Role of Respiratory Syncytial Virus in Pediatric Pneumonia
Abstract
1. Introduction
2. Incidence of Respiratory Syncytial Virus (RSV)
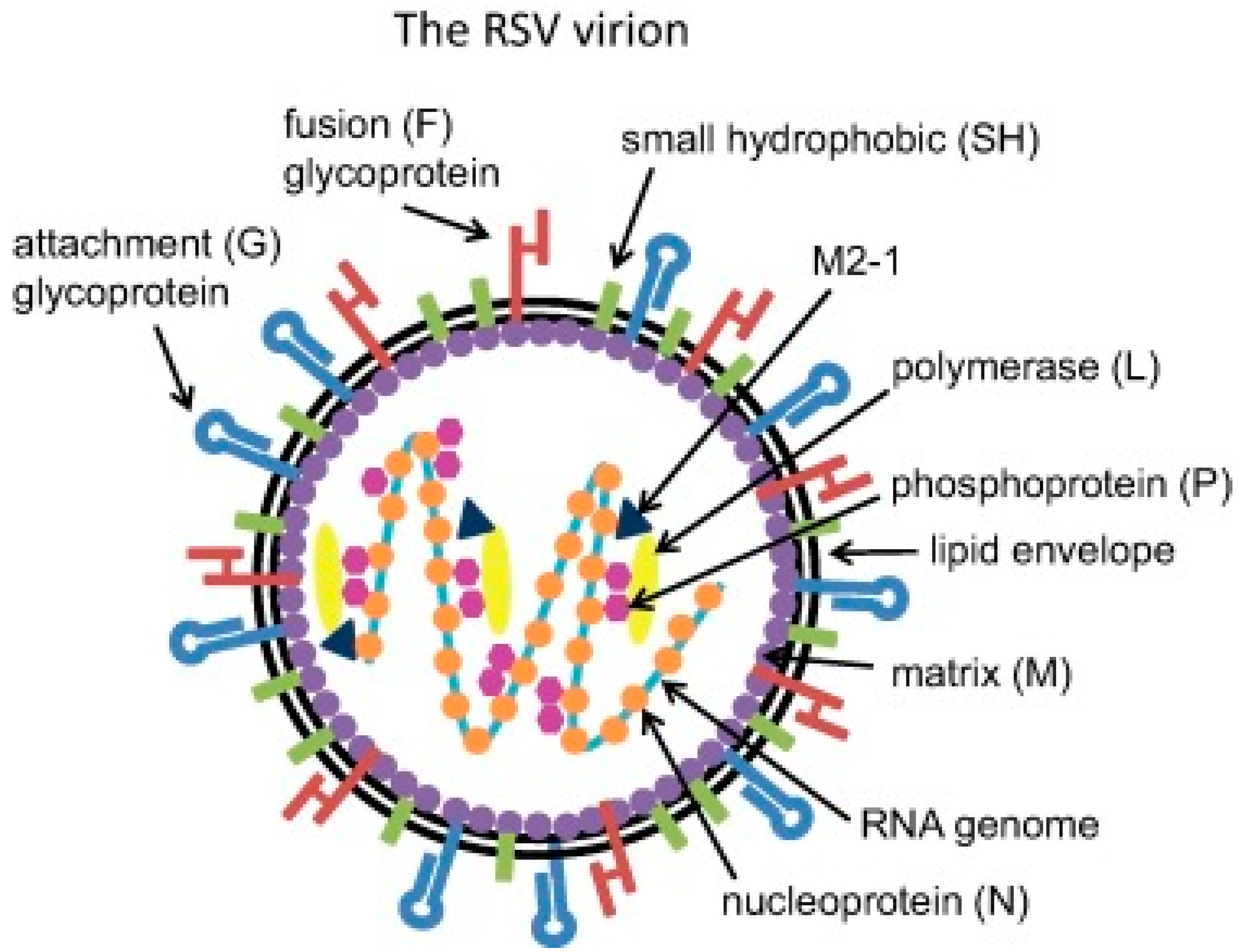
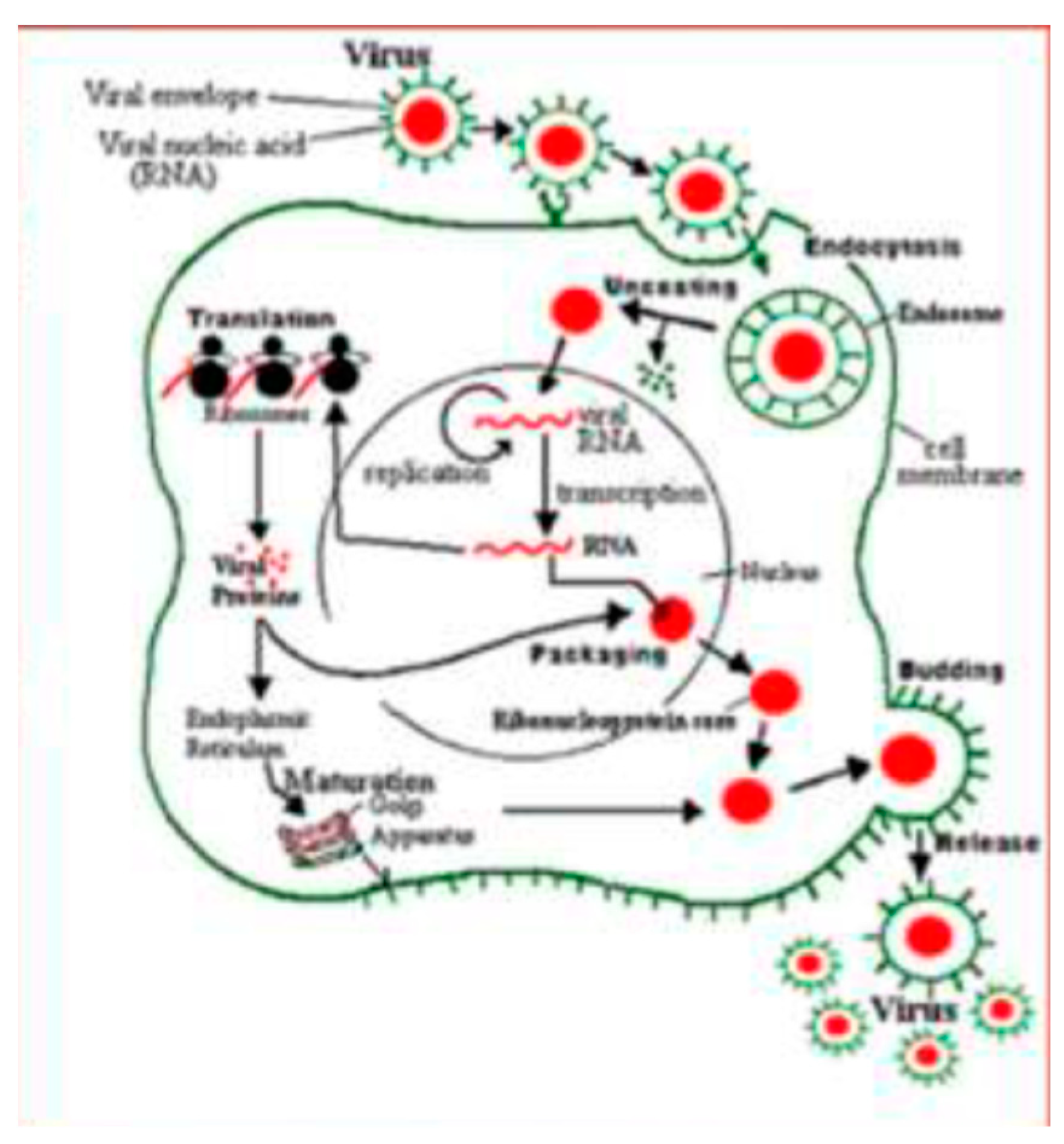
3. Pathophysiology of Respiratory Syncytial Virus (RSV) Infection
4. Clinical Manifestation of Respiratory Syncytial Virus (RSV)
5. Diagnosis of Respiratory Syncytial Virus (RSV)
6. Treatment of Respiratory Syncytial Virus (RSV) Infection
7. Respiratory Syncytial Virus (RSV) Prevention
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Heinonen, S.; Rodriguez-Fernandez, R.; Diaz, A.; Rodriguez-Pastor, S.O.; Ramilo, O.; Mejias, A. Infant immune response to respiratory viral infections. Immunol. Allergy Clin. N. Am. 2019, 39, 361–376. [Google Scholar] [CrossRef] [PubMed]
- Lozano, R.; Naghavi, M.; Foreman, K.; Lim, S.; Shibuya, K.; Aboyans, V.; Abraham, J.; Adair, T.; Aggarwal, R.; Ahn, S.Y.; et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2095–2128. [Google Scholar] [CrossRef]
- Yu, J.; Liu, C.; Xiao, Y.; Xiang, Z.; Zhou, H.; Chen, L.; Shen, K.; Xie, Z.; Ren, L.; Wang, J. Respiratory syncytial virus seasonality, Beijing, China, 2007–2015. Emerg. Infect. Dis. 2019, 25, 1127–1135. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Williams, D.J.; Arnold, S.R.; Ampofo, K.; Bramley, A.M.; Reed, C.; Stockmann, C.; Anderson, E.J.; Grijalva, C.G.; Self, W.H.; et al. Community-Acquired pneumonia requiring hospitalization among U.S. children. N. Engl. J. Med. 2015, 372, 835–845. [Google Scholar] [CrossRef]
- Stockman, L.J.; Curns, A.T.; Anderson, L.J.; Fischer-Langley, G. Respiratory syncytial virus-associated hospitalizations among infants and young children in the United States, 1997–2006. Pediatrics Infect. Dis. J. 2012, 31, 5–9. [Google Scholar] [CrossRef]
- Esposito, S.; Bianchini, S.; Argentiero, A.; Neglia, C.; Principi, N. How does one choose the appropriate pharmacotherapy for children with lower respiratory tract infections? Expert Opin. Pharmacother. 2020, 21, 1–9. [Google Scholar] [CrossRef]
