Abstract
Bacterial stress responses are closely associated with the survival and colonization of anaerobes in the human gut. Mesosutterella multiformis JCM 32464T is a novel member of the family Sutterellaceae, an asaccharolytic bacterium. We previously demonstrated energy generation via heme biosynthesis, which is coupled with nitrate reductase. Here, physiological and morphological changes in M. multiformis induced by exposure to nitrate were investigated. The ability of M. multiformis to reduce nitrate was determined using a colorimetric assay. A unique morphology was observed during nitrate reduction under anaerobic conditions. The association between nitrate concentration and cell size or cellular fatty acid composition was evaluated. Nitrate-induced responses of M. multiformis were compared to those of related species. An increase in cellular filamentation and the ratio of saturated: unsaturated fatty acids was mediated specifically by nitrate. This indicates a decrease in cell fluidity and low leakage. Furthermore, a similar response was not observed in other related species cultured in the presence of nitrate. Hence, the nitrate-induced stress response in new anaerobes such as M. multiformis was demonstrated. The response could also be involved in the conservation of menaquinones and the maximization of nitrate reduction.
1. Introduction
For a variety of microorganisms, it is important to survive in the human gut, a highly complex ecosystem. They may frequently encounter antibiotics or other potentially toxic components secreted by surrounding organisms in nutrient-deficient or energy-limited environments. Many reports have focused on using lactic acid bacteria as starter cultures for the production of fermented food and probiotics, or saccharolytic bacteria acting as foodborne pathogens and causing clinical infections [1]. Bacterial stress responses are diverse and are mediated by various factors (including pH, temperature, osmotic pressure, alcohol, starvation, and antibiotics) in unfavorable environments. This indicates that damage to cellular integrity, cell membrane composition, viability, as well as disruption of gene regulation leads to changes in their phenotypes [2]. With respect to morphological changes, sporulation influences the thickness of the cell wall and cell size. The stress response of Gram-negative bacteria is associated with the integrity of the cell envelope and bacterial toxicity [3]. Understanding the bacterial SOS response in a stress-inducing environment could be the key to revealing their physiological functions and the survival strategy adapted to colonize the human gut.
Mesosutterella multiformis, an asaccharolytic, obligately anaerobic, and Gram-negative bacterium, was isolated from the fecal samples of healthy Japanese individuals [4]. This species is a novel member of the family Sutterellaceae. Based on phylogenetic analyses, it is located between the genera Parasutterella and Sutterella. Sutterellaceae species are anaerobic or microaerophilic and their role in the human gut remains unclear. However, an association between Sutterella species and gastrointestinal disturbances in children with autism [5] or mild pro-inflammatory capacity in the human gastrointestinal tract [6] has been demonstrated. An association between Parasutterella species and irritable bowel syndrome or intestinal chronic inflammation has also been reported [7]. These species are present in abundance in the healthy human colon.
Recently, we demonstrated energy production in M. multiformis mediated by the proton motive force via the heme biosynthesis pathway [8]. In this pathway, the activity of nitrate reductase serves as the key component [9]. The nitrate metabolism of gut bacteria is important in elucidating their symbiotic properties [10].
In this study, we investigated the physiological and morphological changes in M. multiformis under stress conditions in vitro and compared such alterations with related species. To our knowledge, no reports have been published on the stress response of Sutterellaceae species isolated from human feces. Our findings reveal one of the stress responses of asaccharolytic bacteria such as M. multiformis that are difficult to cultivate owing to the unknown metabolic requirements for growth. This could complement the description of the metabolism of M. multiformis provided in our previous report.
2. Materials and Methods
2.1. Bacterial Strains and Growth Conditions
M. multiformis JCM 32464T, Sutterella megalosphaeroides JCM 32470T, Sutterella parvirubra JCM 14724T, Sutterella stercoricanis JCM 32441T, Sutterella wadsworthensis JCM 32440T, Parasutterella excrementihominis JCM 15078T, and Parasutterella secunda JCM 16078T were obtained from the Japan Collection of Microorganisms (JCM), RIKEN BioResource Research Center, Tsukuba, Japan. All strains were maintained on Brucella blood agar supplemented with hemin and menadione (BB; JCM Medium No. 677) for 2–4 days at 37 °C under a H2/CO2/N2 (1:1:8, by vol.) gas mixture. As M. multiformis cannot grow in a liquid medium, TSA plates were used as a solid medium to facilitate growth. In addition, potassium nitrate (KNO3; nitrate) or sodium nitrite (NaNO2; nitrite) were added to the media as required.
2.2. Determination of Nitrite Concentrations
The strain was plated on TSA containing different concentrations of nitrate (0, 0.1, 0.5, 1, 10, and 50 mM) to examine nitrate reduction ability. Nitrite concentrations were determined using a colorimetric assay [11]. The reagents comprised 1 part 0.4% sulfanilic acid plus 30% of acetic acid mixed with H2O and 1 part 0.6% N,N-dimethyl-1-naphthylamine plus 30% of acetic acid mixed with H2O. The bacterial colonies grown on the TSA were collected with a cotton swab after 3, 6, and 10 days of incubation and suspended in 1 mL of distilled water. The optical density of these suspensions was measured at 660 nm (OD660) using an Ultrospec 2100 pro spectrophotometer (Amersham Biosciences, Piscataway, NJ, USA) and adjusted to approximately 0.1. A total of 1 mL of cell suspension was mixed with 20 µL of each reagent and incubated for 1 h at room temperature. The absorbance was measured at 540 nm using a spectrophotometer, and distilled water with reagents was used as a blank. Nitrite concentrations were calculated from a calibration curve that was constructed using several known concentrations of the nitrite solution.
