Cysteine-Capped Hydrogels Incorporating Copper as Effective Antimicrobial Materials against Methicillin-Resistant Staphylococcus aureus
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
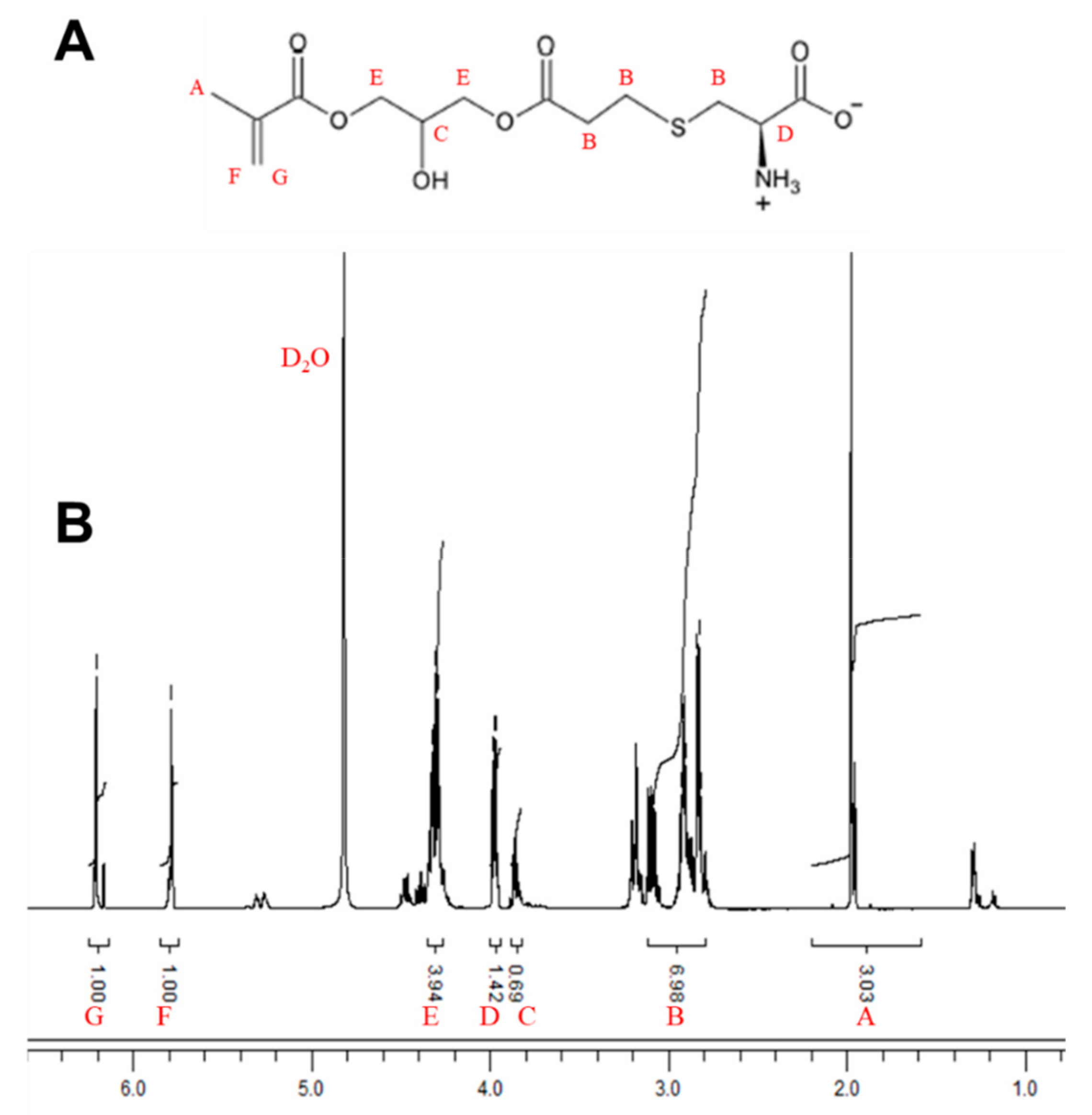
2.2. Synthesis of Cysteine Methacrylate (CysMA)
2.3. Preparation of CysMA/Copper Chloride Hydrogel and 3-(acryloyloxy)-2-hydroxypropyl Methacrylate/Copper Chloride Hydrogel
2.4. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
2.5. Copper Ion Absorption and Release
2.6. The Antibacterial Activity of Hydrogel Containing Copper Ions
2.7. Minimum Bactericidal Concentration (MBC) Assays
2.8. Wound Size Infection on Mouse Animal Model
2.9. Bacterial Counting
2.10. Statistical Analysis
3. Results and Discussion
3.1. Preparation of CysMA Monomer
3.2. Preparation of CysMA and MA Hydrogel and the Hydrogel Appearance
3.3. Absorption and Release Ability of CysMA and MA Hydrogels
3.4. FTIR Analysis
3.5. Antibacterial Test
3.6. Wound Infection Mouse Model
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Balasubramanian, D.; Harper, L.; Shopsin, B.; Torres, V.J. Staphylococcus aureus pathogenesis in diverse host environments. Pathog. Dis. 2017, 75, ftx005. [Google Scholar] [CrossRef] [PubMed]
- Klein, E.; Smith, D.L.; Laxminarayan, R. Hospitalizations and deaths caused by methicillin-resistant Staphylococcus aureus, United States, 1999–2005. Emerg. Infect. Dis. 2007, 13, 1840. [Google Scholar] [CrossRef] [PubMed]
- Otto, M. MRSA virulence and spread. Cell. Microbiol. 2012, 14, 1513–1521. [Google Scholar] [CrossRef] [PubMed]
- Talan, D.A.; Krishnadasan, A.; Gorwitz, R.J.; Fosheim, G.E.; Limbago, B.; Albrecht, V.; Moran, G.J. Comparison of Staphylococcus aureus from skin and soft-tissue infections in US emergency department patients, 2004 and 2008. Clin. Infect. Dis. 2011, 53, 144–149. [Google Scholar] [CrossRef]
- Rayner, C.; Munckhof, W. Antibiotics currently used in the treatment of infections caused by Staphylococcus aureus. Intern. Med. J. 2005, 35, 3–16. [Google Scholar] [CrossRef]
- Stahlmann, R. Antibiotika zur Behandlung von Infektionen durch Methicillin-resistente Staphylococcus aureus (MRSA). Pneumologie 2014, 68, 676–684. [Google Scholar] [CrossRef]
- Ferguson, J. Preventing healthcare-associated infection: Risks, healthcare systems and behaviour. Intern. Med. J. 2009, 39, 574–581. [Google Scholar] [CrossRef]
- Reynolds, C.; Quan, V.; Kim, D.; Peterson, E.; Dunn, J.; Whealon, M.; Terpstra, L.; Meyers, H.; Cheung, M.; Lee, B.; et al. Methicillin-resistant Staphylococcus aureus (MRSA) carriage in 10 nursing homes in Orange County, California. Infect. Control Hosp. Epidemiol. 2011, 32, 91–93. [Google Scholar] [CrossRef]