- World Health Organization. Pneumonia. Available online: https://www.who.int/news-room/fact-sheets/detail/pneumonia (accessed on 7 September 2020).
- Taylor, S.M.; Lopez, P.; Weckx, L.; Borja-Tabora, C.; Ulloa-Gutierrez, R.; Lazcano-Ponce, E.; Kerdpanich, A.; Weber, M.A.R.; Santos, A.M.D.L.; Tinoco, J.-C.; et al. Respiratory viruses and influenza-like illness: Epidemiology and outcomes in children aged 6 months to 10 years in a multi-country population sample. J. Infect. 2017, 74, 29–41. [Google Scholar] [CrossRef]
- Pavia, A.T. Viral infections of the lower respiratory tract: Old viruses, new viruses, and the role of diagnosis. Clin. Infect. Dis. 2011, 52, S284–S289. [Google Scholar] [CrossRef]
- Rey-Jurado, E.; Kalergis, A.M. Immunological features of respiratory syncytial virus-caused pneumonia—Implications for vaccine design. Int. J. Mol. Sci. 2017, 18, 556. [Google Scholar] [CrossRef]
- Bosis, S.; Esposito, S.; Niesters, H.G.M.; Zuccotti, G.V.; Marseglia, G.; Lanari, M.; Zuin, G.; Pelucchi, C.; Osterhaus, A.D.M.E.; Principi, N. Role of respiratory pathogens in infants hospitalized for a first episode of wheezing and their impact on recurrences. Clin. Microbiol. Infect. 2008, 14, 677–684. [Google Scholar] [CrossRef]
- Taleb, S.A.; Al Thani, A.A.; Al Ansari, K.; Yassine, H.M. Human respiratory syncytial virus: Pathogenesis, immune responses, and current vaccine approaches. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 1817–1827. [Google Scholar] [CrossRef] [PubMed]
- Nair, H.; Nokes, D.J.; Gessner, B.D.; Dherani, M.; Madhi, S.A.; Singleton, R.J.; O’Brien, K.L.; Roca, A.; Wright, P.F.; Bruce, N.; et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: A systematic review and meta-analysis. Lancet 2010, 375, 1545–1555. [Google Scholar] [CrossRef]
- World Health Organization. WHO Strategy to Pilot Global Respiratory Syncytial Virus Surveillance Based on the Global Influenza Surveillance and Response System (GISRS). Available online: https://apps.who.int/iris/bitstream/handle/10665/259853/9789241513203-eng.pdf;jsessionid=876497B8465C841C0AA1F4A9F6F8F95D?sequence=1 (accessed on 7 September 2020).