2.3. Evaluation of Cell Morphology
To observe cellular shape during nitrate respiration, M. multiformis JCM 32464T was grown on TSA supplemented with additional nitrate or nitrite as required and on a BB plate for Gram staining. The cells were observed using light microscopy (BIOPHOT, Nikon, Tokyo, Japan). The open-software platform ImageJ (https://imagej.nih.gov/ij/) was used to measure the cell length by direct counts of more than 300 bacterial cells and to compare the dispersion of filamentous cells for each treatment. Subsequently, the morphology of cells was observed after six days of culture on TSA supplemented with 10 mM nitrate using scanning electron microscopy (SEM; JEM-6340F; JEOL, Tokyo, Japan) and transmission electron microscopy (TEM; JEM-1400plus; JEOL, Tokyo, Japan). Sample preparation for SEM and TEM has been described previously [12].
2.4. Effect of Nitrate on the Composition of Cellular Fatty Acids
We next determined the differences in the composition of cellular fatty acids and morphological changes. Fatty acid methyl esters (FAMEs) were obtained from approximately 40 mg of wet cells grown on TSA without nitrate or with 1, 10, and 50 mM of nitrate at 37 °C for 6–8 days by saponification, methylation, and extraction with minor modifications [13] using the method described by Millar [14]. The composition of cellular fatty acid was determined using version 6.2B of the Sherlock Microbial Identification System (MIDI) and version 3.80 of the BHIBLA database.
2.5. Evaluation of the Ability of Other Sutterellaceae Species to Form Filaments
Other reference strains were evaluated for their ability to form filaments on TSA in the presence of nitrate or nitrite. Aliquots of the culture were observed after four days of incubation using light microscopy to determine whether filaments were formed. Cultivation of all strains was performed under the same conditions.
2.6. Genome Analysis
The genome data of S. megalosphaeroides JCM 32470T, S. wadsworthensis 2_1_59BFAA, S. parvirubra YIT 11816T, and P. excrementihominis YIT 11859T were obtained from the NCBI database. S. stercoricanis and P. secunda were excluded from several comparisons owing to the unavailability of genome data in the database. The genes were analyzed by using the Kyoto Encyclopedia of Genes and Genomes (KEGG; release 63.0) [15], Rapid Annotations using Subsystem Technology (RAST; v. 2.0) [16], and the DDBJ Fast Annotation and Submission Tool (DFAST; v. 1.0.2) [17]. Default settings were used.
2.7. Statistical Analyses
Statistical differences and significance were assessed using the ANOVA test. Student’s t-test and post hoc Bonferroni test were used to analyze paired data, and the p-values were obtained. To determined significances in nitrite concentration among each treatment, Tukey’s HSD (honestly significant difference) test was performed.
3. Results
3.1. Effect of Nitrate Concentration on Ability of Nitrate Reduction
The ability of M. multiformis JCM 32464T to reduce nitrate was evaluated using a colorimetric assay. M. multiformis JCM 32464T was able to reduce nitrate to nitrite. The results are in accordance with the prediction of the presence of a nitrate reductase gene based on genome analysis [8]. M. multiformis JCM 32464T was incubated on TSA with different nitrate concentrations (0, 0.1, 0.5, 1, 10, and 50 mM). The detection of a significant nitrite concentration was observed with 10 mM of nitrate (Figure 1), and 122.89 µM nitrite concentration was produced after 10 days. In contrast, cultures with 50 mM nitrate resulted in the production of up to 59.18 µM nitrite, indicating the inhibition of nitrate reduction.
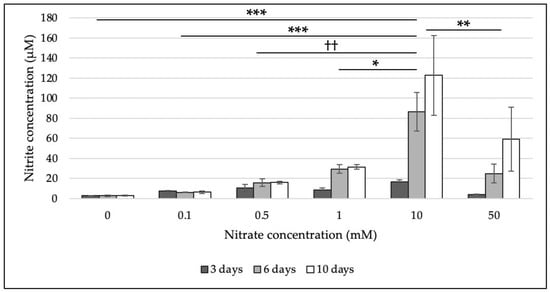
Figure 1.
Determination of nitrite concentration using a colorimetric assay. The bacterial cells grown on TSA supplemented with different nitrate concentrations (0, 0.1, 0.5, 1, 10, and 50 mM) after 3, 6, and 10 days of incubation were used. Nitrite concentrations (µM) were determined by measuring the absorption at 540 nm. Experiments were performed in triplicates. Error bars represent the standard deviation between biological replicates. The statistical significances (p < 0.05) among different concentrations of nitrate that were determined by Turkey’s HSD test are shown. ***, significant differences in all incubation stages; **, significant differences in 3 and 6 days; ††, significant differences in 6 and 10 days; *, significant difference in 6 days.
