Impact of Mediterranean Diet on Disease Activity and Gut Microbiota Composition of Rheumatoid Arthritis Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Sample Collection and DNA Extraction
2.3. Next-Generation Sequencing of Bacterial 16S rRNA Gene
2.4. Bioinformatic Analysis
2.5. Statistical Analysis
3. Results
3.1. Adherence to Mediterranean Diet and Clinical Characteristics of RA Patients
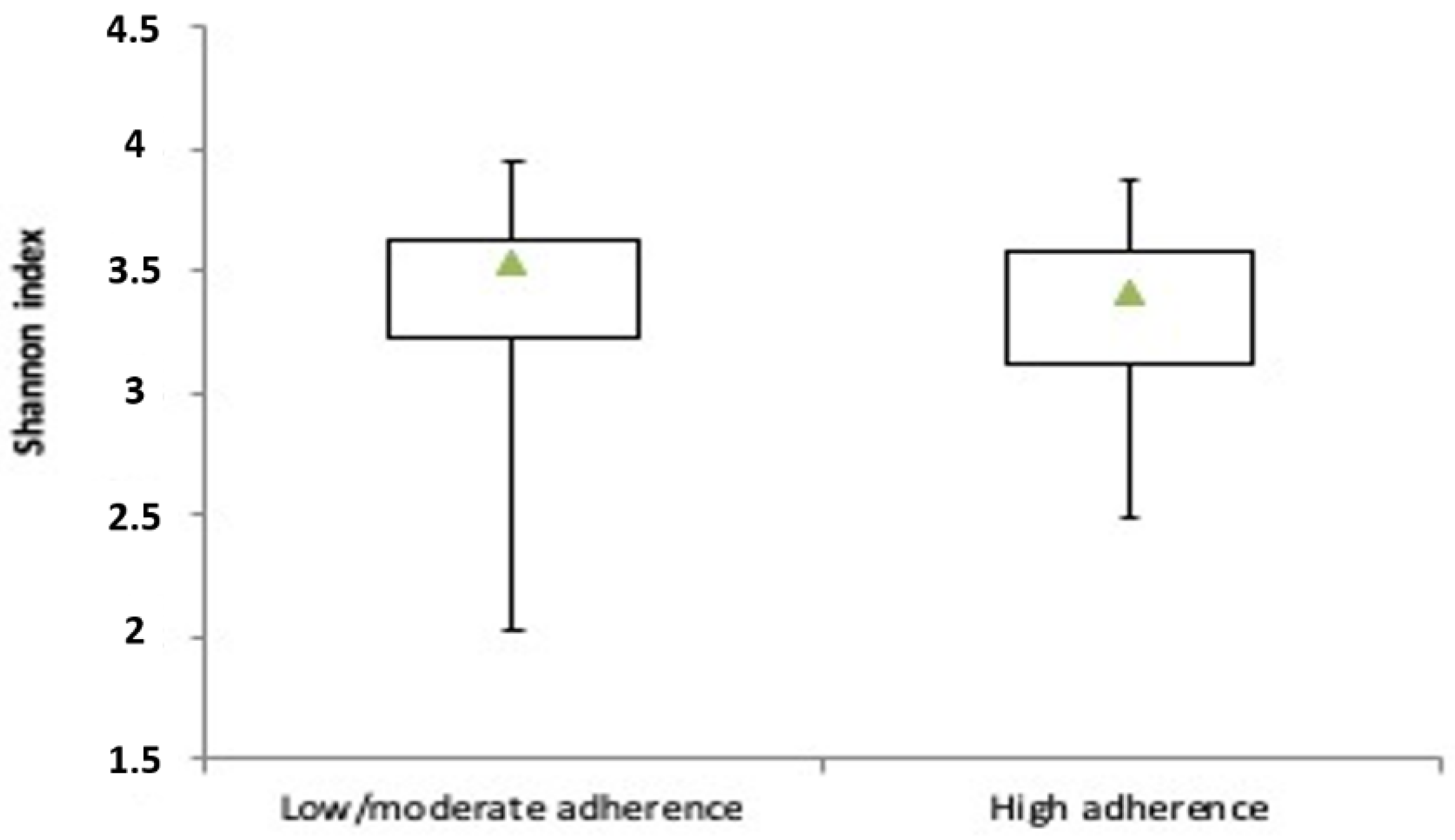
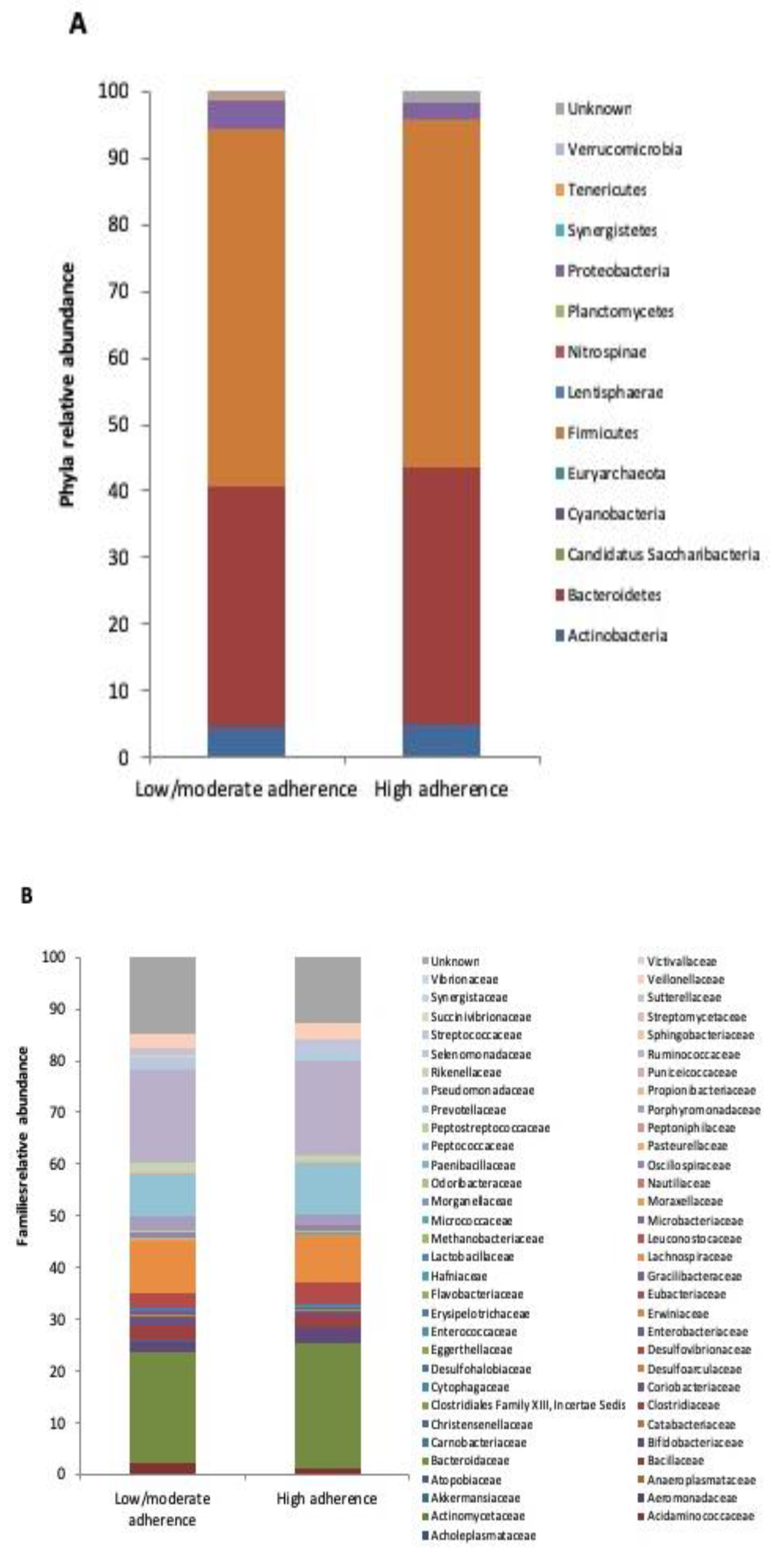
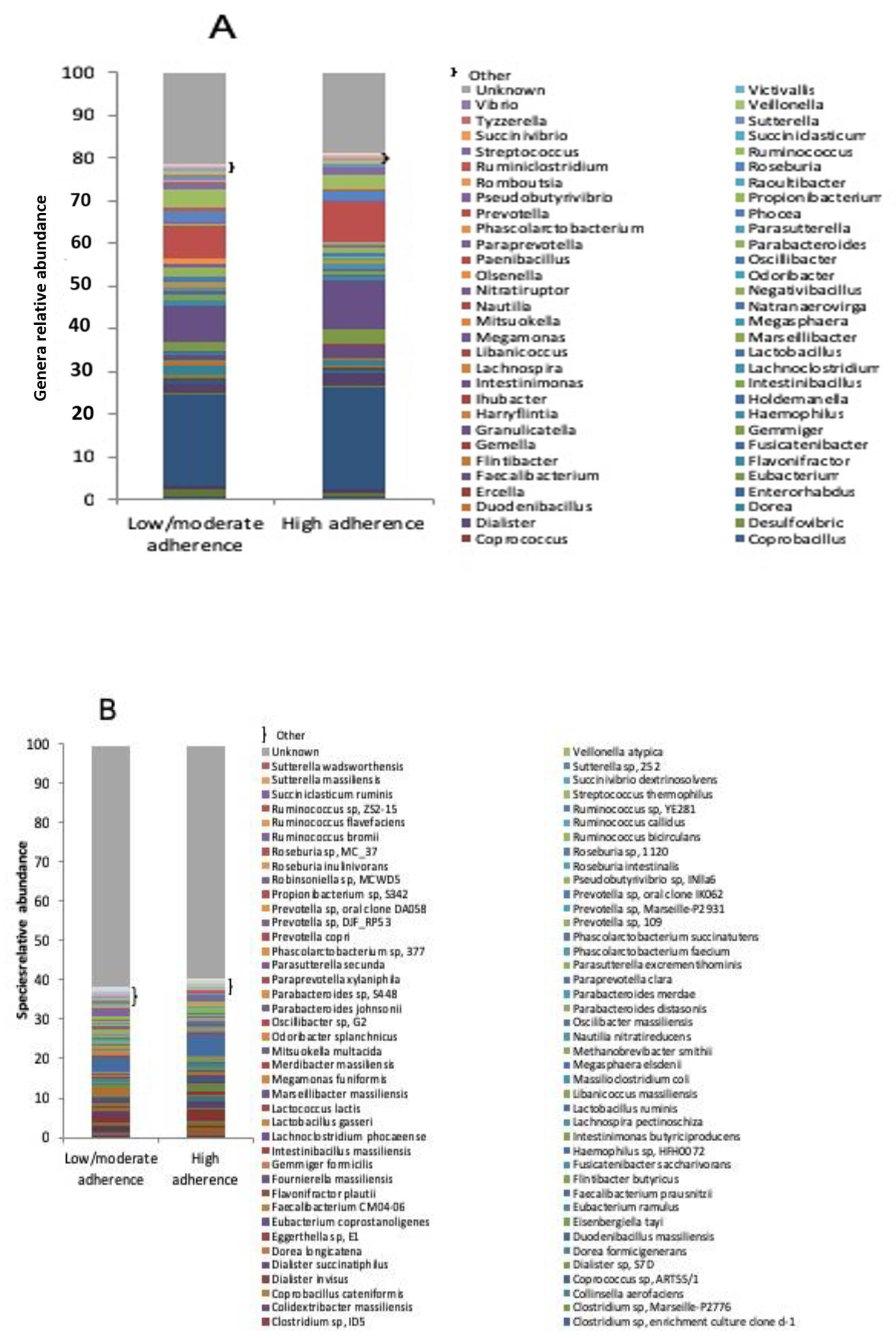
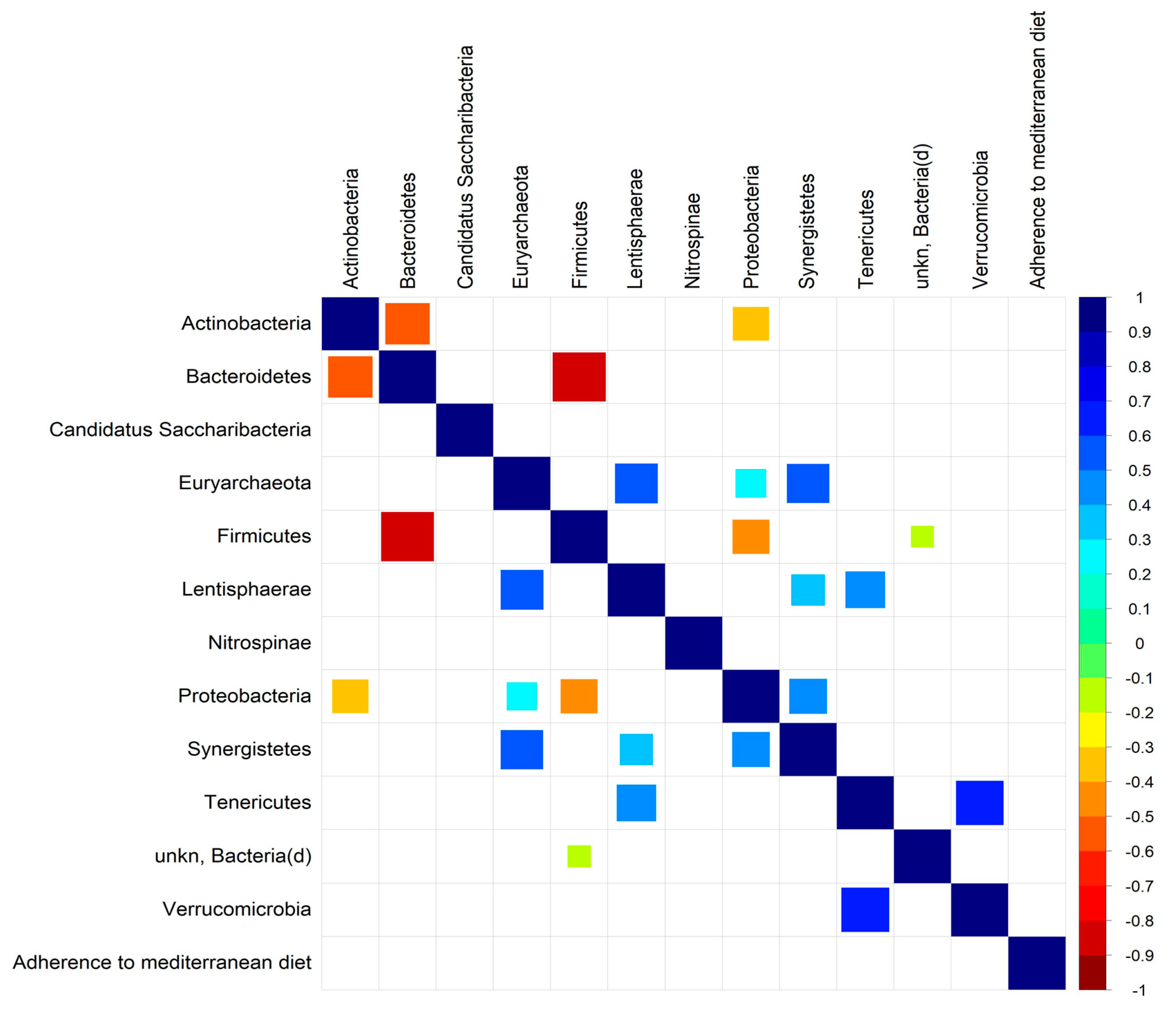
3.2. Microbiota Profile in RA Patients with Low/Moderate and High Adherence to Mediterranean Diet
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Picchianti-Diamanti, A.; Rosado, M.M.; D’Amelio, R. Infectious Agents and Inflammation: The Role of Microbiota in Autoimmune Arthritis. Front. Microbiol. 2018, 8, 2696. [Google Scholar] [CrossRef]
- Vaahtovuo, J.; Munukka, E.; Korkeamäki, M.; Luukkainen, R.; Toivanen, P. Fecal microbiota in early rheumatoid arthritis. J. Rheumatol. 2008, 35, 1500–1505. [Google Scholar] [PubMed]
- Johnson, S.; Sidebottom, D.; Bruckner, F.; Collins, D. Identification of Mycoplasma fermentans in synovial fluid samples from arthritis patients with inflammatory disease. J. Clin. Microbiol. 2000, 38, 90–93. [Google Scholar] [PubMed]
- Newkirk, M.M.; Zbar, A.; Baron, M.; Manges, A.R. Distinct bacterial colonization patterns of Escherichia coli subtypes associate with rheumatoid factor status in early inflammatory arthritis. Rheumatology 2010, 49, 1311–1316. [Google Scholar] [CrossRef] [PubMed]
