metabolism of polycyclic aromatic hydrocarbons by bacterial community from estuarine sediment. Environ. Int. 2019, 129, 308–319. [Google Scholar] [CrossRef]
- Lee, H.; Lee, D.W.; Kwon, S.L.; Heo, Y.M.; Jang, S.; Kwon, B.-O.; Khim, J.S.; Kim, G.-H.; Kim, J.-J. Importance of functional diversity in assessing the recovery of the microbial community after the Hebei Spirit Oil Spill in Korea. Environ. Int. 2019, 128, 89–94. [Google Scholar] [CrossRef]
- Panigrahi, S.; Velraj, P.; Subba Rao, T. Functional microbial diversity in contaminated environment and application in bioremediation. In Microbial Diversity in the Genomic Era; Elsevier: Amsterdam, The Netherlands, 2019; pp. 359–385. ISBN 9780128148495. [Google Scholar]
- Fang, H.; Zhang, H.; Han, L.; Mei, J.; Ge, Q.; Long, Z.; Yu, Y. Exploring bacterial communities and biodegradation genes in activated sludge from pesticide wastewater treatment plants via metagenomic analysis. Environ. Pollut. 2018, 243, 1206–1216. [Google Scholar] [CrossRef]
- Wolińska, A.; Gałązka, A.; Kuźniar, A.; Goraj, W.; Jastrzębska, N.; Grządziel, J.; Stępniewska, Z. Catabolic fingerprinting and diversity of bacteria in mollic gleysol contaminated with petroleum substances. Appl. Sci. 2018, 8, 1970. [Google Scholar] [CrossRef]
- Tusher, T.R.; Shimizu, T.; Inoue, C.; Chien, M.F. Enrichment and analysis of stable 1,4-dioxane- degrading microbial consortia consisting of novel dioxane-degraders. Microorganisms 2020, 8, 50. [Google Scholar] [CrossRef] [PubMed]
- Farber, R.; Rosenberg, A.; Rozenfeld, S.; Benet, G.; Cahan, R. Bioremediation of artificial diesel-contaminated soil using bacterial consortium immobilized to plasma-pretreated wood waste. Microorganisms 2019, 7, 497. [Google Scholar] [CrossRef] [PubMed]
- Calcagno Galvão, T.; Mohn, W.W.; de Lorenzo, V. Exploring the microbial biodegradation and biotransformation gene pool. Trends Biotechnol. 2005, 23, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Bouhajja, E.; Agathos, S.N.; George, I.F. Metagenomics: Probing pollutant fate in natural and engineered ecosystems. Biotechnol. Adv. 2016, 34, 1413–1426. [Google Scholar] [CrossRef] [PubMed]
- Ufarté, L.; Laville, É.; Duquesne, S.; Potocki-Veronese, G. Metagenomics for the discovery of pollutant degrading enzymes. Biotechnol. Adv. 2015, 33, 1845–1854. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, M.; Sanganyado, E.; Li, P.; Liu, W. Distribution of microbial communities in metal-contaminated nearshore sediment from Eastern Guangdong, China. Environ. Pollut. 2019, 250, 482–492. [Google Scholar] [CrossRef]
