Proteolytic Activity of Bacillus subtilis upon κ-Casein Undermines Its “Caries-Safe” Effect
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Growth Media
2.2. Lactose Preparation
2.3. Casein Preparation
2.4. Mono- and Dual-Species Biofilm
2.5. Characterization of Biofilm Structure by Confocal Laser Scanning Microscopy (CLSM)
2.6. Quantification of Biofilm Biomass using DNA Quantification
2.7. Biofilm Visualization by Scanning Electron Microscopy (SEM)
2.8. Proteolytic Activity Assay
2.9. Casein Breakdown in Solution by B. subtilis and S. mutans Lysate
2.10. Casein and κ-Casein Zymography
2.11. Statistical Analysis
3. Results
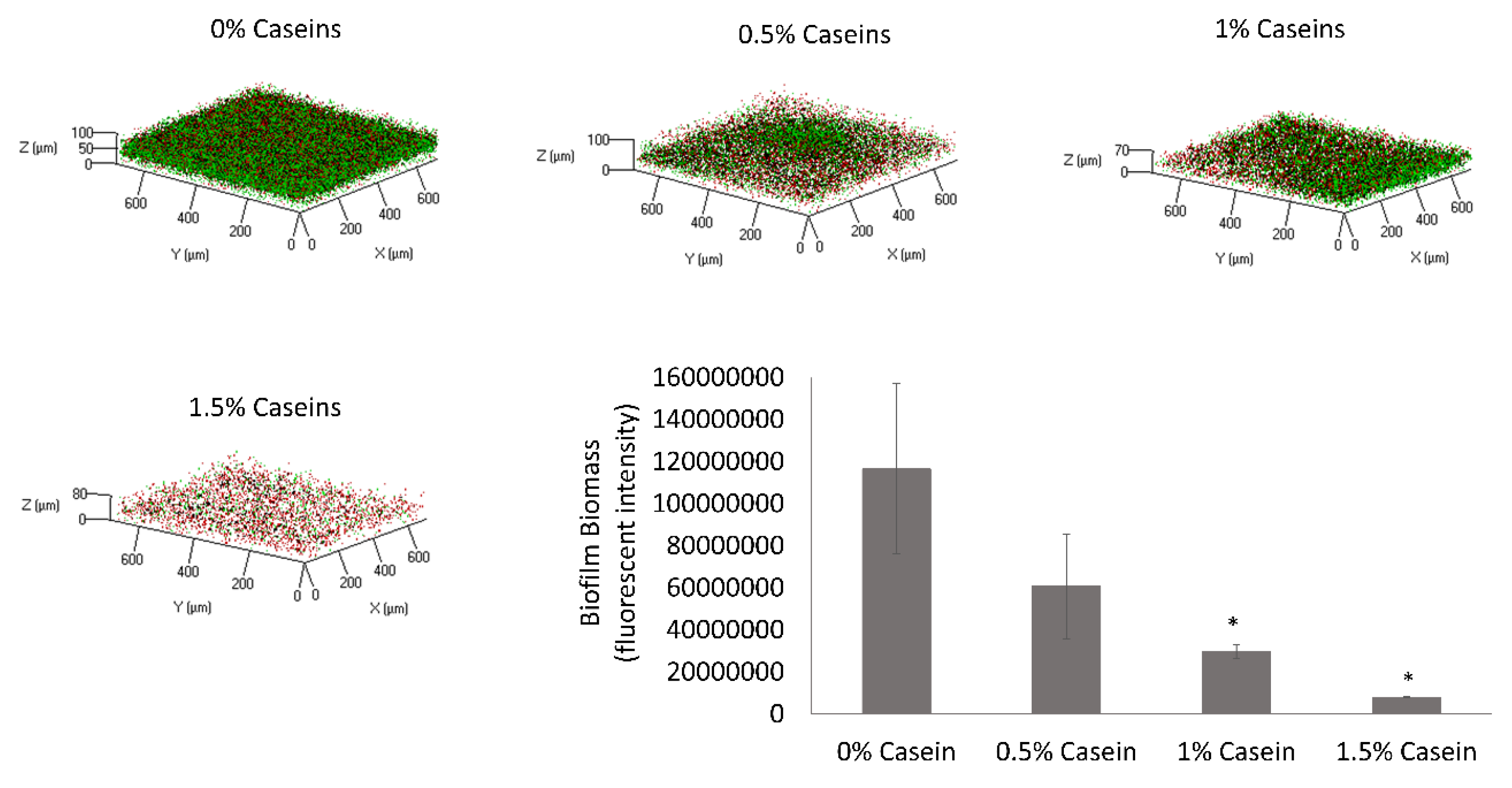
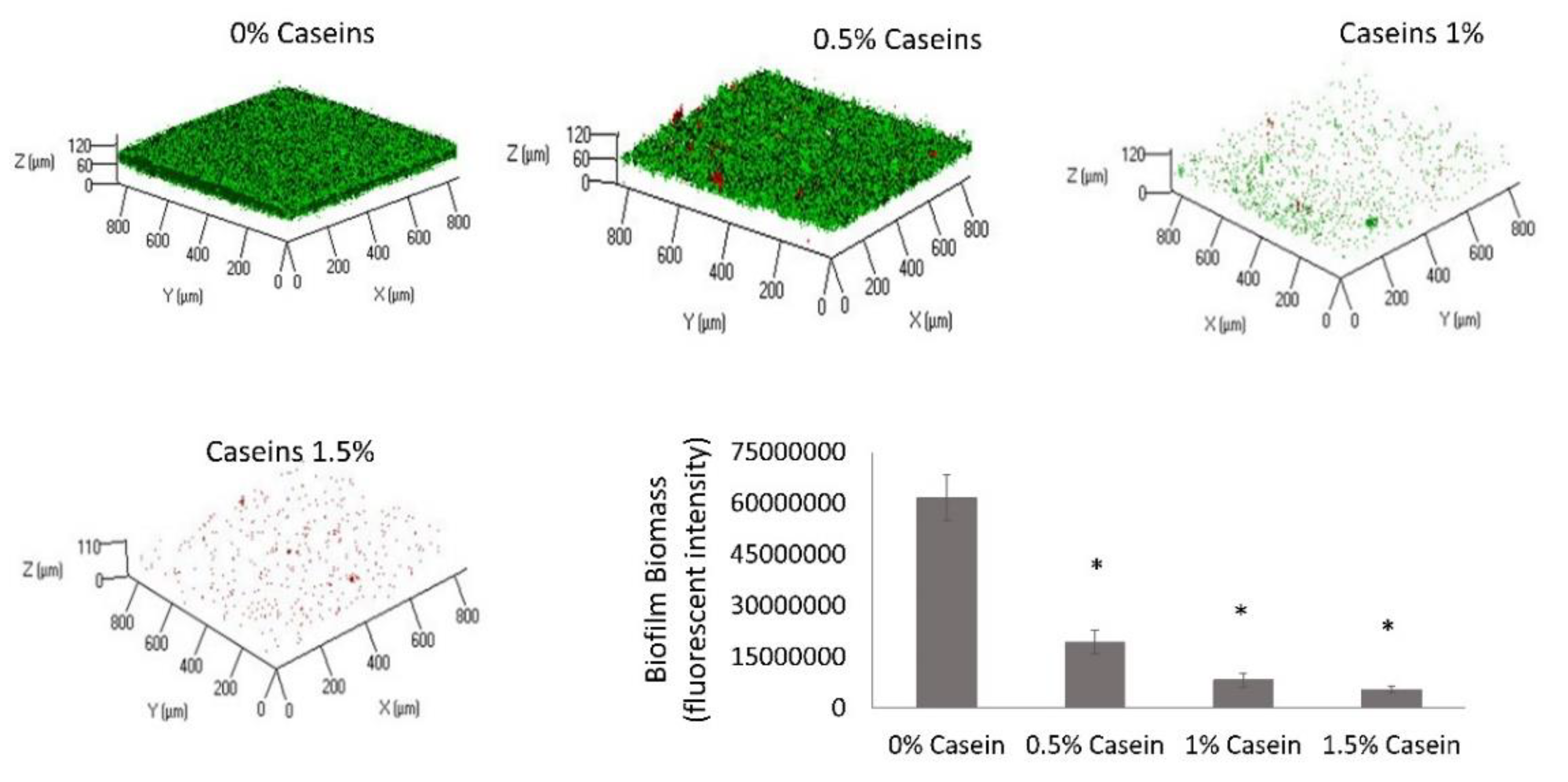
3.1. B. subtilis Enables Biofilm Formation by S. mutans in the Presence of Caseins
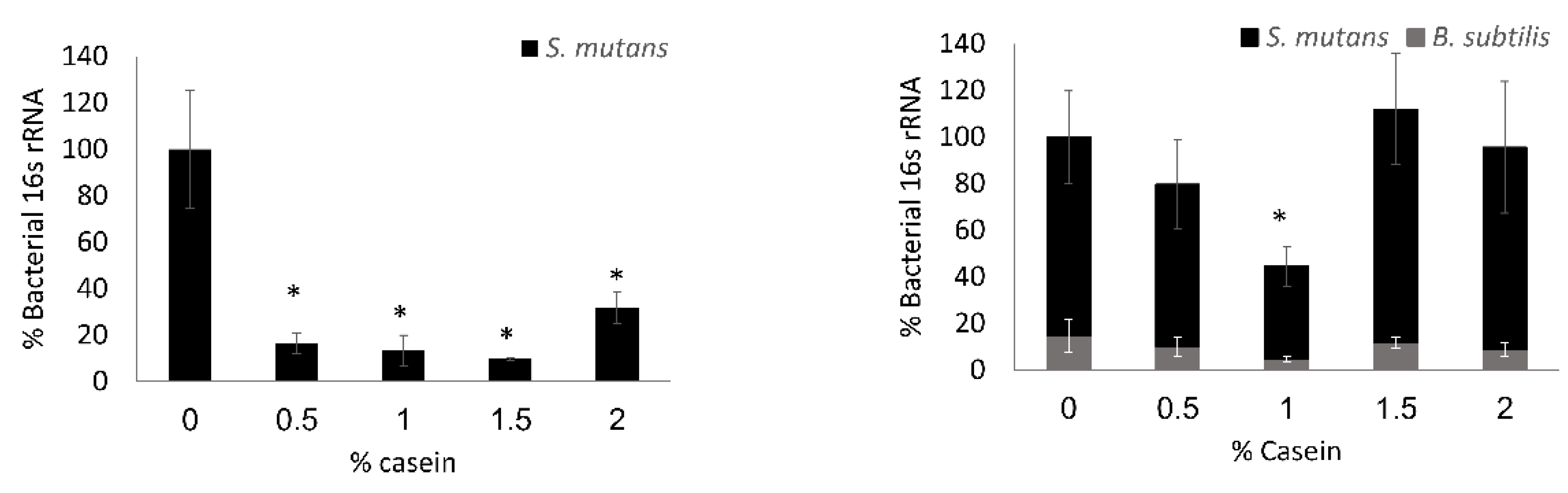
3.2. S. mutans is the Predominant Bacterium in the Dual-Species Biofilm
3.3. B. subtilis Enables the S. mutans Attachment to Hydroxyapatite Discs in the Presence of Casein Proteins
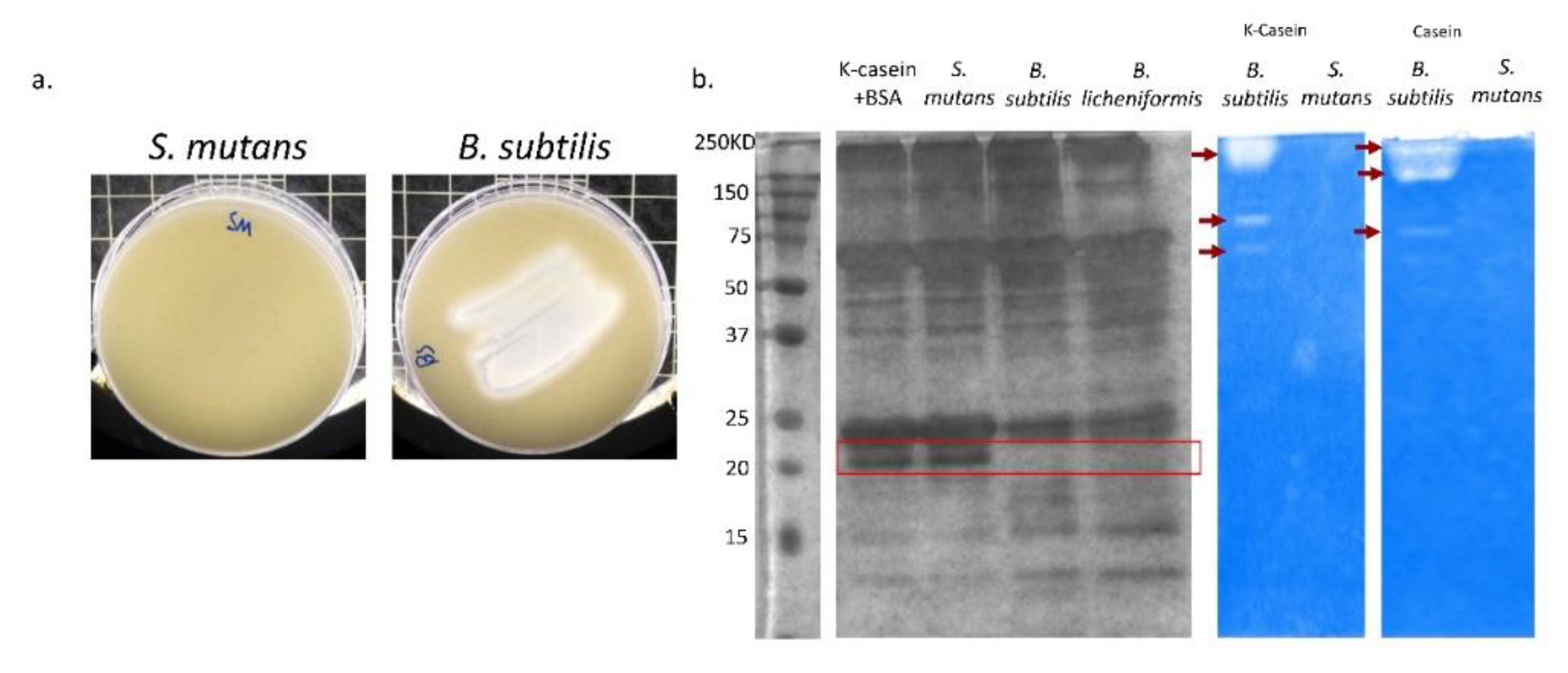
3.4. B. subtilis Has Proteolysis Ability on Milk Proteins
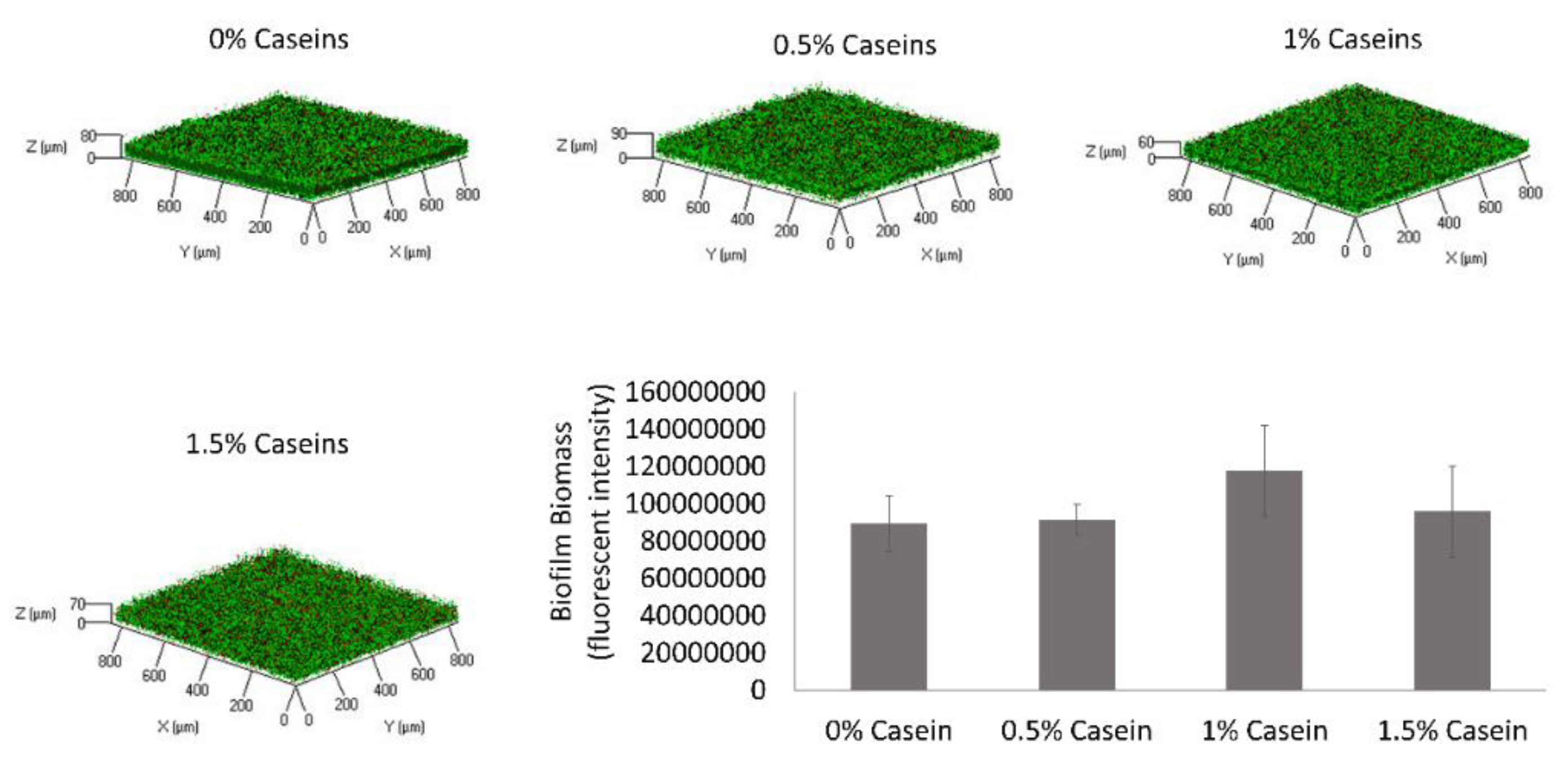