3.2. Effect of Nitrate on Cell Morphology of M. multiformis
The colonies of M. multiformis JCM 32464T cultured on TSA in the presence of nitrate differed from those cultured on TSA without nitrate. Although the total number of colonies was decreased, the formation of colony variants increased. The colonies were larger (0.1–1 mm) than the regular size (0.1–0.2 mm). After long-term incubation with nitrate, the colonies produced a small amount of brown pigment (Figure 2c).
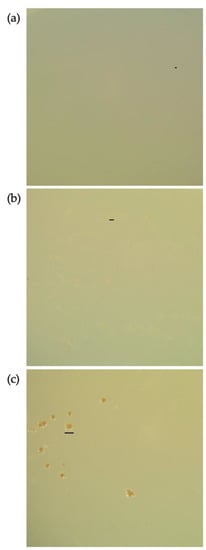
Figure 2.
Pigment production by Mesosutterella multiformis in the presence of nitrate. The strain was cultured on TSA for 15 days at 37 °C under a H2/CO2/N2 (1:1:8, by vol.) gas mixture. (a) Culture in the absence of nitrate, (b) culture in the presence of 1 mM of nitrate, and (c) culture in the presence of 50 mM of nitrate. Bars, 0.2 mm (a), 0.5 mm (b), or 1 mm (c).
The addition of nitrate significantly increased the elongation of M. multiformis JCM 32464T cells compared to the bacterial colonies cultured on TSA in the absence of nitrate (Figure 3). Compared to 1.7–2.1 µm of regular cell length, the length of the filamentous cells was approximately 80 µm. Cell filamentation was frequently observed with 10 mM of nitrate concentration, and the range of cell lengths was higher at 50 mM of nitrate concentrations compared to that observed at all other concentrations. Filamentous cells were not observed when cultured on BB and TSA in the absence of nitrate with no change in cellular morphology.
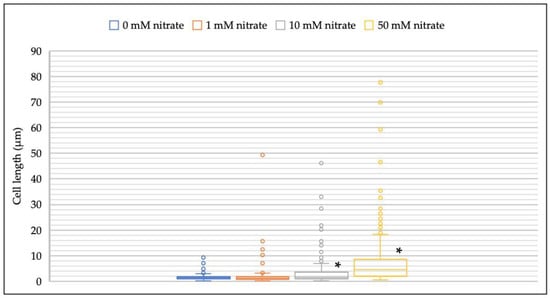
Figure 3.
Distribution of cell lengths of M. multiformis at different nitrate concentrations. The strain was grown on TSA supplemented with 0–50 mM of nitrate. More than 300 cells of M. multiformis were selected and analyzed directly using ImageJ. * indicates significant differences (p < 0.05) compared with control (0 mM nitrate) by Student’s t-test and post hoc Bonferroni test.
To evaluate whether nitrite plays a role in filament formation, nitrite was added to the TSA. The addition of 100 mM nitrite to TSA did not have a significant effect on the morphology of M. multiformis JCM 32464T (Figure 4). Notably, when filamentous cells of M. multiformis JCM 32464T were transferred to fresh BB plates, the cell size was recovered, and a size similar to regular cells was observed.
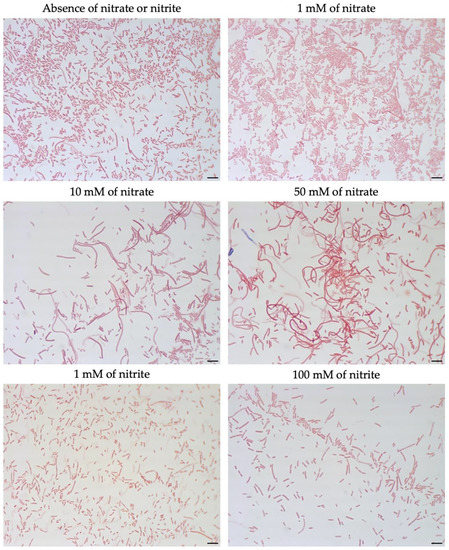
Figure 4.
Morphology of M. multiformis cells grown on TSA in the presence of different concentrations of nitrate and nitrite detected by Gram-staining. Concentrations of 1, 10, and 50 mM of nitrate and 1 and 100 mM of nitrite were added on TSA. The strain was grown on TSA for 4 days at 37 °C under anaerobic conditions. Bars, 10 µm.
Scanning and transmission electron micrographs are shown in Figure 5. Intracellular shading was observed by TEM images. Moreover, entangled masses of cells were also observed sporadically. These results suggest that these morphological changes are caused by nitrate.
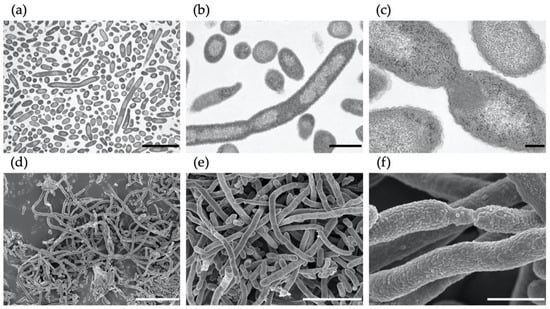
Figure 5.