- Fujishita, S.; Inaba, C.; Tada, S.; Gemmei-Ide, M.; Kitano, H.; Saruwatari, Y. Effect of zwitterionic polymers on wound healing. Biol. Pharm. Bull. 2008, 31, 2309–2315. [Google Scholar] [CrossRef]
- Chien, H.-W.; Xu, X.; Ella-Menye, J.-R.; Tsai, W.-B.; Jiang, S. High viability of cells encapsulated in degradable poly (carboxybetaine) hydrogels. Langmuir 2012, 28, 17778–17784. [Google Scholar] [CrossRef]
- Tong, X.; Yang, F. Engineering interpenetrating network hydrogels as biomimetic cell niche with independently tunable biochemical and mechanical properties. Biomaterials 2014, 35, 1807–1815. [Google Scholar] [CrossRef] [PubMed]
- Ning, J.; Li, G.; Haraguchi, K. Effects of Polymer Concentration on Structure and Properties of Zwitterionic Nanocomposite Gels. MacroMol. Chem. Phys. 2014, 215, 235–244. [Google Scholar] [CrossRef]
- Morris, C. Wound management and dressing selection. Wound Essent. 2006, 1, 178–183. [Google Scholar]
- Haraguchi, K.; Farnworth, R.; Ohbayashi, A.; Takehisa, T. Compositional effects on mechanical properties of nanocomposite hydrogels composed of poly (N, N-dimethylacrylamide) and clay. Macromolecules 2003, 36, 5732–5741. [Google Scholar] [CrossRef]
- Azari, S.; Zou, L. Fouling resistant zwitterionic surface modification of reverse osmosis membranes using amino acid l-cysteine. Desalination 2013, 324, 79–86. [Google Scholar] [CrossRef]
- Shimazaki, Y.; Takani, M.; Yamauchi, O. Metal complexes of amino acids and amino acid side chain groups. Structures and properties. Dalton Trans. 2009, 38, 7854–7869. [Google Scholar] [CrossRef]
- Romanski, J.; Karbarz, M.; Pyrzynska, K.; Jurczak, J.; Stojek, Z. Polymeric hydrogels modified with ornithine and lysine: Sorption and release of metal cations and amino acids. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 542–550. [Google Scholar] [CrossRef]
- Brisson, E.R.; Xiao, Z.; Connal, L.A. Amino Acid Functional Polymers: Biomimetic Polymer Design Enabling Catalysis, Chiral Materials, and Drug Delivery. Aust. J. Chem. 2016, 69, 705–716. [Google Scholar] [CrossRef]
- Borkow, G.; Gabbay, J. Copper as a biocidal tool. Curr. Med. Chem. 2005, 12, 2163–2175. [Google Scholar] [CrossRef]
- Jayaramudu, T.; Varaprasad, K.; Reddy, K.K.; Pyarasani, R.D.; Akbari-Fakhrabadi, A.; Amalraj, J. Chitosan-pluronic based Cu nanocomposite hydrogels for prototype antimicrobial applications. Int. J. Biol. Macromol. 2019, 143, 825–832. [Google Scholar] [CrossRef]
- Klinkajon, W.; Supaphol, P. Novel copper (II) alginate hydrogels and their potential for use as anti-bacterial wound dressings. Biomed. Mater. 2014, 9, 045008. [Google Scholar] [CrossRef] [PubMed]