- Histoshi, T.; McAllister, D.A.; O’Brien, K.L.; Simoes, E.A.F.; Madhi, S.A.; Gessner, B.D.; Polack, F.P.; Balsells, E.; Acacio, S.; Aguayo, C.; et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: A systematic review and modelling study. Lancet 2017, 390, 946–958. [Google Scholar] [CrossRef]
- Rath, B.; Conrad, T.; Myles, P.; Alchikh, M.; Ma, X.; Hoppe, C.; Tief, F.; Chen, X.; Obermeier, P.; Kisler, B.; et al. Influenza and other respiratory viruses: Standardizing disease severity in surveillance and clinical trials. Expert Rev. Anti-Infect. Ther. 2017, 15, 545–568. [Google Scholar] [CrossRef]
- Hodgson, D.; Pebody, R.; Panovska-Griffiths, J.; Baguelin, M.; Atkins, K.E. Evaluating the next generation of RSV intervention strategies: A mathematical modelling study and cost-effectiveness analysis. BMC Med. 2020, 18, 348. [Google Scholar] [CrossRef]
- Korsun, N.; Angelova, S.; Tzotcheva, I.; Georgieva, I.; Lazova, S.; Parina, S.; Alexiev, I.; Perenovska, P. Prevalence and genetic characterisation of respiratory syncytial viruses circulating in Bulgaria during the 2014/15 and 2015/16 winter seasons. Pathog. Glob. Health 2017, 111, 351–361. [Google Scholar] [CrossRef]
- Baymakova, M. Fever of unknown origin in a bulgarian hospital: Evaluation of 54 cases for a four year-period. J. Clin. Anal. Med. 2016, 7, 70–75. [Google Scholar] [CrossRef]
- Korsun, N.; Angelova, S.; Trifonova, I.; Voleva, S.; Grigorova, I.; Tzotcheva, I.; Mileva, S.; Alexiev, I.; Perenovska, P. Predominance of ON1 and BA9 genotypes of Respiratory Syncytial Virus (RSV) in Bulgaria, 2016–2018. J. Med. Virol. 2020, 11. [Google Scholar] [CrossRef]
- Pavlova, S.; Hadzhiolova, T.; Abadjieva, P.; Kotseva, R. Application of RT-PCR for diagnosis of respiratory syncytial virus and human metapneumovirus infections in Bulgaria, 2006–7 and 2007–8. Eurosurveillance 2009, 14, 19233. [Google Scholar] [CrossRef][Green Version]
- Dimova-Yaneva, D.N.; Helms, P.J. The role of leukotrienes and eosinophil cationic protein in acute respiratory syncytial virus bronchiolitis. Folia Med. 2003, 45, 5–11. [Google Scholar]
- Stein, R.T.; Bont, L.J.; Zar, H.; Polack, F.P.; Park, C.; Claxton, A.; Borok, G.; Butylkova, Y.; Wegzyn, C.M. Respiratory syncytial virus hospitalization and mortality: Systematic review and meta-analysis. Pediatrics Pulmonol. 2016, 52, 556–569. [Google Scholar] [CrossRef] [PubMed]
- Blanken, M.O.; Rovers, M.M.; Molenaar, J.M.; Winkler-Seinstra, P.L.; Meijer, A.; Kimpen, J.L.; Bont, L.; Dutch RSV Neonatal Network. Respiratory syncytial virus and recurrent wheeze in healthy preterm infants. N. Engl. J. Med. 2013, 368, 1791–1799. [Google Scholar] [CrossRef] [PubMed]
- Feddema, J.J.; Claassen, E. Prevalence of viral respiratory infections amongst asthmatics: Results of a meta-regression analysis. Respir. Med. 2020, 173, 106020. [Google Scholar] [CrossRef] [PubMed]
- Kitcharoensakkul, M.; Bacharier, L.B.; Schweiger, T.L.; Wilson, B.; Goss, C.W.; Lew, D.; Schechtman, K.B.; Castro, M. Lung function trajectories and bronchial hyperresponsiveness during childhood following severe RSV bronchiolitis in infancy. Pediatrics Allergy Immunol. 2020. [Google Scholar] [CrossRef]
- Beigelman, A.; Bacharier, L.B. The role of early life viral bronchiolitis in the inception of asthma. Curr. Opin. Allergy Clin. Immunol. 2013, 13, 211–216. [Google Scholar] [CrossRef]
- Lim, A.; Butt, M.L.; Dix, J.; Elliott, L.; Paes, B. Respiratory syncytial virus (RSV) infection in children with medical complexity. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 38, 171–176. [Google Scholar] [CrossRef]
- Bont, L.; Checchia, P.A.; Fauroux, B.; Figueras-Aloy, J.; Manzoni, P.; Paes, B.; Simões, E.A.F.; Carbonell-Estrany, X. Defining the epidemiology and burden of severe respiratory syncytial virus infection among infants and children in western countries. Infect. Dis. Ther. 2016, 5, 271–298. [Google Scholar] [CrossRef]
- Huang, Y.; Hua, J.; Wang, D.; Chen, L.; Zhang, J.; Zhu, H.; Tian, J.; Zhang, T.; Zhao, G. Risk factors of respiratory syncytial virus infection among pediatric influenza-like illness and severe acute respiratory infections in Suzhou, China. J. Med. Virol. 2018, 90, 397–404. [Google Scholar] [CrossRef]
- Hall, C.B. Respiratory syncytial virus in young children. Lancet 2010, 375, 1500–1502. [Google Scholar] [CrossRef]
- Griffiths, C.; Drews, S.J.; Marchant, D.J. Respiratory Syncytial Virus: Infection, Detection, and New Options for Prevention and Treatment. Clin. Microbiol. Rev. 2017, 30, 277–319. [Google Scholar] [CrossRef]
- Mazur, N.I.; Martinón-Torres, F.; Baraldi, E.; Fauroux, B.; Greenough, A.; Heikkinen, T.; Manzoni, P.; Mejias, A.; Nair, H.; Papadopoulos, N.G.; et al. Lower respiratory tract infection caused by respiratory syncytial virus: Current management and new therapeutics. Lancet Respir. Med. 2015, 3, 888–900. [Google Scholar] [CrossRef]
- Green, C.A.; Sande, C.; De Lara, C.; Thompson, A.; Silva-Reyes, L.; Napolitano, F.; Pierantoni, A.; Capone, S.; Vitelli, A.; Klenerman, P.; et al. Humoral and cellular immunity to RSV in infants, children and adults. Vaccine 2018, 36, 6183–6190. [Google Scholar] [CrossRef] [PubMed]
- Beckhaus, A.A.; Castro-Rodriguez, J.A. Down Syndrome and the Risk of Severe RSV Infection: A Meta-analysis. Pediatrics 2018, 142, e20180225. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; McLean, K.; Campbell, H.; Nair, H. Aetiological role of common respiratory viruses in acute lower respiratory infections in children under five years: A systematic review and meta-analysis. J. Glob. Health 2015, 5, 010408. [Google Scholar] [CrossRef] [PubMed]
- Murray, A.; Chu, H.Y. RSV, antibodies and the developing world. Pediatrics Infect. Dis. J. 2019, 38, S24–S27. [Google Scholar] [CrossRef]
- Cohen, E.; Kuo, D.Z.; Agrawal, R.; Berry, J.G.; Bhagat, S.K.M.; Simon, T.D.; Srivastava, R. Children with medical complexity: An emerging population for clinical and research initiatives. Pediatrics 2011, 127, 529–538. [Google Scholar] [CrossRef]
- Leyenaar, J.K.; Lagu, T.; Shieh, M.-S.; Pekow, P.S.; Lindenauer, P.K. Management and outcomes of pneumonia among children with complex chronic conditions. Pediatr. Infect. Dis. J. 2014, 33, 907–911. [Google Scholar] [CrossRef]
- Manzoni, P.; Figueras-Aloy, J.; Simões, E.A.F.; Checchia, P.A.; Fauroux, B.; Bont, L.; Paes, B.; Carbonell-Estrany, X. Defining the incidence and associated morbidity and mortality of severe respiratory syncytial virus infection among children with chronic diseases. Infect. Dis. Ther. 2017, 6, 383–411. [Google Scholar] [CrossRef]
- Murray, J.; Bottle, A.; Sharland, M.; Modi, N.; Aylin, P.; Majeed, A.; Saxena, S.; On Behalf of the Medicines for Neonates Investigator Group. Risk factors for hospital admission with RSV bronchiolitis in England: A population-based birth cohort study. PLoS ONE 2014, 9, e89186. [Google Scholar] [CrossRef]
- World Health Organization. WHO RSV Surveillance Phase-2 (2019–2021). Available online: https://www.who.int/influenza/rsv/RSV_surveillance_phase2/en/ (accessed on 7 September 2020).