Electron micrographs of M. multiformis cells cultured with 10 mM nitrate after 6 days of incubation. Images were obtained using transmission electron microscopy (a–c) and scanning electron microscopy (d–f). Bars, 5 µm (a,e), 1 µm (b,f), 200 nm (c), or 10 µm (d).
3.3. Effect of Nitrate-Induced Filamentation on the Composition of Cellular Fatty Acids
The differences in cellular fatty acid composition are shown in Table 1. C16:0, summed feature 10 (C18:1c11/t9/t6 and/or unknown 17.834), C14:0, and C16:1ω7c were the major cellular fatty acids (>10%) of M. multiformis JCM 32464T cultured on TSA in the presence of nitrate. Interestingly, with 10 mM of nitrate, the amount of C14:0 observed was higher than that observed with other treatments (2.3 to 23.0% compared to the control). As expected, this pattern was different from the profiles of M. multiformis JCM 32464T cultured without nitrate. Instead of the increase in the amount of C16:0 (32.8% to 48.5%), the amount of summed feature 10 decreased from 44.1% to 18.1% upon the addition of 50 mM of nitrate. Furthermore, the ratio of saturated fatty acid to unsaturated fatty acid (S/U ratio) significantly increased with the addition of 10 or 50 mM nitrate (Figure 6). These results indicated that the fluidity of cell membranes was decreased [18]. However, a lower S/U ratio was observed when 1 mM of nitrate was used as a relative control.

Table 1.
Differential compositions of cellular fatty acids of M. multiformis cultured with or without nitrate.
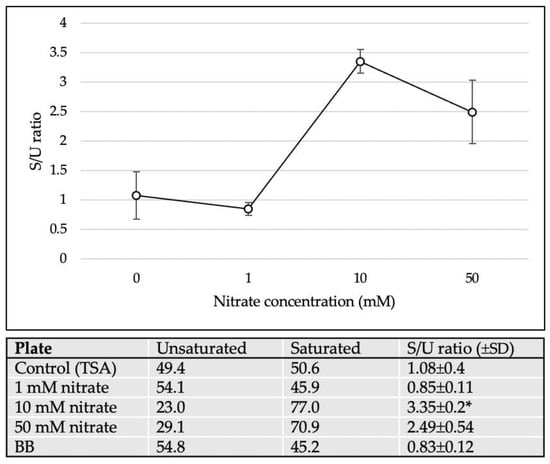
Figure 6.
The S/U ratio of M. multiformis cultured with different nitrate concentrations. The saturated fatty acids, namely, C9:0, C12:0, C14:0, C16:0, C18:0, C16:0 ALDE, C16:0 DMA and summed feature 5 (C15:0 DMA and/or unknown 17.834); the unsaturated fatty acids, namely, C16:1ω7c and summed feature 10 (C18:1c11/t9/t6 and/or unknown 17.834). All values and error bars represent the mean ± standard deviation of three experiments. * indicates significant differences (p < 0.05) compared with control (0 mM nitrate) by Student’s t-test and post hoc Bonferroni test.
3.4. Effect of Nitrate or Nitrite on Other Sutterellaceae Species
To compare filamentation ability, other Sutterellaceae species were exposed to nitrate or nitrite. No similar elongation was observed in the presence of 50 mM nitrate with other reference strains. However, in the presence of 1 mM of nitrite, S. megalosphaeroides JCM 32470T and P. excrementihominis JCM 15078T cells demonstrated swelling and burst-like morphology (Figure 7). The swelling cells of P. excrementihominis JCM 15078T showed 2.0–2.6 µm (nitrite) from 0.8 to 1 µm (absence of nitrate or nitrite) and S. megalosphaeroides JCM 32470T with nitrite showed 3.0–8.7 µm from 1 to 2 µm (absence of nitrate or nitrite).
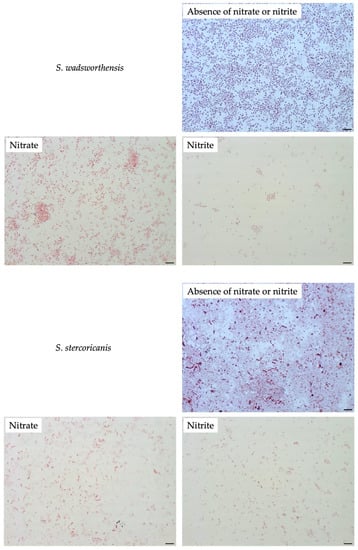
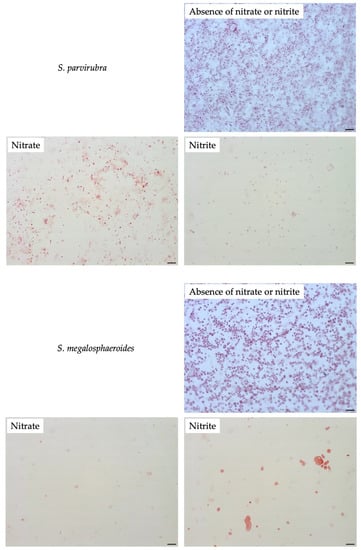
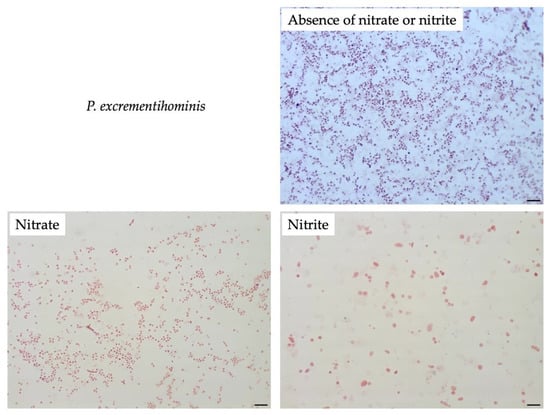
Figure 7.