3.5. B. subtilis Proteolysis Ability Enables Biofilm Formation in the Presence of Caseins
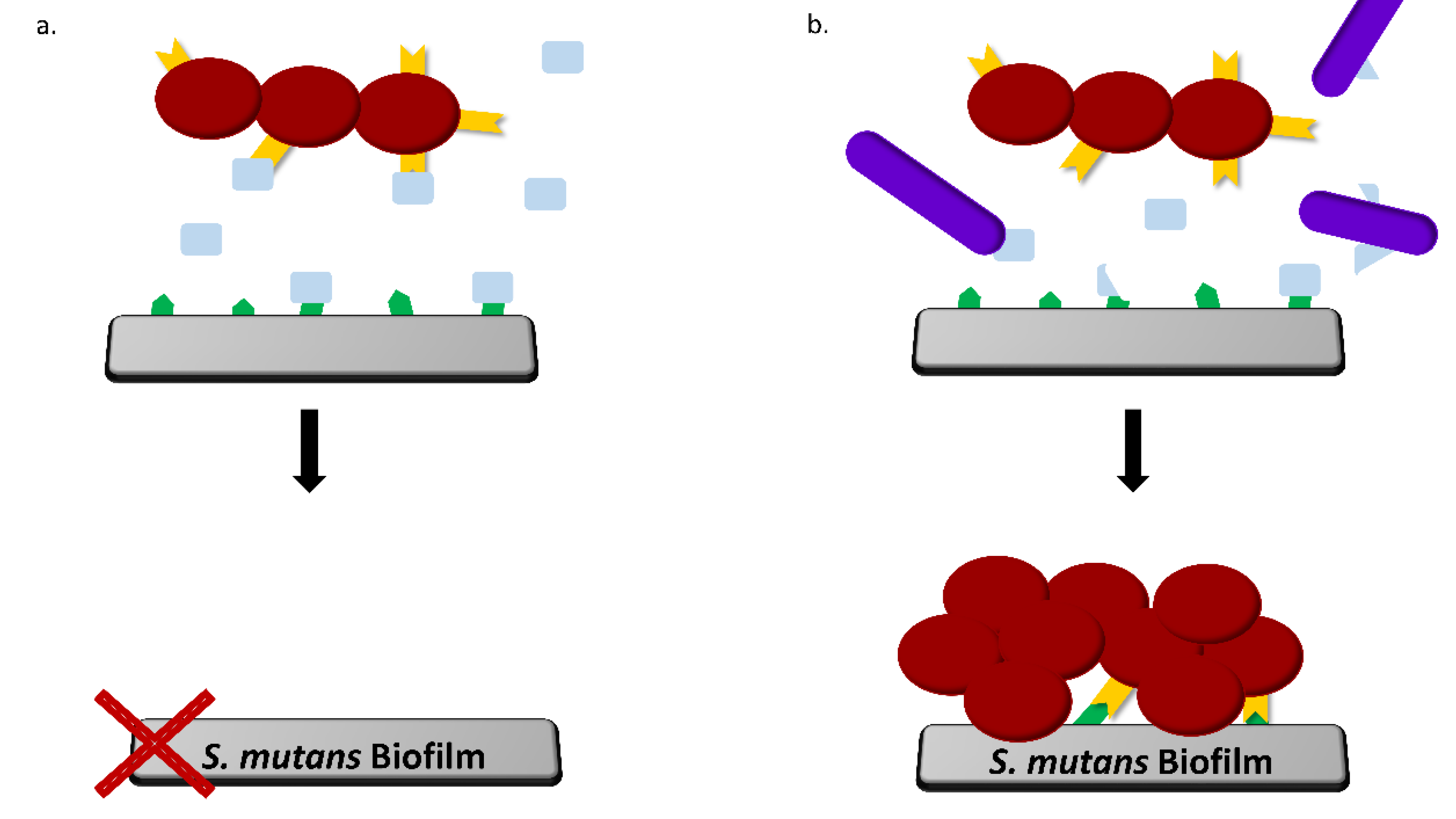
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Grenby, T.; Andrews, A.; Mistry, M.; Williams, R. Dental caries-protective agents in milk and milk products: Investigations in vitro. J. Dent. 2001, 29, 83–92. [Google Scholar] [CrossRef]
- Zeng, L.; Das, S.; Burne, R.A. Utilization of lactose and galactose by Streptococcus mutans: Transport, toxicity, and carbon catabolite repression. J. Bacteriol. 2010, 192, 2434–2444. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Sandoval, C.; Muñoz-Cifuentes, M.J.; Giacaman, R.A.; Ccahuana-Vasquez, R.A.; Cury, J.A. Effect of bovine milk on Streptococcus mutans biofilm cariogenic properties and enamel and dentin demineralization. Pediatric Dent. 2012, 34, 197E–201E. [Google Scholar]
- Pereira, P.C. Milk nutritional composition and its role in human health. Nutrition 2014, 30, 619–627. [Google Scholar] [CrossRef]
- Bowden, G.H.; Hamilton, I.R. Survival of oral bacteria. Crit. Rev. Oral Biol. Med. 1998, 9, 54–85. [Google Scholar] [CrossRef]