- Senior, B.W.; Anderson, G.A.; Morley, K.D.; Kerr, M.A. Evidence that patients with rheumatoid arthritis have asymptomatic ’non-significant’ Proteus mirabilis bacteriuria more frequently than healthy controls. J. Infect. 1999, 38, 99–106. [Google Scholar] [CrossRef]
- Liu, X.; Zou, Q.; Zeng, B.; Fang, Y.; Wei, H. Analysis of fecal lactobacillus community structure in patients with early rheumatoid arthritis. Curr. Microbiol. 2013, 67, 170–176. [Google Scholar] [CrossRef]
- Chen, J.; Wright, K.; Davis, J.M.; Jeraldo, P.; Marietta, E.V.; Murray, J.; Nelson, H.; Matteson, E.L.; Taneja, V. An expansion of rare lineage intestinal microbes characterizes rheumatoid arthritis. Genome Med. 2016, 8, 43. [Google Scholar] [CrossRef]
- Scher, J.U.; Sczesnak, A.; Longman, R.S.; Segata, N.; Ubeda, C.; Bielski, C.; Rostron, T.; Cerundolo, V.; Pamer, E.G.; Abramson, S.B.; et al. Expansion of intestinal prevotella copri correlates with enhanced susceptibility to arthritis. Elife 2013, 2, e01202. [Google Scholar] [CrossRef]
- Picchianti-Diamanti, A.; Panebianco, C.; Salemi, S.; Sorgi, M.L.; Di Rosa, R.; Tropea, A.; Sgrulletti, M.; Salerno, G.; Terracciano, F.; D’Amelio, R.; et al. Analysis of Gut Microbiota in Rheumatoid Arthritis Patients: Disease-Related Dysbiosis and Modifications Induced by Etanercept. Int. J. Mol. Sci. 2018, 19, 2938. [Google Scholar] [CrossRef]