Cellular morphology of reference strains cultured in the presence of nitrate or nitrite observed by Gram-staining after four days of incubation at 37 °C under anaerobic conditions. None represent TSA only; Nitrate represents supplementation with 50 mM of nitrate; Nitrite represents supplementation with 1 mM of nitrite. Bars, 10 µm.
3.5. Differences of Genes in Sutterellaceae Species
The draft genomes of M. multiformis and P. excrementihominis demonstrated genes involved in heme biosynthesis (Table 2), such as nitrate reductase, hemG, ATPase, and menaquinones that were coupled to electron transport chains for energy generation [9,19]. M. multiformis and P. excrementihominis showed longer chain menaquinones (MK-6 and MMK-6) [4]. In addition, S. megalosphaeroides, S. wadsworthensis, and S. parvirubra were predicted to possess a heme biosynthesis pathway, but did not possess genes encoding nitrate reductase. The genes encoding nitrite reductase were located in the genome of M. multiformis. This strain has the ability to convert nitrite to ammonia in the nitrogen cycle, but not the ability to produce nitric oxide.

Table 2.
Differential characteristics of M. multiformis and related taxa.
4. Discussion
M. multiformis JCM 32464T showed a unique cellular morphology influenced by nitrate that has not been explored before.
Although M. multiformis formed small pinpoint colonies on common blood agar plates, cell growth was arrested in the liquid medium. Related Sutterella spp. grow poorly and show no visible turbidity in broth [24]. These findings indicate a lack of growth factors in the liquid medium to facilitate the growth of Sutterella spp.
A recent study examined the ability of Sutterella spp. to adhere to intestinal epithelial cells [6]; however, the association between their adherence and growth in the gut remains unclear.
We previously suggested that M. multiformis generated a trace amount of ATP via heme biosynthesis [8]. Menaquinone is utilized as an electron donor for the reduction of nitrate to nitrite mediated by nitrate reductase, which is the key enzyme in this pathway [9]. However, in this study, their growth was not enhanced by additional nitrate, indicating that nitrate was not adapted to growth stimulation.
The addition of nitrate induced filamentation in M. multiformis JCM 32464T cells. This unique morphology was adapted to cope with nitrate stress. Morphological changes such as filament formation are a type of stress response observed under specific conditions. For instance, changes are observed in Salmonella enterica with pelargonic acid [25], Pseudomonas aeruginosa during anaerobic respiration [26], Corynebacterium glutamicum with nitrate [27], and Bacillus subtilis during anaerobic nitrate respiratory [28]. The changes might be attributed to defects in cell division, DNA damage, or the induction of SOS gene expression. A higher concentration of nitrate in the medium demonstrated a lower nitrite production and increased cell length, suggesting that the elongation of M. multiformis was a stress response toward nitrate. Once the filamentous cells were cultured on TSA without nitrate, cellular morphology was almost recovered, and morphology similar to regular cells was observed. This result demonstrated that the filamentous cells could be fragmented again and their biological activity could be recovered.
SEM images showed that the elongation of M. multiformis was accompanied by abnormal cell division, with intracellular light and shade. Clump formation was occasionally observed in the presence of nitrate (Figure 5) similar to S. wadsworthensis [29], which can be attributed to both the presence of excessive nitrate and microaerobic conditions.
These morphological changes affected the structure or surface of bacterial cells. One of the stress responses altered the composition of cellular fatty acids. The level of unsaturated fatty acids is correlated with the fluidity of the cell membrane [30]. In a previous study, the association between cell membrane composition and synthesis of menaquinones in Escherichia coli was investigated [18]. Liu et al. showed that an increase in the S/U ratio promoted the expression of intracellular menaquinones, and unsaturated fatty acids stimulated the secretion of extracellular menaquinones. The shorter lipids (C16:0, C14:0) and the S/U ratio increased upon the addition of 10 or 50 mM nitrate (Table 1, Figure 6). This indicated that the membrane of filamentous cells demonstrated low leakage and conserved menaquinones. This result is consistent with nitrite production and the S/U ratio as inferred from the role of menaquinones involved in nitrate respiration [31]. Consequently, it might be one of the strategies employed for maximizing menaquinone utilization for nitrate reduction.