- Burne, R. Oral streptococci... products of their environment. J. Dent. Res. 1998, 77, 445–452. [Google Scholar] [CrossRef]
- Jenkinson, H.F. Adherence and accumulation of oral streptococci. Trends Microbiol. 1994, 2, 209–212. [Google Scholar] [CrossRef]
- Loesche, W.J. Role of Streptococcus mutans in human dental decay. Microbiol. Rev. 1986, 50, 353. [Google Scholar] [CrossRef]
- Liljemark, W.F.; Bloomquist, C. Human oral microbial ecology and dental caries and periodontal diseases. Crit. Rev. Oral Biol. Med. 1996, 7, 180–198. [Google Scholar] [CrossRef]
- Steinberg, D. Studying plaque biofilms on various dental surfaces. In Handbook of Bacterial Adhesion; Humana Press: Totowa, NJ, USA, 2000; pp. 353–370. [Google Scholar]
- Robinson, C.; Strafford, S.; Rees, G.; Brookes, S.; Kirkham, J.; Shore, R.; Watson, P.; Wood, S. Plaque biofilms: The effect of chemical environment on natural human plaque biofilm architecture. Arch. Oral Biol. 2006, 51, 1006–1014. [Google Scholar] [CrossRef]
- Assaf, D.; Steinberg, D.; Shemesh, M. Lactose triggers biofilm formation by Streptococcus mutans. Int. Dairy J. 2015, 42, 51–57. [Google Scholar] [CrossRef]