- Lam, T.T.; Tang, J.W.; Lai, F.Y.; Zaraket, H.; Dbaibo, G.; Bialasiewicz, S.; Tozer, S.; Heraud, J.-M.; Drews, S.J.; Hachette, T.; et al. Comparative global epidemiology of influenza, respiratory syncytial and parainfluenza viruses, 2010–2015. J. Infect. 2019, 79, 373–382. [Google Scholar] [CrossRef]
- Walker, C.L.; Rudan, I.; Liu, L.; Nair, H.; Theodoratou, E.; Bhutta, Z.A.; O’Brien, K.L.; Campbell, H.; Black, R.E. Global burden of childhood pneumonia and diarrhea. Lancet 2013, 381, 1405–1416. [Google Scholar] [CrossRef]
- Liu, L.; Oza, S.; Hogan, D.; Chu, Y.; Perin, J.; Zhu, J.; Lawn, J.E.; Cousens, S.; Mathers, C.; Black, R.E. Global, regional, and national causes of under-5 mortality in 2000–2015: An updated systematic analysis with implications for the Sustainable Development Goals. Lancet 2016, 388, 3027–3035. [Google Scholar] [CrossRef]
- Prayle, A.P.; Atkinson, M.; Smyth, A.R. Pneumonia in the developed world. Paediatr. Respir. Rev. 2011, 12, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Berg, A.S.; Inchley, C.S.; Aase, A.; Fjaerli, H.O.; Bull, R.; Aaberge, I.; Leegaard, T.M.; Nakstad, B. Etiology of pneumonia in a pediatric population with high pneumococcal vaccine coverage: A prospective study. Pediatrics Infect. Dis. J. 2016, 35, e69–e75. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.-D.; Jiang, R.; Chen, X.-J.; Ye, Q. Meteorological factors on the incidence of MP and RSV pneumonia in children. PLoS ONE 2017, 12, e0173409. [Google Scholar] [CrossRef] [PubMed]
- Du Prel, J.; Puppe, W.; Gröndahl, B.; Knuf, M.; Weigl, J.A.I.; Schaaff, F.; Schmitt, H. Are meteorological parameters associated with acute respiratory tract infections? Clin. Infect. Dis. 2009, 49, 861–868. [Google Scholar] [CrossRef]
- O’Brien, K.L.; Baggett, H.C.; Brooks, W.A.; Feikin, D.R.; Hammitt, L.L.; Higdon, M.M.; Howie, S.R.; Knoll, M.D.; Kotloff, K.L.; Levine, O.S.; et al. Causes of severe pneumonia requiring hospital admission in children without HIV infection from Africa and Asia: The PERCH multi-country case-control study. Lancet 2019, 394, 757–779. [Google Scholar] [CrossRef]
- Levine, O.S.; O’Brien, K.L.; Deloria-Knoll, M.; Murdoch, D.R.; Feikin, D.R.; DeLuca, A.N.; Driscoll, A.J.; Baggett, H.C.; Brooks, W.A.; Howie, S.R.C.; et al. The pneumonia etiology research for child health project: A 21st century childhood pneumonia etiology study. Clin. Infect. Dis. 2012, 54, S93–S101. [Google Scholar] [CrossRef]
- Scott, J.A.G.; Wonodi, C.; Moïsi, J.C.; Deloria-Knoll, M.; DeLuca, A.N.; Karron, R.A.; Bhat, N.; Murdoch, D.R.; Crawley, J.; Levine, O.S.; et al. The definition of pneumonia, the assessment of severity, and clinical standardization in the pneumonia etiology research for child health study. Clin. Infect. Dis. 2012, 54, S109–S116. [Google Scholar] [CrossRef]
- Knoll, M.D.; Feikin, D.R.; Scott, J.A.G.; O’Brien, K.L.; DeLuca, A.; Driscoll, A.J.; Levine, O.S.; The Pneumonia Methods Working Group. Identification and selection of cases and controls in the pneumonia etiology research for child health project. Clin. Infect. Dis. 2012, 54, S117–S123. [Google Scholar] [CrossRef]
- Higdon, M.M.; Le, T.; O’Brien, K.L.; Murdoch, D.R.; Prosperi, C.; Baggett, H.C.; Brooks, W.A.; Feikin, D.R.; Hammitt, L.L.; Howie, S.R.C.; et al. Association of C-reactive protein with bacterial and respiratory syncytial virus-associated pneumonia among children aged <5 years in the PERCH Study. Clin. Infect. Dis. 2017, 64, S378–S386. [Google Scholar] [PubMed]
- Bénet, T.; Picot, V.S.; Messaoudi, M.; Chou, M.; Eap, T.; Wang, J.; Shen, K.; Pape, J.-W.; Rouzier, V.; Awasthi, S.; et al. Microorganisms associated with pneumonia in children <5 years of age in developing and emerging countries: The GABRIEL Pneumonia Multicenter, prospective, case-control study. Clin. Infect. Dis. 2017, 65, 604–612. [Google Scholar] [PubMed]
- Bhuiyan, M.U.; Snelling, T.L.; West, R.; Lang, J.; Rahman, T.; Granland, C.; De Gier, C.; Borland, M.L.; Thornton, R.B.; Kirkham, L.-A.S.; et al. The contribution of viruses and bacteria to community-acquired pneumonia in vaccinated children: A case-control study. Thorax 2019, 74, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Nascimento-Carvalho, A.C.; Vilas-Boas, A.-L.; Fontoura, M.-S.H.; Vuorinen, T.; Nascimento-Carvalho, C.M.; PNEUMOPAC-Efficacy Study Group. Respiratory viruses among children with non-severe community-acquired pneumonia: A prospective cohort study. J. Clin. Virol. 2018, 105, 77–83. [Google Scholar] [CrossRef]
- Khuri-Bulos, N.; Lawrence, L.; Piya, B.; Wang, L.; Fonnesbeck, C.; Faouri, S.; Shehabi, A.; Vermund, S.H.; Williams, J.V.; Halasa, N.B. Severe outcomes associated with respiratory viruses in newborns and infants: A prospective viral surveillance study in Jordan. BMJ Open 2018, 8, e021898. [Google Scholar] [CrossRef]