Normally, BB plates are used for the cultivation of M. multiformis. The cellular fatty acid composition (S/U ratio) on BB plates was similar to that observed on TSA in the presence of 1 mM nitrate compared to the control [4]. This result indicated that an appropriate amount of nitrate supported the growth and cellular stability of M. multiformis.
These stress mechanisms causing changes in cell surface and membrane composition may be associated with adhesion to intestinal cells [32,33]. As mentioned above, the adherence of Sutterella spp. is closely related to human intestinal health.
The genome of all related species was predicted to contain hemG involved in the heme biosynthesis pathway. Only M. multiformis and P. excrementihominis possess the gene narGHJI encoding nitrate reductase, similar to the longer-chain menaquinones (MK-6 and MMK-6). However, some discrepancies were observed in the nitrite tolerance of P. excrementihominis compared to M. multiformis. Cells of M. multiformis did not demonstrate any changes when cultured on the TSA containing 100 mM of nitrite, whereas S. megalosphaeroides and P. excrementihominis cells showed burst morphology in the presence of 1 mM of nitrite (Figure 7). Moreover, P. secunda did not form colonies with nitrite. Nitrite is known to have antimicrobial properties, and the ability to exert toxic effects might be a possible mechanism [34]. Nitrite is added to inhibit the growth of bacterial pathogens in processed meat. Nitrate is also included in many edible plants; hence, the dietary intake of nitrate can be a precursor to nitrite production in the gut.
Only M. multiformis showed elongated cell morphology influenced by nitrate among all related species. These morphological changes were specifically observed in the presence of nitrate; however, filamentation might be induced by certain environmental factors in the human gut [35,36,37]. The family of Sutterellaceae was highly detected in the biopsy of patients of IBD and autism by metagenomic analysis [38]. However, we have isolated several strains within the family Sutterellaceae including S. parvirubra, S. wadsworthensis, S. megalosphaeroides, and M. multiformis from healthy human feces. Thus, this species was commonly abundant in the human gut regardless of the presence or absence of disease. This suggested that this physiological and morphological alteration induced by various intestinal environments such as high nitrate concentration correlated their abundance and biological activity in the human gut. Host–microbe interactions with filamentous cells should be examined in further studies.
Considering the difficulty to obtain a sufficient amount of RNAs because of their poor growth of M. multiformis, gene expression could not be confirmed by transcriptome analysis, as the current growth condition. Further studies are required to determine the growth factors that are needed to prepare enough bacterial samples for more multidimensional experiments.
These findings revealed that excessive nitrate could be a factor involved in inducing the stress response in M. multiformis, leading to filamentation and the inhibition of enzyme activity. The cellular fatty acid compositions were significantly altered during cell elongation as a stress response, suggesting strategies for maximizing nitrate respiration and menaquinone conservation to compete with the excessive nitrate.
We believe that our findings have provided substantial insights into M. multiformis and will lead to a more comprehensive understanding of this uncharted organism.
Author Contributions
Conceptualization, N.I. and M.S.; methodology, N.I. and M.S.; validation, N.I. and M.S.; formal analysis, N.I.; investigation, N.I.; resources, M.S.; data curation, N.I. and M.S.; writing—original draft preparation, N.I.; writing—review and editing, M.S.; visualization, N.I. and M.S.; supervision, M.O. and M.S.; project administration, M.S.; funding acquisition, M.O. and M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by PRIME, the Japan Agency for Medical Research and Development (AMED) under Grant Number JP19gm6010007 to M.S. and by a RIKEN Competitive Program for Creative Science and Technology to M.O.
Acknowledgments
We thank Wakako Bunryo and Naomi Sakurai for their technical assistance. We would like to thank Editage for the English-language editing.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Papadimitriou, K.; Alegría, Á.; Bron, P.A.; de Angelis, M.; Gobbetti, M.; Kleerebezem, M.; Lemos, J.A.; Linares, D.M.; Ross, P.; Stanton, C.; et al. Stress physiology of lactic acid bacteria. Microbiol. Mol. Biol. Rev. 2016, 80, 837–890. [Google Scholar] [CrossRef] [PubMed]