- Smith, A.V.; Bowen, W. The Effects of Milk and Kappa–Casein on Salivary Pellicle Formed on Hydroxyapatite Discs in situ. Caries Res. 2000, 34, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Vacca-Smith, A.; Van Wuyckhuyse, B.; Tabak, L.; Bowen, W. The effect of milk and casein proteins on the adherence of Streptococcus mutans to saliva-coated hydroxyapatite. Arch. Oral Biol. 1994, 39, 1063–1069. [Google Scholar] [CrossRef]
- Vacca-Smith, A.; Bowen, W. The effect of milk and kappa casein on streptococcal glucosyltransferase. Caries Res. 1995, 29, 498–506. [Google Scholar] [CrossRef]
- Oho, T.; Shimazaki, Y.; Mitoma, M.; Yoshimura, M.; Yamashita, Y.; Okano, K.; Nakano, Y.; Kawagoe, H.; Fukuyama, M.; Fujihara, N. Bovine milk antibodies against cell surface protein antigen PAc-glucosyltransferase fusion protein suppress cell adhesion and alter glucan synthesis of Streptococcus mutans. J. Nutr. 1999, 129, 1836–1841. [Google Scholar] [CrossRef]
- Aimutis, W.R. Bioactive properties of milk proteins with particular focus on anticariogenesis. J. Nutr. 2004, 134, 989S–995S. [Google Scholar] [CrossRef]
- Leonil, J.; Maubois, J.-L. Milk-derived bioactive peptides and proteins: Future perspectives. Sci. Aliment. 2002, 22, 383–392. [Google Scholar] [CrossRef]