- Shi, J.; Li, X.; He, T.; Wang, J.; Wang, Z.; Li, P.; Lai, Y.; Sanganyado, E.; Liu, W. Integrated assessment of heavy metal pollution using transplanted mussels in eastern Guangdong, China. Environ. Pollut. 2018, 243, 601–609. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S.; Sato, Y.; Kawashima, M.; Furumichi, M.; Tanabe, M. Data, information, knowledge and principle: Back to metabolism in KEGG. Nucleic Acids Res. 2014, 42, 199–205. [Google Scholar] [CrossRef]
- Langille, M.G.I.; Zaneveld, J.; Caporaso, J.G.; McDonald, D.; Knights, D.; Reyes, J.A.; Clemente, J.C.; Burkepile, D.E.; Vega Thurber, R.L.; Knight, R.; et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotechnol. 2013, 31, 814–821. [Google Scholar] [CrossRef]
- Zhu, D.; Tanabe, S.-H.; Yang, C.; Zhang, W.; Sun, J. Bacterial community composition of South China Sea sediments through pyrosequencing-based analysis of 16S rRNA genes. PLoS ONE 2013, 8, e78501. [Google Scholar]
- Feng, B.W.; Li, X.R.; Wang, J.H.; Hu, Z.Y.; Meng, H.; Xiang, L.Y.; Quan, Z.X. Bacterial diversity of water and sediment in the Changjiang estuary and coastal area of the East China Sea. FEMS Microbiol. Ecol. 2009, 70, 236–248. [Google Scholar] [CrossRef] [PubMed]
- Quero, G.M.; Cassin, D.; Botter, M.; Perini, L.; Luna, G.M. Patterns of benthic bacterial diversity in coastal areas contaminated by heavy metals, polycyclic aromatic hydrocarbons (PAHs) and polychlorinated biphenyls (PCBs). Front. Microbiol. 2015, 6, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Sekiguchi, Y.; Imachi, H.; Kamagata, Y.; Ohashi, A.; Harada, H. Diversity, localization, and physiological properties of filamentous microbes belonging to Chloroflexi Subphylum I in mesophilic and thermophilic methanogenic sludge granules. Appl. Environ. Microbiol. 2005, 71, 7493–7503. [Google Scholar] [CrossRef] [PubMed]
- Edlund, A.; Hrdeman, F.; Jansson, J.K.; Sjling, S. Active bacterial community structure along vertical redox gradients in Baltic Sea sediment. Environ. Microbiol. 2008, 10, 2051–2063. [Google Scholar] [CrossRef] [PubMed]
- Sinkko, H.; Lukkari, K.; Sihvonen, L.M.; Sivonen, K.; Leivuori, M.; Rantanen, M.; Paulin, L.; Lyra, C. Bacteria contribute to sediment nutrient release and reflect progressed eutrophication-driven hypoxia in an organic-rich continental sea. PLoS ONE 2013, 8, e67061. [Google Scholar] [CrossRef]
- Acosta-González, A.; Rosselló-Móra, R.; Marqués, S. Characterization of the anaerobic microbial community in oil-polluted subtidal sediments: Aromatic biodegradation potential after the Prestige Oil Spill. Environ. Microbiol. 2013, 15, 77–92. [Google Scholar] [CrossRef]