- Picchianti-Diamanti, A.; Rosado, M.M.; Laganà, B.; D’Amelio, R. Microbiota and chronic inflammatory arthritis: An interwoven link. J. Transl. Med. 2016, 14, 233. [Google Scholar] [CrossRef]
- Salonen, A.; de Vos, W.M. Impact of diet on human intestinal microbiota and health. Ann. Rev. Food Sci. Technol. 2014, 5, 239–262. [Google Scholar] [CrossRef] [PubMed]
- Sofi, F.; Cesari, F.; Abbate, R.; Gensini, G.F.; Casini, A. Adherence to Mediterranean diet and health status: Meta-analysis. BMJ 2008, 337, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Willett, W.C.; Sacks, F.; Trichopoulou, A.; Drescher, G.; Ferro-Luzzi, A.; Helsing, E.; Trichopoulous, D. Mediterranean diet pyramid: A cultural model for healthy eating. Am. J. Clin. Nutr. 1995, 61, 1402S–1406S. [Google Scholar] [CrossRef] [PubMed]
- Dinu, M.; Pagliai, G.; Casini, A.; Sofi, F. Mediterranean diet and multiple health outcomes: An umbrella review of meta-analyses of observational studies and randomised trials. Eur. J. Clin. Nutr. 2018, 72, 30–43. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Hoffmann, G. Adherence to Mediterranean diet and risk of cancer: An updated systematic review and meta-analysis of observational studies. Cancer Med. 2015, 4, 1933–1947. [Google Scholar] [CrossRef] [PubMed]
- Schröder, H. Protective mechanisms of the Mediterranean diet in obesity and type 2 diabetes. J. Nutr. Biochem. 2007, 18, 149–160. [Google Scholar] [CrossRef]
- Mitsou, E.K.; Kakali, A.; Antonopouolu, S.; Mountzoris, K.C.; Yannakoulia, M.; Panagiotakos, D.B.; Kyriacou, A. Adherence to the Mediterranean diet is associated with the gut microbiota pattern and gastrointestinal characteristics in an adult population. Br. J. Nutr. 2017, 117, 1645–1655. [Google Scholar] [CrossRef]