- Bullappa, D.; Puranik, M.P.; Uma, S. Casein phosphopeptide-Amorphous calcium phosphate: A review. Int. J. Dent. Health Sci. 2015, 2, 116–125. [Google Scholar]
- Duanis-Assaf, D.; Duanis-Assaf, T.; Zeng, G.; Meyer, R.L.; Reches, M.; Steinberg, D.; Shemesh, M. Cell wall associated protein TasA provides an initial binding component to extracellular polysaccharides in dual-species biofilm. Sci. Rep. 2018, 8, 9350. [Google Scholar] [CrossRef]
- Pasvolsky, R.; Zakin, V.; Ostrova, I.; Shemesh, M. Butyric acid released during milk lipolysis triggers biofilm formation of Bacillus species. Int. J. Food Microbiol. 2014, 181, 19–27. [Google Scholar] [CrossRef]
- Sauer, A.; Moraru, C. Heat stability of micellar casein concentrates as affected by temperature and pH. J. Dairy Sci. 2012, 95, 6339–6350. [Google Scholar] [CrossRef] [PubMed]
- Periasamy, S.; Kolenbrander, P.E. Mutualistic biofilm communities develop with Porphyromonas gingivalis and initial, early, and late colonizers of enamel. J. Bacteriol. 2009, 191, 6804–6811. [Google Scholar] [CrossRef] [PubMed]