- Xie, X.; Liu, N.; Yang, B.; Yu, C.; Zhang, Q.; Zheng, X.; Xu, L.; Li, R.; Liu, J. Comparison of microbial community in hydrolysis acidification reactor depending on different structure dyes by Illumina MiSeq sequencing. Int. Biodeterior. Biodegrad. 2016, 111, 14–21. [Google Scholar] [CrossRef]
- Cui, D.; Zhang, H.; He, R.; Zhao, M. The comparative study on the rapid decolorization of azo, anthraquinone and triphenylmethane dyes by anaerobic sludge. Int. J. Environ. Res. Public Health 2016, 13, 1053. [Google Scholar] [CrossRef]
- Forss, J.; Pinhassi, J.; Lindh, M.; Welander, U. Microbial diversity in a continuous system based on rice husks for biodegradation of the azo dyes Reactive Red 2 and Reactive Black 5. Bioresour. Technol. 2013, 130, 681–688. [Google Scholar] [CrossRef]
- Wolińska, A.; Kuźniar, A.; Zielenkiewicz, U.; Izak, D.; Szafranek-Nakonieczna, A.; Banach, A.; Błaszczyk, M. Bacteroidetes as a sensitive biological indicator of agricultural soil usage revealed by a culture-independent approach. Appl. Soil Ecol. 2017, 119, 128–137. [Google Scholar] [CrossRef]
- Vilchez-Vargas, R.; Junca, H.; Pieper, D.H. Metabolic networks, microbial ecology and ‘omics’ technologies: Towards understanding in situ biodegradation processes. Environ. Microbiol. 2010, 12, 3089–3104. [Google Scholar] [CrossRef] [PubMed]
- Yuan, K.; Chen, B.; Qing, Q.; Zou, S.; Wang, X.; Luan, T. Polycyclic aromatic hydrocarbons (PAHs) enrich their degrading genera and genes in human-impacted aquatic environments. Environ. Pollut. 2017, 230, 936–944. [Google Scholar] [CrossRef] [PubMed]
- de Menezes, A.; Clipson, N.; Doyle, E. Comparative metatranscriptomics reveals widespread community responses during phenanthrene degradation in soil. Environ. Microbiol. 2012, 14, 2577–2588. [Google Scholar] [CrossRef] [PubMed]
- Urata, M.; Uchida, E.; Nojiri, H.; Omori, T.; Obo, R.; Miyaura, N.; Ouchiyama, N. Genes involved in aniline degradation by Delftia acidovorans strain 7N and its distribution in the natural environment. Biosci. Biotechnol. Biochem. 2004, 68, 2457–2465. [Google Scholar] [CrossRef]
- Liu, J.; Yang, H.; Zhao, M.; Zhang, X.H. Spatial distribution patterns of benthic microbial communities along the Pearl Estuary, China. Syst. Appl. Microbiol. 2014, 37, 578–589. [Google Scholar] [CrossRef]
- Bunse, C.; Pinhassi, J. Marine bacterioplankton seasonal succession dynamics. Trends Microbiol. 2017, 25, 494–505. [Google Scholar] [CrossRef]
- Achtman, M.; Wagner, M. Microbial diversity and the genetic nature of microbial species. Nat. Rev. Microbiol. 2008, 6, 431–440. [Google Scholar] [CrossRef]
- Martiny, J.B.H.; Eisen, J.A.; Penn, K.; Allison, S.D.; Horner-Devine, M.C. Drivers of bacterial β-diversity depend on spatial scale. Proc. Natl. Acad. Sci. USA 2011, 108, 7850–7854. [Google Scholar] [CrossRef]