- Lerner, A.; Patricia, J.; Mathias, T. Nutrients, bugs and us: The short-chain fatty acids story in celiac disease. Int. J. Celiac. Dis. 2016, 4, 92–94. [Google Scholar]
- De Santis, S.; Cavalcanti, E.; Mastronardi, M.; Jirillo, E.; Chieppa, M. Nutritional keys for intestinal barrier modulation. Front. Immunol. 2015, 6, 612. [Google Scholar] [CrossRef]
- Pedersen, M.; Stripp, C.; Klarlund, M.; Olsen, F.S.; Tjonneland, A.M.; Frisch, M. Diet and risk of rheumatoid arthritis in a prospective cohort. J. Rheum. 2005, 32, 1249–1252. [Google Scholar]
- Cerhan, J.R.; Saag, K.J.; Merlino, M.A.; Mikuls, T.R.; Criswell, L.A. Antioxidant micronutrients and risk of rheumatoid arthritis in a cohort of older women. Am. J. Epidemiol. 2003, 157, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Pattison, D.J.; Symmons, D.P.; Lunt, M.; Welch, A.; Luben, R.; Bingham, S.A.; Khhaw, K.T.; Day, N.E.; Silman, A.J. Dietary risk factors for the development of inflammatory polyarthritis: Evidence for a role of high level of red meat consumption. Arthritis Rheum. 2004, 50, 3804–3812. [Google Scholar] [CrossRef] [PubMed]
- Pattison, D.J.; Silman, A.J.; Goodson, N.J.; Lunt, M.; Bunn, D.; Luben, R.; Welch, A.; Bingham, S.; Khaw, K.-T.; Day, N.; et al. Vitamin C and the risk of developing inflammatory polyarthritis: Prospective nested case-control study. Ann. Rheum. Dis. 2004, 63, 843–847. [Google Scholar] [CrossRef] [PubMed]