- Catoira, M.C.; Fusaro, L.; Di Francesco, D.; Ramella, M.; Boccafoschi, F. Overview of natural hydrogels for regenerative medicine applications. J. Mater. Sci. Mater. Med. 2019, 30, 115. [Google Scholar] [CrossRef] [PubMed]
- Mirastschijski, U.; Martin, A.; Jorgensen, L.N.; Sampson, B.; Ågren, M.S. Zinc, copper, and selenium tissue levels and their relation to subcutaneous abscess, minor surgery, and wound healing in humans. Biol. Trace Elem. Res. 2013, 153, 76–83. [Google Scholar] [CrossRef]
- Moynahan, E. Acrodermatitis enteropathica: A lethal inherited human zinc-deficiency disorder. Lancet 1974, 2, 399. [Google Scholar] [CrossRef]
- Lansdown, A.B. Physiological and toxicological changes in the skin resulting from the action and interaction of metal ions. Crit. Rev. Toxicol. 1995, 25, 397–462. [Google Scholar] [CrossRef] [PubMed]
- Mathews, S.; Hans, M.; Mücklich, F.; Solioz, M. Contact Killing of Bacteria on Copper Is Suppressed if Bacterial-Metal Contact Is Prevented and Is Induced on Iron by Copper Ions. Appl. Environ. Microbiol. 2013, 79, 2605–2611. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-J.; Chang, T.-W.; Jiang, Y.; Kao, H.-J.; Chiou, B.-H.; Kao, M.-S.; Huang, C.-M. Commensal Staphylococcus aureus Provokes Immunity to Protect against Skin Infection of Methicillin-Resistant Staphylococcus aureus. Int. J. Mol. Sci. 2018, 19, 1290. [Google Scholar] [CrossRef]
- Alswieleh, A.M.; Cheng, N.; Canton, I.; Ustbas, B.; Xue, X.; Ladmiral, V.; Xia, S.; Ducker, R.E.; El Zubir, O.; Cartron, M.L. Zwitterionic Poly (amino acid methacrylate) Brushes. J. Am. Chem. Soc. 2014, 136, 9404–9413. [Google Scholar] [CrossRef]
- Huang, K.-T.; Fang, Y.-L.; Hsieh, P.-S.; Li, C.-C.; Dai, N.-T.; Huang, C.-J. Zwitterionic nanocomposite hydrogels as effective wound dressings. J. Mater. Chem. B 2016, 4, 4206–4215. [Google Scholar] [CrossRef]
- Säbel, C.E.; Neureuther, J.M.; Siemann, S. A spectrophotometric method for the determination of zinc, copper, and cobalt ions in metalloproteins using Zincon. Anal. Biochem. 2010, 397, 218–226. [Google Scholar] [CrossRef]
- Liu, P.-F.; Cheng, J.-S.; Sy, C.-L.; Huang, W.-C.; Yang, H.-C.; Gallo, R.L.; Huang, C.-M.; Shu, C.-W. IsaB inhibits autophagic flux to promote host transmission of methicillin-resistant Staphylococcus aureus. J. Investig. Dermatol. 2015, 135, 2714–2722. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.-T.; Huang, C.-J. Novel zwitterionic nanocomposite hydrogel as effective chronic wound healing dressings. In Proceedings of the 1st Global Conference on Biomedical Engineering & 9th Asian-Pacific Conference on Medical and Biological Engineering, Tainan, Taiwan, 9–12 October 2014; IFMBE Proceedings. Springer: Cham, Switzerland, 2015; Volume 47, pp. 35–38. [Google Scholar]