- Luo, L.; Wu, R.N.; Meng, H.; Li, X.Y.; Gu, J.D. Seasonal and spatial variations in diversity and abundance of bacterial laccase-like genes in sediments of a subtropical mangrove ecosystem. Int. Biodeterior. Biodegrad. 2016, 114, 260–267. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, T.; Shen, T.; Gao, G. Seasonal and spatial variation in the sediment bacterial community and diversity of Lake Bosten, China. J. Basic Microbiol. 2019, 59, 224–233. [Google Scholar] [CrossRef]
- Marzinelli, E.M.; Qiu, Z.; Dafforn, K.A.; Johnston, E.L.; Steinberg, P.D.; Mayer-Pinto, M. Coastal urbanisation affects microbial communities on a dominant marine holobiont. NPJ Biofilms Microbiomes 2018, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Heery, E.C.; Dafforn, K.A.; Smith, J.A.; Ushiama, S.; Mayer-Pinto, M. Not all artificial structures are created equal: Pilings linked to greater ecological and environmental change in sediment communities than seawalls. Mar. Environ. Res. 2018, 142, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Hiraoka, S.; Miyahara, M.; Fujii, K.; Machiyama, A.; Iwasaki, W. Seasonal analysis of microbial communities in precipitation in the greater Tokyo Area, Japan. Front. Microbiol. 2017, 8, 1506. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, J.A.; Steele, J.A.; Caporaso, J.G.; Steinbrück, L.; Reeder, J.; Temperton, B.; Huse, S.; McHardy, A.C.; Knight, R.; Joint, I.; et al. Defining seasonal marine microbial community dynamics. ISME J. 2012, 6, 298–308. [Google Scholar] [CrossRef]
- Sheeba, V.A.; Abdulaziz, A.; Gireeshkumar, T.R.; Ram, A.; Rakesh, P.S.; Jasmin, C.; Parameswaran, P.S. Role of heavy metals in structuring the microbial community associated with particulate matter in a tropical estuary. Environ. Pollut. 2017, 231, 589–600. [Google Scholar] [CrossRef]
- Sanganyado, E.; Gwenzi, W. Antibiotic resistance in drinking water systems: Occurrence, removal, and human health risks. Sci. Total Environ. 2019, 669, 785–797. [Google Scholar] [CrossRef]
- Liang, Y.; Van Nostrand, J.D.; Deng, Y.; He, Z.; Wu, L.; Zhang, X.; Li, G.; Zhou, J. Functional gene diversity of soil microbial communities from five oil-contaminated fields in China. ISME J. 2011, 5, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, S.; Shah, M.H.; Shaheen, N.; Khalique, A.; Jaffar, M. Multivariate analysis of trace metals in textile effluents in relation to soil and groundwater. J. Hazard. Mater. 2006, 137, 31–37. [Google Scholar] [CrossRef]