- Benito-Garcia, E.; Feskanich, D.; Hu, F.B.; Mandl, L.A.; Krlson, E.W. Protein, iron, and meat consumption and risk for rheumatoid arthritis: A prospective cohort study. Arthritis Res. Ther. 2007, 9, R16. [Google Scholar] [CrossRef]
- Costenbader, K.H.; Kang, J.H.; Karlson, E.W. Antioxidant intake and risks of rheumatoid arthritis and systemic lupus erythematosus in women. Am. J. Epidemiol. 2010, 172, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B. The role of meat in the expression of rheumatoid arthritis. Br. J. Nutr. 2000, 84, 589–595. [Google Scholar] [CrossRef]
- Gioia, C.; Lucchino, B.; Tarsitano, M.G.; Iannucelli, C.; Di Franco, M. Dietary Habits and Nutrition in Rheumatoid Arthritis: Can Diet Influence Disease Development and Clinical Manifestations? Nutrients 2020, 12, 1456. [Google Scholar] [CrossRef]
- Dourado, E.; Ferro, M.; Guerreiro, C.S.; Fonseca, J.E. Diet as a Modulator of Intestinal Microbiota in Rheumatoid Arthritis. Nutrients 2020, 12, 3504. [Google Scholar] [CrossRef]
- Aletaha, D.; Neogi, T.; Silman, A.J.; Funovits, J.; Felson, D.T.; O Bingham, C., 3rd; Birnbaum, N.S.; Burmester, G.R.; Bykerk, V.P.; Cohen, M.D.; et al. 2010 rheumatoid arthritis classification criteria: An american college of rheumatology/european league against rheumatism collaborative initiative. Ann. Rheum Dis. 2010, 69, 1580–1588. [Google Scholar] [CrossRef]