- Romero-Espinoza, J.A.; Moreno-Valencia, Y.; Coronel-Tellez, R.H.; Torres-Espíndola, L.M.; Hernández, A.; Dominguez, A.; Miliar-García, A.; Barbachano-Guerrero, A.; Perez-Padilla, R.; Alejandre-Garcia, A.; et al. Virome and bacteriome characterization of children with pneumonia and asthma in Mexico City during winter seasons 2014 and 2015. PLoS ONE 2018, 13, e0192878. [Google Scholar] [CrossRef]
- Ching, N.S.; Kotsanas, D.; Easton, M.L.; Francis, M.J.; Korman, T.M.; Buttery, J.P. Respiratory virus detection and co-infection in children and adults in a large Australian hospital in 2009–2015. J. Paediatr. Child. Health 2018, 54, 1321–1328. [Google Scholar] [CrossRef]
- Esposito, S.; Piralla, A.; Zampiero, A.; Bianchini, S.; Di Pietro, G.; Scala, A.; Pinzani, R.; Fossali, E.; Baldanti, F.; Principi, N. Characteristics and their clinical relevance of respiratory syncytial virus types and genotypes circulating in northern Italy in five consecutive winter seasons. PLoS ONE 2015, 10, e0129369. [Google Scholar] [CrossRef]
- Kazakova, A.; Kakkola, L.; Päkkilä, H.; Teros-Jaakkola, T.; Soukka, T.; Peltola, V.; Waris, M.; Julkunen, I. Serological array-in-well multiplex assay reveals a high rate of respiratory virus infections and reinfections in young children. mSphere 2019, 4, e00447-19. [Google Scholar] [CrossRef]
- Hall, C.B.; Weinberg, G.A.; Iwane, M.K.; Blumkin, A.K.; Edwards, K.M.; Staat, M.A.; Auinger, P.; Griffin, M.R.; Poehling, K.A.; Erdman, D.; et al. The burden of respiratory syncytial virus infection in young children. N. Engl. J. Med. 2009, 360, 588–598. [Google Scholar] [CrossRef]
- Graham, B.S. Vaccine development for respiratory syncytial virus. Curr. Opin. Virol. 2017, 23, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Welliver, T.P.; Garofalo, R.P.; Hosakote, Y.; Hintz, K.H.; Avendano, L.; Sanchez, K.; Velozo, L.; Jafri, H.; Chavez-Bueno, S.; Ogra, P.L.; et al. Severe human lower respiratory tract illness caused by respiratory syncytial virus and influenza virus is characterized by the absence of pulmonary cytotoxic lymphocyte responses. J. Infect. Dis. 2007, 195, 1126–1136. [Google Scholar] [CrossRef] [PubMed]
- Villenave, R.; Thavagnanam, S.; Sarlang, S.; Parker, J.; Douglas, I.; Skibinski, G.; Heaney, L.G.; McKaigue, J.P.; Coyle, P.V.; Shields, M.D.; et al. In vitro modeling of respiratory syncytial virus infection of pediatric bronchial epithelium, the primary target of infection in vivo. Proc. Natl. Acad. Sci. USA 2012, 109, 5040–5045. [Google Scholar] [CrossRef] [PubMed]
- Teng, M.N.; Whitehead, S.S.; Collins, P.L. Contribution of the respiratory syncytial virus g glycoprotein and its secreted and membrane-bound forms to virus replication in vitro and in vivo. Virology 2001, 289, 283–296. [Google Scholar] [CrossRef]
- Lay, M.K.; González, P.A.; León, M.A.; Céspedes, P.F.; Bueno, S.M.; Riedel, C.A.; Kalergis, A.M. Advances in understanding respiratory syncytial virus infection in airway epithelial cells and consequential effects on the immune response. Microbes Infect. 2013, 15, 230–242. [Google Scholar] [CrossRef]
- Rossi, G.A.; Colin, A.A. Infantile respiratory syncytial virus and human rhinovirus infections: Respective role in inception and persistence of wheezing. Eur. Respir. J. 2014, 45, 774–789. [Google Scholar] [CrossRef]
- Guo-Parke, H.; Canning, P.; Douglas, I.; Villenave, R.; Heaney, L.G.; Coyle, P.V.; Lyons, J.D.; Shields, M.D.; Power, U.F. Relative respiratory syncytial virus cytopathogenesis in upper and lower respiratory tract epithelium. Am. J. Respir. Crit. Care Med. 2013, 188, 842–851. [Google Scholar] [CrossRef]
- Legg, J.P.; Hussain, I.R.; Warner, J.A.; Johnston, S.L.; Warner, J.O. Type 1 and Type 2 cytokine imbalance in acute respiratory syncytial virus bronchiolitis. Am. J. Respir. Crit. Care Med. 2003, 168, 633–639. [Google Scholar] [CrossRef]
- Johnson, J.E.; A Gonzales, R.; Olson, S.J.; Wright, P.F.; Graham, B.S. The histopathology of fatal untreated human respiratory syncytial virus infection. Mod. Pathol. 2007, 20, 108–119. [Google Scholar] [CrossRef]
- Noor, A.; Krilov, L.R. Respiratory syncytial virus vaccine: Where are we now and what comes next? Expert Opin. Biol. Ther. 2018, 18, 1247–1256. [Google Scholar] [CrossRef]
- González-Parra, G.; Dobrovolny, H.M. A quantitative assessment of dynamical differences of RSV infections in vitro and in vivo. Virology 2018, 523, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Roe, M.; O’Donnell, D.; Callif, C.G. Respiratory viruses in the intensive care unit. Paediatr. Respir. Rev. 2003, 4, 166–171. [Google Scholar] [CrossRef]
- Cherian, T.; Mulholland, E.K.; Carlin, J.B.; Ostensen, H.; Amin, R.; De Campo, M.; Greenberg, D.; Lagos, R.; Lucero, M.; Madhi, S.A.; et al. Standardized interpretation of paediatric chest radiographs for the diagnosis of pneumonia in epidemiological studies. Bull. World Health Organ. 2005, 83, 353–359. [Google Scholar] [PubMed]