- Liang, C.; Huang, Y.; Wang, H. pahE, a functional marker gene for polycyclic aromatic hydrocarbon-degrading bacteria. Appl. Environ. Microbiol. 2019, 85, 1–17. [Google Scholar] [CrossRef]
- Ferrero, M.; Llobet-Brossa, E.; Lalucat, J.; García-Valdés, E.; Rosselló-Mora, R.; Bosch, R. Coexistence of two distinct copies of naphthalene degradation genes in Pseudomonas strains isolated from the western Mediterranean region. Appl. Environ. Microbiol. 2002, 68, 957–962. [Google Scholar] [CrossRef]
- Misal, S.A.; Gawai, K.R. Azoreductase: A key player of xenobiotic metabolism. Bioresour. Bioprocess. 2018, 5, 17. [Google Scholar] [CrossRef]
- Kohl, K.D. An introductory “how-to” guide for incorporating microbiome research into integrative and comparative biology. Integr. Comp. Biol. 2017, 57, 674–681. [Google Scholar] [CrossRef] [PubMed]
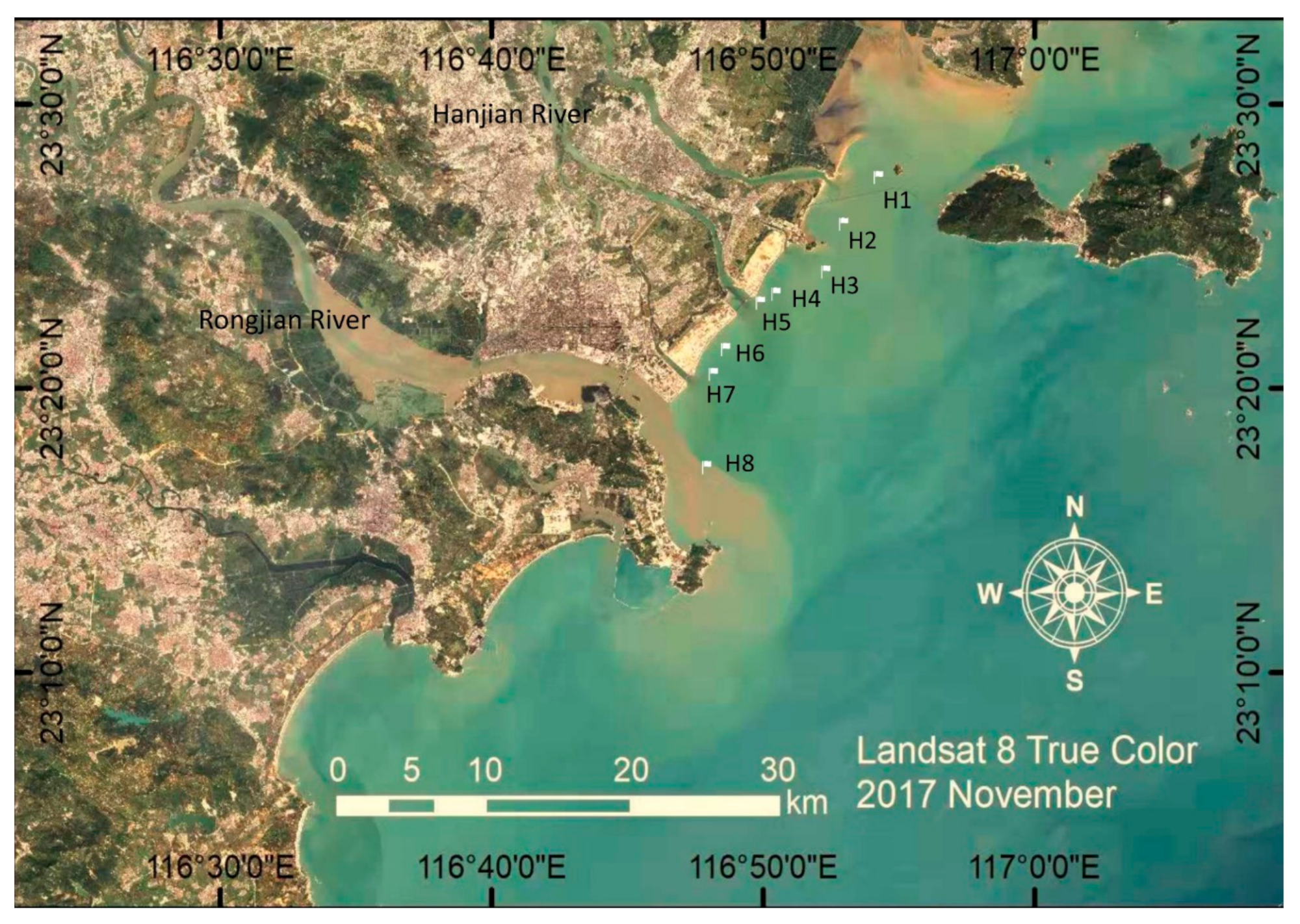
| Sites | Salinity (‰) | TOC (g/kg) | Inorganic Nutrients (mg/L) | Metal Concentration in Sediment (mg/kg) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NO3− | NO2− | NH4+ | PO43 | SO32 | Cu | Zn | Cr | Pb | As | Hg | |||
| H1 | 30.3 | 4.2 | 0.096 | 0.001 | 0.063 | 0.027 | 0.695 | 13.8 | 59.6 | 16.7 | 29.6 | 5.8 | 0.045 |
| H2 | 20.4 | 6.5 | 0.335 | 0.009 | 0.085 | 0.028 | 1.382 | 20.4 | 76.1 | 24.3 | 53.0 | 8.3 | 0.090 |
| H3 | 27.7 | 5.8 | 0.042 | 0.001 | 0.037 | 0.029 | 0.132 | 21.9 | 87.6 | 29.7 | 38.1 | 8.2 | 0.075 |
| H4 | 30.4 | 9.0 | 0.122 | 0.004 | 0.08 | 0.018 | 0.538 | 35.0 | 109.0 | 36.8 | 53.7 | 10.1 | 0.080 |
| H5 | 22.9 | 7.5 | 0.296 | 0.006 | 0.086 | 0.039 | 1.007 | 75.8 | 155.0 | 24.3 | 95.7 | 11.5 | 0.116 |
| H6 | 20.5 | 2.0 | 0.421 | 0.009 | 0.104 | 0.043 | 1.866 | 10.3 | 51.2 | 16.7 | 24.0 | 5.9 | 0.046 |
| H7 | 27.8 | 9.0 | 0.072 | 0.007 | 0.061 | 0.028 | 0.616 | 43.0 | 123.0 | 39.1 | 57.9 | 10.9 | 0.084 |
| H8 | 29.6 | 9.3 | 0.316 | 0.042 | 0.142 | 0.057 | 0.866 | 37.6 | 135.0 | 52.5 | 43.1 | 9.0 | 0.097 |
| AzoR | Laccase | nahAb | nahAc | nahAa | nahC | nahD | nahP | nahF | Tyrosinase | Glutamine | Cytochrome | NADH | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TOC | −0.482 * | 0.036 | −0.448 * | −0.292 | −0.225 | 0.011 | −0.359 | −0.379 | −0.240 | −0.292 | −0.543 * | 0.031 | −0.062 |
| Cu | −0.286 | 0.112 | −0.303 | 0.014 | −0.072 | 0.501 * | −0.094 | −0.444 * | −0.291 | −0.325 | −0.504 * | 0.164 | 0.105 |
| Zn | −0.375 | −0.030 | −0.390 | −0.117 | −0.093 | 0.266 | −0.209 | −0.436 * | −0.333 | −0.329 | −0.524 * | 0.203 | 0.049 |
| Cr | −0.468 * | −0.231 | −0.437 * | −0.376 | −0.142 | −0.263 | −0.394 | −0.393 | −0.249 | −0.287 | −0.478 * | 0.170 | −0.073 |
| Pb | −0.262 | 0.143 | −0.222 | 0.024 | −0.080 | 0.516 * | −0.078 | −0.334 | −0.092 | −0.198 | −0.405 | −0.072 | −0.004 |