- Feldman, M.; Shenderovich, J.; Lavy, E.; Friedman, M.; Steinberg, D. A sustained-release membrane of thiazolidinedione-8: Effect on formation of a Candida/Bacteria mixed biofilm on hydroxyapatite in a continuous flow model. BioMed Res. Int. 2017, 2017, 3510124. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Sarkar, P.K. In vitro model study for biofilm formation by Bacillus cereus in dairy chilling tanks and optimization of clean-in-place (CIP) regimes using response surface methodology. Food Control 2014, 36, 153–158. [Google Scholar] [CrossRef]
- Haddadi, K.; Moussaoui, F.; Hebia, I.; Laurent, F.; Le Roux, Y.E. coli proteolytic activity in milk and casein breakdown. Reprod. Nutr. Dev. 2005, 45, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Choi, N.-S.; Yoon, K.-S.; Lee, J.-Y.; Han, K.-Y.; Kim, S.-H. Comparison of three substrates (casein, fibrin, and gelatin) in zymographic gel. BMB Rep. 2001, 34, 531–536. [Google Scholar]
- Ostrov, I.; Sela, N.; Belausov, E.; Steinberg, D.; Shemesh, M. Adaptation of Bacillus species to dairy associated environment facilitates their biofilm forming ability. Food Microbiol. 2019, 82, 316–324. [Google Scholar] [CrossRef]