- Fancourt, N.; Knoll, M.D.; Barger-Kamate, B.; De Campo, J.; De Campo, M.; Diallo, M.; Ebruke, B.E.; Feikin, D.R.; Gleeson, F.; Gong, W.; et al. standardized interpretation of chest radiographs in cases of pediatric pneumonia from the PERCH study. Clin. Infect. Dis. 2017, 64, S253–S261. [Google Scholar] [CrossRef] [PubMed]
- Esposito, S.; Mencacci, A.; Cenci, E.; Camilloni, B.; Silvestri, E.; Principi, N. Multiplex platforms for the identification of respiratory pathogens: Are they useful in pediatric clinical practice? Front. Cell. Infect. Microbiol. 2019, 9, 196. [Google Scholar] [CrossRef]
- Rodriguez-Fernandez, R.; Tapia, L.I.; Yang, C.-F.; Torres, J.P.; Chavez-Bueno, S.; Garcia, C.; Jaramillo, L.M.; Moore-Clingenpeel, M.; Jafri, H.S.; Peeples, M.E.; et al. Respiratory syncytial virus genotypes, host immune profiles, and disease severity in young children hospitalized with bronchiolitis. J. Infect. Dis. 2018, 217, 24–34. [Google Scholar] [CrossRef]
- Shi, T.; Balsells, E.; Wastnedge, E.; Singleton, R.; Rasmussen, Z.A.; Zar, H.J.; Rath, B.A.; Madhi, S.A.; Campbell, S.; Vaccari, L.C.; et al. Risk factors for respiratory syncytial virus associated with acute lower respiratory infection in children under five years: Systematic review and meta–analysis. J. Glob. Health 2015, 5, 020416. [Google Scholar] [CrossRef]
- Ravindranath, T.M.; Gomez, A.; Harwayne-Gidansky, I.; Connors, T.J.; Neill, N.; Levin, B.; Howell, J.D.; Saiman, L.; Baird, J.S. Pediatric acute respiratory distress syndrome associated with human metapneumovirus and respiratory syncytial virus. Pediatrics Pulmonol. 2018, 53, 929–935. [Google Scholar] [CrossRef]
- Agoti, C.N.; Mwihuri, A.G.; Sande, C.J.; Onyango, C.O.; Medley, G.F.; Cane, P.A.; Nokes, D.J. Genetic relatedness of infecting and reinfecting respiratory syncytial virus strains identified in a birth cohort from rural Kenya. J. Infect. Dis. 2012, 206, 1532–1541. [Google Scholar] [CrossRef]
- Openshaw, P.J.; Chiu, C.; Culley, F.J.; Johansson, C. Protective and harmful immunity to RSV infection. Annu. Rev. Immunol. 2017, 35, 501–532. [Google Scholar] [CrossRef]
- Netea, M.G.; Joosten, L.A.B.; Latz, E.; Mills, K.H.G.; Natoli, G.; Stunnenberg, H.G.; O’Neill, L.A.J.; Xavier, R.J. Trained immunity: A program of innate immune memory in health and disease. Science 2016, 352, aaf1098. [Google Scholar] [CrossRef] [PubMed]
- Kusel, M.M.; Kebadze, T.; Johnston, S.L.; Holt, P.G.; Sly, P.D. Febrile respiratory illnesses in infancy and atopy are risk factors for persistent asthma and wheeze. Eur. Respir. J. 2011, 39, 876–882. [Google Scholar] [CrossRef]
- Coverstone, A.M.; Wang, L.; Sumino, K. Beyond respiratory syncytial virus and rhinovirus in the pathogenesis and exacerbation of asthma: The role of metapneumovirus, bocavirus and influenza virus. Immunol. Allergy Clin. N. Am. 2019, 39, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Bacharier, L.B.; Cohen, R.; Schweiger, T.; Yin-DeClue, H.; Christie, C.; Zheng, J.; Schechtman, K.B.; Strunk, R.C.; Castro, M. Determinants of asthma after severe respiratory syncytial virus bronchiolitis. J. Allergy Clin. Immunol. 2012, 130, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Sigurs, N.; AlJassim, F.; Kjellman, B.; Robinson, P.D.; Sigurbergsson, F.; Bjarnason, R.; Gustafsson, P.M. Asthma and allergy patterns over 18 years after severe RSV bronchiolitis in the first year of life. Thorax 2010, 65, 1045–1052. [Google Scholar] [CrossRef]
- Mohapatra, S.S.; Boyapalle, S. Epidemiologic, experimental, and clinical links between respiratory syncytial virus infection and asthma. Clin. Microbiol. Rev. 2008, 21, 495–504. [Google Scholar] [CrossRef]
- Daley, D.; Park, J.E.; He, J.-Q.; Yan, J.; Akhabir, L.; Stefanowicz, D.; Becker, A.; Chan-Yeung, M.; Bossé, Y.; Kozyrskyj, A.L.; et al. Associations and interactions of genetic polymorphisms in innate immunity genes with early viral infections and susceptibility to asthma and asthma-related phenotypes. J. Allergy Clin. Immunol. 2012, 130, 1284–1293. [Google Scholar] [CrossRef]
- James, K.M.; Peebles, R.S., Jr.; Hartert, T.V. Response to infections in patients with asthma and atopic disease: An epiphenomenon or reflection of host susceptibility? J. Allergy Clin. Immunol. 2012, 130, 343–351. [Google Scholar] [CrossRef]
- Ferronato, Â.E.; Gilio, A.E.; Ferraro, A.A.; De Paulis, M.; Vieira, S.E. Etiological diagnosis reduces the use of antibiotics in infants with bronchiolitis. Clinics 2012, 67, 1007–1011. [Google Scholar] [CrossRef]
- Hogan, C.A.; Caya, C.; Papenburg, J. Rapid and simple molecular tests for the detection of respiratory syncytial virus: A review. Expert Rev. Mol. Diagn. 2018, 18, 617–629. [Google Scholar] [CrossRef]
- Mahony, J.B.; Blackhouse, G.; Babwah, J.; Smieja, M.; Buracond, S.; Chong, S.; Ciccotelli, W.; O’Shea, T.; Alnakhli, D.; Griffiths-Turner, M.; et al. Cost analysis of multiplex PCR testing for diagnosing respiratory virus infections. J. Clin. Microbiol. 2009, 47, 2812–2817. [Google Scholar] [CrossRef] [PubMed]