- Kao, M.S.; Huang, S.; Chang, W.L.; Hsieh, M.F.; Huang, C.J.; Gallo, R.L.; Huang, C.M. Microbiome precision editing: Using PEG as a selective fermentation initiator against methicillin-resistant Staphylococcus aureus. Biotechnol. J. 2017, 12, 1600399. [Google Scholar] [CrossRef] [PubMed]
- Shu, M.; Wang, Y.; Yu, J.; Kuo, S.; Coda, A.; Jiang, Y.; Gallo, R.L.; Huang, C.-M. Fermentation of Propionibacterium acnes, a commensal bacterium in the human skin microbiome, as skin probiotics against methicillin-resistant Staphylococcus aureus. PLoS ONE 2013, 8, e55380. [Google Scholar] [CrossRef] [PubMed]
- Zhi, X.; Li, P.; Gan, X.; Zhang, W.; Shen, T.; Yuan, J.; Shen, J. Hemocompatibility and anti-biofouling property improvement of poly (ethylene terephthalate) via self-polymerization of dopamine and covalent graft of lysine. J. Biomater. Sci. Polym. Ed. 2014, 25, 1619–1628. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Hu, X.; Wang, J.; Zhang, Y.-M.; Liu, X.-J.; Xie, B.-B.; Yao, C.; Li, Y.; Li, X.-S. Library of antifouling surfaces derived from natural amino acids by click reaction. ACS Appl. Mater. Interfaces 2015, 7, 17337–17345. [Google Scholar] [CrossRef]
- Sood, A.; Granick, M.S.; Tomaselli, N.L. Wound dressings and comparative effectiveness data. Adv. Wound Care 2014, 3, 511–529. [Google Scholar] [CrossRef]
- Grass, G.; Rensing, C.; Solioz, M. Metallic copper as an antimicrobial surface. Appl. Environ. Microbiol. 2011, 77, 1541–1547. [Google Scholar] [CrossRef]
- Ndokoye, P.; Ke, J.; Liu, J.; Zhao, Q.; Li, X. L-Cysteine-modified gold nanostars for SERS-based copper ions detection in aqueous media. Langmuir 2014, 30, 13491–13497. [Google Scholar] [CrossRef]
- Qi, L.; Xu, Z.; Jiang, X.; Hu, C.; Zou, X. Preparation and antibacterial activity of chitosan nanoparticles. Carbohydr. Res. 2004, 339, 2693–2700. [Google Scholar] [CrossRef]
- Stohs, S.J.; Bagchi, D. Oxidative mechanisms in the toxicity of metal ions. Free Radic. Biol. Med. 1995, 18, 321–336. [Google Scholar] [CrossRef]
- Howlett, N.; Avery, S. Relationship between cadmium sensitivity and degree of plasma membrane fatty acid unsaturation in Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 1997, 48, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Kavoosi, G.; Dadfar, S.M.M.; Purfard, A.M. Mechanical, physical, antioxidant, and antimicrobial properties of gelatin films incorporated with thymol for potential use as nano wound dressing. J. Food Sci. 2013, 78, E244–E250. [Google Scholar] [CrossRef] [PubMed]