| As | −0.379 | 0.118 | −0.287 | −0.119 | −0.130 | 0.190 | −0.190 | −0.448 * | −0.208 | −0.242 | −0.549 ** | −0.065 | −0.133 |
| Hg | −0.480 * | 0.047 | −0.308 | −0.258 | −0.113 | 0.033 | −0.287 | −0.529 * | −0.059 | −0.299 | −0.650 ** | −0.238 | −0.391 |
| Salinity | 0.199 | −0.553 ** | 0.103 | 0.118 | 0.023 | −0.278 | 0.153 | 0.134 | −0.348 | 0.125 | 0.191 | 0.418 | 0.259 |
| AzoR | Laccase | nahAb | nahAc | nahAa | nahC | nahD | nahP | nahF | Tyrosinase | Glutamine | Cytochrome | NADH | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AzoR | 1 | ||||||||||||
| laccase | 0.016 | 1 | |||||||||||
| nahAb | 0.941 ** | −0.047 | 1 | ||||||||||
| nahAc | 0.899 ** | 0.137 | 0.835 ** | 1 | |||||||||
| nahAa | 0.854 ** | −0.026 | 0.892 ** | 0.810 ** | 1 | ||||||||
| nahC | 0.313 | 0.380 | 0.229 | 0.590 ** | 0.243 | 1 | |||||||
| nahD | 0.942 ** | 0.044 | 0.912 ** | 0.982 ** | 0.859 ** | 0.468 * | 1 | ||||||
| nahP | 0.335 | −0.197 | 0.358 | 0.159 | 0.251 | −0.164 | 0.212 | 1 | |||||
| nahF | 0.177 | −0.189 | 0.331 | −0.100 | 0.206 | −0.145 | −0.004 | 0.403 | 1 | ||||
| tyrosinase | 0.899 ** | −0.128 | 0.820 ** | 0.622 ** | 0.783 ** | −0.020 | 0.683 ** | 0.486 * | 0.463 * | 1 | |||
| glutamine | 0.589 ** | −0.212 | 0.616 ** | 0.362 | 0.446 * | −0.023 | 0.440 * | 0.898 ** | 0.521 * | 0.615 ** | 1 | ||
| cytochrome | −0.072 | 0.152 | −0.318 | 0.072 | −0.287 | 0.220 | −0.018 | −0.536 * | −0.768 ** | −0.422 | −0.456 * | 1 | |
| NADH | 0.016 | 0.057 | −0.144 | −0.069 | −0.223 | 0.103 | −0.085 | −0.027 | −0.172 | −0.192 | 0.105 | 0.432 | 1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhuang, M.; Sanganyado, E.; Xu, L.; Zhu, J.; Li, P.; Liu, W. High Throughput Sediment DNA Sequencing Reveals Azo Dye Degrading Bacteria Inhabit Nearshore Sediments. Microorganisms 2020, 8, 233. https://doi.org/10.3390/microorganisms8020233
Zhuang M, Sanganyado E, Xu L, Zhu J, Li P, Liu W. High Throughput Sediment DNA Sequencing Reveals Azo Dye Degrading Bacteria Inhabit Nearshore Sediments. Microorganisms. 2020; 8(2):233. https://doi.org/10.3390/microorganisms8020233
Chicago/Turabian StyleZhuang, Mei, Edmond Sanganyado, Liang Xu, Jianming Zhu, Ping Li, and Wenhua Liu. 2020. "High Throughput Sediment DNA Sequencing Reveals Azo Dye Degrading Bacteria Inhabit Nearshore Sediments" Microorganisms 8, no. 2: 233. https://doi.org/10.3390/microorganisms8020233
APA StyleZhuang, M., Sanganyado, E., Xu, L., Zhu, J., Li, P., & Liu, W. (2020). High Throughput Sediment DNA Sequencing Reveals Azo Dye Degrading Bacteria Inhabit Nearshore Sediments. Microorganisms, 8(2), 233. https://doi.org/10.3390/microorganisms8020233