- Chartrand, C.; Tremblay, N.; Renaud, C.; Papenburg, J. Diagnostic accuracy of rapid antigen detection tests for respiratory syncytial virus infection: Systematic review and meta-analysis. J. Clin. Microbiol. 2015, 53, 3738–3749. [Google Scholar] [CrossRef] [PubMed]
- Papenburg, J.; Buckeridge, D.L.; De Serres, G.; Boivin, G. Host and viral factors affecting clinical performance of a rapid diagnostic test for respiratory syncytial virus in hospitalized children. J. Pediatrics 2013, 163, 911–913. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.; Koo, S.H.; Jiang, B.; Lim, P.Q.; Tan, T.-Y. Comparison of the biofire filmarray respiratory panel, seegene AnyplexII RV16, and Argene for the detection of respiratory viruses. J. Clin. Virol. 2018, 106, 13–17. [Google Scholar] [CrossRef]
- Beckmann, C.; Hirsch, H.H. Comparing luminex NxTAG-respiratory pathogen panel and respifinder-22 for multiplex detection of respiratory pathogens. J. Med. Virol. 2016, 88, 1319–1324. [Google Scholar] [CrossRef]
- Moe, N.; Pedersen, B.; Nordbø, S.A.; Skanke, L.H.; Krokstad, S.; Smyrnaios, A.; Døllner, H. Respiratory virus detection and clinical diagnosis in children attending day care. PLoS ONE 2016, 11, e0159196. [Google Scholar] [CrossRef]
- Turi, K.N.; Romick-Rosendale, L.; Gebretsadik, T.; Watanabe, M.; Brunwasser, S.; Anderson, L.J.; Moore, M.L.; Larkin, E.K.; Peebles, R.S.; Hartert, T.V. Using urine metabolomics to understand the pathogenesis of infant respiratory syncytial virus (RSV) infection and its role in childhood wheezing. Metabolomics 2018, 14, 135. [Google Scholar] [CrossRef]
- Barr, R.; Green, C.A.; Sande, C.J.; Drysdale, S.B. Respiratory syncytial virus: Diagnosis, prevention and management. Ther. Adv. Infect. Dis. 2019, 6. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence. Bronchiolitis in Children: Diagnosis and Management. NICE Guideline [NG9]. 2015. Available online: https://www.nice.org.uk/guidance/NG9 (accessed on 7 September 2020).
- Xing, Y.; Proesmans, M. New therapies for acute RSV infections: Where are we? Eur. J. Pediatrics 2019, 178, 131–138. [Google Scholar] [CrossRef]
- Simões, E.A.F.; DeVincenzo, J.P.; Boeckh, M.; Bont, L.; Crowe, J.E.; Griffiths, P.; Hayden, F.G.; Hodinka, R.L.; Smyth, R.L.; Spencer, K.; et al. Challenges and opportunities in developing respiratory syncytial virus therapeutics. J. Infect. Dis. 2015, 211, S1–S20. [Google Scholar] [CrossRef]
- Jorquera, P.A.; Tripp, R.A. Respiratory syncytial virus: Prospects for new and emerging therapeutics. Expert Rev. Respir. Med. 2017, 11, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Heylen, E.; Neyts, J.; Jochmans, D. Drug candidates and model systems in respiratory syncytial virus antiviral drug discovery. Biochem. Pharmacol. 2017, 127, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ke, Z.; Dillard, R.S.; Chirkova, T.; Leon, F.; Stobart, C.C.; Hampton, C.M.; Strauss, J.D.; Rajan, D.; Rostad, C.A.; Taylor, J.V.; et al. The morphology and assembly of respiratory syncytial virus revealed by cryo-electron tomography. Viruses 2018, 10, 446. [Google Scholar] [CrossRef] [PubMed]
- Graham, B.S.; Modjarrad, K.; McLellan, J.S. Novel antigens for RSV vaccines. Curr. Opin. Immunol. 2015, 35, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Ralston, S.L.; Lieberthal, A.S.; Meissner, H.C.; Alverson, B.K.; Baley, J.E.; Gadomski, A.M.; Johnson, D.W.; Light, M.J.; Maraqa, N.F.; Mendonca, E.A.; et al. Clinical practice guideline: The diagnosis, management, and prevention of bronchiolitis. Pediatrics 2014, 134, e1474–e1502. [Google Scholar] [CrossRef] [PubMed]
- Walpert, A.S.; Thomas, I.D.; Lowe, M.C., Jr.; Seckeler, M.D. RSV prophylaxis guideline changes and outcomes in children with congenital heart disease. Congenit. Heart Dis. 2018, 13, 428–431. [Google Scholar] [CrossRef] [PubMed]
- Blake, S.M.; Tanaka, D.; Bendz, L.M.; Staebler, S.; Brandon, D. Evaluation of the financial and health burden of infants at risk for respiratory syncytial virus. Adv. Neonatal Care 2017, 17, 292–298. [Google Scholar] [CrossRef]
- Ambrose, C.S.; Chen, X.; Kumar, V.R. A population-weighted, condition-adjusted estimate of palivizumab efficacy in preventing RSV-related hospitalizations among US high-risk children. Hum. Vaccines Immunother. 2014, 10, 2785–2788. [Google Scholar] [CrossRef]
- Domachowske, J.B.; Khan, A.A.; Esser, M.T.; Jensen, K.; Takas, T.; Villafana, T.; Dubovsky, F.; Griffin, M.P. Safety, Tolerability and Pharmacokinetics of MEDI8897, an Extended Half-life Single-dose Respiratory Syncytial Virus Prefusion F-targeting Monoclonal Antibody Administered as a Single Dose to Healthy Preterm Infants. Pediatrics Infect. Dis. J. 2018, 37, 886–892. [Google Scholar] [CrossRef]
- Bianchini, S.; Argentiero, A.; Camilloni, B.; Silvestri, E.; Alunno, A.; Esposito, S. Vaccination against paediatric respiratory pathogens. Vaccines 2019, 7, 168. [Google Scholar] [CrossRef]