- Raghavendra, G.M.; Jung, J.; Seo, J. Microwave assisted antibacterial chitosan–silver nanocomposite films. Int. J. Biol. Macromol. 2016, 84, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Reller, L.B.; Weinstein, M.; Jorgensen, J.H.; Ferraro, M.J. Antimicrobial susceptibility testing: A review of general principles and contemporary practices. Clin. Infect. Dis. 2009, 49, 1749–1755. [Google Scholar] [CrossRef]
- Uçkay, I.; Bernard, L.; Buzzi, M.; Harbarth, S.; François, P.; Huggler, E.; Ferry, T.; Schrenzel, J.; Renzoni, A.; Vaudaux, P.; et al. High prevalence of isolates with reduced glycopeptide susceptibility in persistent or recurrent bloodstream infections due to methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2012, 56, 1258–1264. [Google Scholar] [CrossRef][Green Version]
- Kassim, A.; Omuse, G.; Premji, Z.; Revathi, G. Comparison of Clinical Laboratory Standards Institute and European Committee on Antimicrobial Susceptibility Testing guidelines for the interpretation of antibiotic susceptibility at a University teaching hospital in Nairobi, Kenya: A cross-sectional study. Ann. Clin. Microbiol. Antimicrob. 2016, 15, 21. [Google Scholar] [CrossRef]
- Borkow, G.; Gabbay, J.; Zatcoff, R.C. Could chronic wounds not heal due to too low local copper levels? Med. Hypotheses 2008, 70, 610–613. [Google Scholar] [CrossRef]







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.J.; Huang, Y.-C.; Chuang, T.-H.; Herr, D.R.; Hsieh, M.-F.; Huang, C.-J.; Huang, C.-M. Cysteine-Capped Hydrogels Incorporating Copper as Effective Antimicrobial Materials against Methicillin-Resistant Staphylococcus aureus. Microorganisms 2020, 8, 149. https://doi.org/10.3390/microorganisms8020149
Yang JJ, Huang Y-C, Chuang T-H, Herr DR, Hsieh M-F, Huang C-J, Huang C-M. Cysteine-Capped Hydrogels Incorporating Copper as Effective Antimicrobial Materials against Methicillin-Resistant Staphylococcus aureus. Microorganisms. 2020; 8(2):149. https://doi.org/10.3390/microorganisms8020149
Chicago/Turabian StyleYang, John Jackson, Yung-Chi Huang, Tsung-Hsien Chuang, Deron Raymond Herr, Ming-Fa Hsieh, Chun-Jen Huang, and Chun-Ming Huang. 2020. "Cysteine-Capped Hydrogels Incorporating Copper as Effective Antimicrobial Materials against Methicillin-Resistant Staphylococcus aureus" Microorganisms 8, no. 2: 149. https://doi.org/10.3390/microorganisms8020149
APA StyleYang, J. J., Huang, Y.-C., Chuang, T.-H., Herr, D. R., Hsieh, M.-F., Huang, C.-J., & Huang, C.-M. (2020). Cysteine-Capped Hydrogels Incorporating Copper as Effective Antimicrobial Materials against Methicillin-Resistant Staphylococcus aureus. Microorganisms, 8(2), 149. https://doi.org/10.3390/microorganisms8020149