- Bucka-Kolendo, J.; Sokołowska, B. Lactic acid bacteria stress response to preservation processes in the beverage and juice industry. Acta Biochim. Pol. 2017, 64, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Hews, C.L.; Cho, T.; Rowley, G.; Raivio, T.L. Maintaining integrity under stress: Envelope stress response regulation of pathogenesis in Gram-negative bacteria. Front. Cell. Infect. Microbiol. 2019, 9, 313. [Google Scholar] [CrossRef]
- Sakamoto, M.; Ikeyama, N.; Kunihiro, T.; Iino, T.; Yuki, M.; Ohkuma, M. Mesosutterella multiformis gen. nov., sp. nov., a member of the family Sutterellaceae and Sutterella megalosphaeroides sp. nov., isolated from human faeces. Int. J. Syst. Evol. Microbiol. 2018, 68, 3942–3950. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.L.; Hornig, M.; Parekh, T.; Lipkin, W.I. Application of novel PCR-based methods for detection, quantitation, and phylogenetic characterization of Sutterella species in intestinal biopsy samples from children with autism and gastrointestinal disturbances. mBio 2012, 3, e00261-11. [Google Scholar] [CrossRef] [PubMed]
- Hiippala, K.; Kainulainen, V.; Kalliomäki, M.; Arkkila, P.; Satokari, R. Mucosal prevalence and interactions with the epithelium indicate commensalism of Sutterella spp. Front. Microbiol. 2016, 7, 1706. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.J.; Wu, H.; Wu, S.D.; Lu, N.; Wang, Y.T.; Liu, H.N.; Dong, L.; Liu, T.T.; Shen, X.Z. Parasutterella, in association with irritable bowel syndrome and intestinal chronic inflammation. J. Gastroenterol. Hepatol. 2018, 33, 1844–1852. [Google Scholar] [CrossRef] [PubMed]
- Ikeyama, N.; Ohkuma, M.; Sakamoto, M. Draft genome sequence of Mesosutterella multiformis JCM 32464T, a member of the family Sutterellaceae, isolated from human feces. Microbiol. Resour. Announc. 2019, 8, e00478-19. [Google Scholar] [CrossRef]
- Möbius, K.; Arias-Cartin, R.; Breckau, D.; Hännig, A.L.; Riedmann, K.; Biedendieck, R.; Schröder, S.; Becher, D.; Magalon, A.; Moser, J.; et al. Heme biosynthesis is coupled to electron transport chains for energy generation. Proc. Natl. Acad. Sci. USA 2010, 107, 10436–10441. [Google Scholar] [CrossRef]
- Tiso, M.; Schechter, A.N. Nitrate reduction to nitrite, nitric oxide and ammonia by gut bacteria under physiological conditions. PLoS ONE 2015, 10, e0119712. [Google Scholar] [CrossRef]
- Platzen, L.; Koch-Koerfges, A.; Weil, B.; Brocker, M.; Bott, M. Role of flavohaemoprotein Hmp and nitrate reductases NarGHJI of Corynebacterium glutamicum for coping with nitrite and nitrosative stress. FEMS Microbiol. Lett. 2014, 350, 239–248. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sakamoto, M.; Iino, T.; Ohkuma, M. Faecalimonas umbilicata gen. nov., sp. nov., isolated from human faeces, and reclassification of Eubacterium contortum, Eubacterium fissicatena and Clostridium oroticum as Faecalicatena contorta gen. nov., comb. nov., Faecalicatena fissicatena comb. nov. and Faecalicatena orotica comb. nov. Int. J. Syst. Evol. Microbiol. 2017, 67, 1219–1227. [Google Scholar] [CrossRef] [PubMed]
- Kuykendall, L.D.; Roy, M.A.; O’Neill, J.J.; Devine, T.E. Fatty acids, antibiotic resistance, and deoxyribonucleic acid homology groups of Bradyrhizobium japonicum. Int. J. Syst. Bacteriol. 1988, 38, 358–361. [Google Scholar] [CrossRef]
- Miller, L.T. Single derivatization method for routine analysis of bacterial whole-cell fatty acid methyl esters, including hydroxy acids. J. Clin. Microbiol. 1982, 16, 584–586. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Furumichi, M.; Tanabe, M.; Sato, Y.; Morishima, K. KEGG: New perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017, 45, D353–D361. [Google Scholar] [CrossRef] [PubMed]
- Aziz, R.K.; Bartels, D.; Best, A.A.; DeJongh, M.; Disz, T.; Edwards, R.A.; Formsma, K.; Gerdes, S.; Glass, E.M.; Kubal, M.; et al. The RAST Server: Rapid annotations using subsystems technology. BMC Genom. 2008, 9, 75. [Google Scholar] [CrossRef]
- Tanizawa, Y.; Fujisawa, T.; Nakamura, Y. DFAST: A flexible prokaryotic genome annotation pipeline for faster genome publication. Bioinformatics 2018, 34, 1037–1039. [Google Scholar] [CrossRef]
- Liu, Y.; Ding, X.M.; Xue, Z.L.; Hu, L.X.; Zhang, N.J.; Wang, Z.; Yang, J.W.; Cheng, Q.; Chen, M.H.; Zhang, Z.Z.; et al. The change of the state of cell membrane can enhance the synthesis of menaquinone in Escherichia coli. World J. Microbiol. Biotechnol. 2017, 33, 52. [Google Scholar] [CrossRef]
- Heinemann, I.U.; Jahn, M.; Jahn, D. The biochemistry of heme biosynthesis. Arch. Biochem. Biophys. 2008, 474, 238–251. [Google Scholar] [CrossRef]