- Esposito, S.; Scarselli, E.; Lelii, M.; Scala, A.; Vitelli, A.; Capone, S.; Fornili, M.; Biganzoli, E.; Orenti, A.; Nicosia, A.; et al. Antibody response to respiratory syncytial virus infection in children <18 months old. Hum. Vaccines Immunother. 2016, 12, 1700–1706. [Google Scholar]
- Rostad, C.A.; Stobart, C.C.; Gilbert, B.E.; Pickles, R.J.; Hotard, A.L.; Meng, J.; Blanco, J.C.G.; Moin, S.M.; Graham, B.S.; Piedra, P.A.; et al. A recombinant respiratory syncytial virus vaccine candidate attenuated by a low-fusion F protein is immunogenic and protective against challenge in cotton rats. J. Virol. 2016, 90, 7508–7518. [Google Scholar] [CrossRef]
- Melero, J.A.; Mas, V.; McLellan, J.S. Structural, antigenic and immunogenic features of respiratory syncytial virus glycoproteins relevant for vaccine development. Vaccine 2017, 35, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Mazur, N.I.; Higgins, D.; Nunes, M.C.; Melero, J.A.; Langedijk, A.C.; Horsley, N.; Buchholz, U.J.; Openshaw, P.J.; McLellan, J.S.; A Englund, J.; et al. The respiratory syncytial virus vaccine landscape: Lessons from the graveyard and promising candidates. Lancet Infect. Dis. 2018, 18, e295–e311. [Google Scholar] [CrossRef]
- Crank, M.C.; Ruckwardt, T.J.; Chen, M.; Morabito, K.M.; Phung, E.; Costner, P.J.; Holman, L.A.; Hickman, S.P.; Berkowitz, N.M.; Gordon, I.J.; et al. A proof of concept for structure-based vaccine design targeting RSV in humans. Science 2019, 365, 505–509. [Google Scholar] [CrossRef]
- Giersing, B.K.; Modjarrad, K.; Kaslow, D.C.; Moorthy, V.S.; WHO Product Development for Vaccines Advisory Committee; WHO Product Development for Vaccines Product Development Advisory Committee. Report from the World Health Organization’s Product Development for Vaccines Advisory Committee (PDVAC) meeting, Geneva, 7–9th September 2015. Vaccine 2016, 34, 2865–2869. [Google Scholar] [CrossRef]
- Abu-Raya, B.; Maertens, K.; Edwards, K.M.; Omer, S.B.; Englund, J.A.; Flanagan, K.L.; Snape, M.D.; Amirthalingam, G.; Leuridan, E.; Van Damme, P.; et al. Global perspectives on immunization during pregnancy and priorities for future research and development: An international consensus statement. Front. Immunol. 2020, 11, 1282. [Google Scholar] [CrossRef]
- Esposito, S.; Principi, N. Strategies to develop vaccines of pediatric interest. Expert Rev. Vaccines 2017, 175–186. [Google Scholar] [CrossRef]
- Chirkova, T.; Lin, S.; Oomens, A.G.P.; Gaston, K.A.; Boyoglu-Barnum, S.; Meng, J.; Stobart, C.C.; Cotton, C.U.; Hartert, T.V.; Moore, M.L.; et al. CX3CR1 is an important surface molecule for respiratory syncytial virus infection in human airway epithelial cells. J. Gen. Virol. 2015, 96, 2543–2556. [Google Scholar] [CrossRef]
- Marr, N.; Turvey, S.E. Role of human TLR4 in respiratory syncytial virus-induced NF-κB activation, viral entry and replication. Innate Immun. 2012, 18, 856–865. [Google Scholar] [CrossRef]
- Tayyari, F.; Marchant, D.R.; Moraes, T.J.; Duan, W.; Mastrangelo, P.; Hegele, R.G. Identification of nucleolin as a cellular receptor for human respiratory syncytial virus. Nat. Med. 2011, 17, 1132–1135. [Google Scholar] [CrossRef] [PubMed]
- Clinical Trials Gov. RSV Infection. Available online: https://clinicaltrials.gov/ct2/results?cond=RSV+Infection&Search=Apply&recrs=a&age_v=&age=0&gndr=&type=&rslt= (accessed on 7 September 2020).
| Test | Turnaround Time | Sensitivity | Specificity | Note |
|---|---|---|---|---|
| Cell culture | Days | Low | Excellent | Traditional gold standard; long time for results |
| RT-PCR | Hours | Excellent | Excellent | New gold standard; expensive; needs expertise; positive results may not indicate active infection; possibility of multiplex tests |
| DFAs | <1 h | Low | Low | Needs expertise; rapid results |
| RADTs | Minutes | Low | Variable | Traditional tests depend on operator skills; newer platforms have better sensitivity/specificity |
| Novel rapid molecular diagnostic assays | Minutes–few hours | Excellent | Good | Variability; not detection of RSV types; qualitative but not quantitative results |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bianchini, S.; Silvestri, E.; Argentiero, A.; Fainardi, V.; Pisi, G.; Esposito, S. Role of Respiratory Syncytial Virus in Pediatric Pneumonia. Microorganisms 2020, 8, 2048. https://doi.org/10.3390/microorganisms8122048
Bianchini S, Silvestri E, Argentiero A, Fainardi V, Pisi G, Esposito S. Role of Respiratory Syncytial Virus in Pediatric Pneumonia. Microorganisms. 2020; 8(12):2048. https://doi.org/10.3390/microorganisms8122048
Chicago/Turabian StyleBianchini, Sonia, Ettore Silvestri, Alberto Argentiero, Valentina Fainardi, Giovanna Pisi, and Susanna Esposito. 2020. "Role of Respiratory Syncytial Virus in Pediatric Pneumonia" Microorganisms 8, no. 12: 2048. https://doi.org/10.3390/microorganisms8122048
APA StyleBianchini, S., Silvestri, E., Argentiero, A., Fainardi, V., Pisi, G., & Esposito, S. (2020). Role of Respiratory Syncytial Virus in Pediatric Pneumonia. Microorganisms, 8(12), 2048. https://doi.org/10.3390/microorganisms8122048