- Martínez-González, M.A.; Garcıa-Arellano, A.; Toledo, E.; Salas Salvado, J.; Buil-Cosiale, P.; Corella, D.; Covas, M.I.; Schroder, H.; Aros, F. PREDIMED Study Investigators. A 14-item Mediterranean diet assessment tool and obesity indexes among high-risk subjects: The Predimed trial. PLoS ONE. 2012, 7, e43134. [Google Scholar] [CrossRef]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glöckner, F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013, 7, e1. [Google Scholar] [CrossRef] [PubMed]
- Fontana, A.; Panebianco, C.; Picchianti-Diamanti, A.; Laganà, B.; Cavalieri, D.; Potenza, A.; Pracella, R.; Binda, E.; Copetti, M.; Pazienza, V. Gut Microbiota Profiles Differ among Individuals Depending on Their Region of Origin: An Italian Pilot Study. Int. J. Environ. Res. Public Health 2019, 16, 4065. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Costenbader, K.H.; Gao, X.; Hu, F.B.; Karlson, E.W.; Lu, B. Mediterranean diet and incidence of rheumatoid arthritis in women. Arthritis Care Res. 2015, 67, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Sundstrom, B.; Johansson, I.; Rantapaa-Dahlqvist, S. Diet and alcohol as risk factors for rheumatoid arthritis: A nested case-control study. Rheumatol. Int. 2015, 35, 533–539. [Google Scholar] [CrossRef]
- Johansson, K.; Askling, J.; Alfredsson, L.; Di Giuseppe, D.; EIRA study group. Mediterranean diet and risk of rheumatoid arthritis: A population-based case-control study. Arthritis Res. Ther. 2018, 20, 175. [Google Scholar] [CrossRef]