- Wexler, H.M.; Reeves, D.; Summanen, P.H.; Molitoris, E.; McTeague, M.; Duncan, J.; Wilson, K.H.; Finegold, S.M. Sutterella wadsworthensis gen. nov., sp. nov., bile-resistant microaerophilic Campylobacter gracilis-like clinical isolates. Int. J. Syst. Bacteriol. 1996, 46, 252–258. [Google Scholar] [CrossRef]
- Greetham, H.L.; Collins, M.D.; Gibson, G.R.; Giffard, C.; Falsen, E.; Lawson, P.A. Sutterella stercoricanis sp. nov., isolated from canine faeces. Int. J. Syst. Evol. Microbiol. 2004, 54, 1581–1584. [Google Scholar] [CrossRef] [PubMed]
- Sakon, H.; Nagai, F.; Morotomi, M.; Tanaka, R. Sutterella parvirubra sp. nov. and Megamonas funiformis sp. nov., isolated from human faeces. Int. J. Syst. Evol. Microbiol. 2008, 58, 970–975. [Google Scholar] [CrossRef] [PubMed]
- Nagai, F.; Morotomi, M.; Sakon, H.; Tanaka, R. Parasutterella excrementihominis gen. nov., sp. nov., a member of the family Alcaligenaceae isolated from human faeces. Int. J. Syst. Evol. Microbiol. 2009, 59, 1793–1797. [Google Scholar] [CrossRef] [PubMed]
- Morotomi, M.; Nagai, F.; Watanabe, Y. Parasutterella secunda sp. nov., isolated from human faeces and proposal of Sutterellaceae fam. nov. in the order Burkholderiales. Int. J. Syst. Evol. Microbiol. 2011, 61, 637–643. [Google Scholar] [CrossRef]
- Dev Kumar, G.; Macarisin, D.; Micallef, S.A. Salmonella enterica filamentation induced by pelargonic acid is a transient morphotype. Appl. Environ. Microbiol. 2019, 85, e02191-18. [Google Scholar] [CrossRef]
- Yoon, M.Y.; Lee, K.M.; Park, Y.; Yoon, S.S. Contribution of cell elongation to the biofilm formation of Pseudomonas aeruginosa during anaerobic respiration. PLoS ONE 2011, 6, e16105. [Google Scholar] [CrossRef]
- Nishimura, T.; Teramoto, H.; Inui, M.; Yukawa, H. Gene expression profiling of Corynebacterium glutamicum during anaerobic nitrate respiration: Induction of the SOS response for cell survival. J. Bacteriol. 2011, 193, 1327–1333. [Google Scholar] [CrossRef]
- Hoffmann, T.; Troup, B.; Szabo, A.; Hungerer, C.; Jahn, D. The anaerobic life of Bacillus subtilis: Cloning of the genes encoding the respiratory nitrate reductase system. FEMS Microbiol. Lett. 1995, 131, 219–225. [Google Scholar] [CrossRef]
- Mukhopadhya, I.; Hansen, R.; Nicholl, C.E.; Alhaidan, Y.A.; Thomson, J.M.; Berry, S.H.; Pattinson, C.; Stead, D.A.; Russell, R.K.; El-Omar, E.M.; et al. A comprehensive evaluation of colonic mucosal isolates of Sutterella wadsworthensis from inflammatory bowel disease. PLoS ONE 2011, 6, e27076. [Google Scholar] [CrossRef]
- Szalontai, B.; Nishiyama, Y.; Gombos, Z.; Murata, N. Membrane dynamics as seen by fourier transform infrared spectroscopy in a cyanobacterium, Synechocystis PCC 6803. The effects of lipid unsaturation and the protein-to-lipid ratio. Biochim. Biophys. Acta 2000, 1509, 409–419. [Google Scholar] [CrossRef]
- Sasarman, A.; Purvis, P.; Portelance, V. Role of menaquinone in nitrate respiration in Staphylococcus aureus. J. Bacteriol. 1974, 117, 911–913. [Google Scholar] [CrossRef] [PubMed]
- Haddaji, N.; Mahdhi, A.K.; Krifi, B.; Ismail, M.B.; Bakhrouf, A. Change in cell surface properties of Lactobacillus casei under heat shock treatment. FEMS Microbiol. Lett. 2015, 362, fnv047. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, A.; Shah, N.P. Effect of salt stress on morphology and membrane composition of Lactobacillus acidophilus, Lactobacillus casei, and Bifidobacterium bifidum, and their adhesion to human intestinal epithelial-like Caco-2 cells. J. Dairy Sci. 2016, 99, 2594–2605. [Google Scholar] [CrossRef] [PubMed]
- Archer, D.L. Evidence that ingested nitrate and nitrite are beneficial to health. J. Food Prot. 2002, 65, 872–875. [Google Scholar] [CrossRef]
- Shaw, M.K. Formation of filaments and synthesis of macromolecules at temperatures below the minimum for growth of Escherichia coli. J. Bacteriol. 1968, 95, 221–230. [Google Scholar] [CrossRef]
- Sajbidor, J. Effect of some environmental factors on the content and composition of microbial membrane lipids. Crit. Rev. Biotechnol. 1997, 17, 87–103. [Google Scholar] [CrossRef]
- Crompton, M.J.; Dunstan, R.H.; Macdonald, M.M.; Gottfries, J.; von Eiff, C.; Roberts, T.K. Small changes in environmental parameters lead to alterations in antibiotic resistance, cell morphology and membrane fatty acid composition in Staphylococcus lugdunensis. PLoS ONE 2014, 9, e92296. [Google Scholar] [CrossRef][Green Version]
- Wang, L.; Christophersen, C.T.; Sorich, M.J.; Gerber, J.P.; Angley, M.T.; Conlon, M.A. Increased abundance of Sutterella spp. and Ruminococcus torques in feces of children with autism spectrum disorder. Mol. Autism 2013, 4, 42. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).