- Nikawa, H.; Makihira, S.; Fukushima, H.; Nishimura, H.; Ozaki, Y.; Ishida, K.; Darmawan, S.; Hamada, T.; Hara, K.; Matsumoto, A. Lactobacillus reuteri in bovine milk fermented decreases the oral carriage of mutans streptococci. Int. J. Food Microbiol. 2004, 95, 219–223. [Google Scholar] [CrossRef]
- Meer, R.; Baker, J.; Bodyfelt, F.; Griffiths, M. Psychrotrophic Bacillus spp. in fluid milk products: A review. J. Food Prot. 1991, 54, 969–979. [Google Scholar] [CrossRef]
- Sharma, M.; Anand, S. Biofilms evaluation as an essential component of HACCP for food/dairy processing industry–a case. Food Control 2002, 13, 469–477. [Google Scholar] [CrossRef]
- Perry, J.A.; Cvitkovitch, D.G.; Lévesque, C.M. Cell death in Streptococcus mutans biofilms: A link between CSP and extracellular DNA. FEMS Microbiol. Lett. 2009, 299, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, E.C.; Wong, A. Effect of adsorbed protein on hydroxyapatite zeta potential and Streptococcus mutans adherence. Infect. Immun. 1983, 39, 1285–1290. [Google Scholar] [CrossRef] [PubMed]
- Halpin, R.; Brady, D.; O’Riordan, E.; O’Sullivan, M. The effect of untreated and enzyme-treated commercial dairy powders on the growth and adhesion of Streptococcus mutans. LWT-Food Sci. Technol. 2011, 44, 1525–1532. [Google Scholar] [CrossRef][Green Version]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duanis-Assaf, D.; Kenan, E.; Sionov, R.; Steinberg, D.; Shemesh, M. Proteolytic Activity of Bacillus subtilis upon κ-Casein Undermines Its “Caries-Safe” Effect. Microorganisms 2020, 8, 221. https://doi.org/10.3390/microorganisms8020221
Duanis-Assaf D, Kenan E, Sionov R, Steinberg D, Shemesh M. Proteolytic Activity of Bacillus subtilis upon κ-Casein Undermines Its “Caries-Safe” Effect. Microorganisms. 2020; 8(2):221. https://doi.org/10.3390/microorganisms8020221
Chicago/Turabian StyleDuanis-Assaf, Danielle, Eli Kenan, Ronit Sionov, Doron Steinberg, and Moshe Shemesh. 2020. "Proteolytic Activity of Bacillus subtilis upon κ-Casein Undermines Its “Caries-Safe” Effect" Microorganisms 8, no. 2: 221. https://doi.org/10.3390/microorganisms8020221
APA StyleDuanis-Assaf, D., Kenan, E., Sionov, R., Steinberg, D., & Shemesh, M. (2020). Proteolytic Activity of Bacillus subtilis upon κ-Casein Undermines Its “Caries-Safe” Effect. Microorganisms, 8(2), 221. https://doi.org/10.3390/microorganisms8020221