- Hagen, K.B.; Byfuglien, M.G.; Falzon, L.; Olsen, S.U.; Smedslund, G. Dietary interventions for rheumatoid arthritis. Cochrane Database Syst. Rev. 2009, 1, CD006400. [Google Scholar] [CrossRef]
- Forsyth, C.; Kouvari, M.; D’Cunha, N.M.; Georgousopoulou, E.N.; Panagiotakos, D.B.; Mellor, D.D.; Kellett, J.; Naumovski, N. The effects of the Mediterranean diet on rheumatoid arthritis prevention and treatment: A systematic review of human prospective studies. Rheumatol. Int. 2018, 38, 737–747. [Google Scholar] [CrossRef]
- Sköldstam, L.; Hagfors, L.; Johansson, G. An experimental study of a Mediterranean diet intervention for patients with rheumatoid arthritis. Ann. Rheum Dis. 2003, 62, 208–214. [Google Scholar]
- Nelson, J.; Sjöblom, H.; Gjertsson, I.; Ulven, M.S.; Lindqvist, M.H.; Bärebring, L. Do Interventions with Diet or Dietary Supplements Reduce the Disease Activity Score in Rheumatoid Arthritis? A Systematic Review of Randomized Controlled Trials. Nutrients 2020, 12, 2991. [Google Scholar] [CrossRef]
- McKellar, G.; Morrison, E.; McEntegart, A.; Hampson, R.; Tierney, A.; Mackle, G.; Scoular, J.; Scott, J.A.; Capell, H.A. A pilot study of a Mediterranean type diet intervention in female patients with rheumatoid arthritis living in areas of social deprivation in Glasgow. Ann. Rheum. Dis. 2007, 66, 1239–1243. [Google Scholar] [CrossRef]
- Vadell, K.E.A.; Bärebring, L.; Hulander, E.; Gjertsson, I.; Lindqvist, M.H.; Winkvist, A. Anti-inflammatory Diet In Rheumatoid Arthritis (ADIRA)—A randomized, controlled crossover trial indicating dieffects on disease activity. Am. J. Clin. Nutr. 2020, 111, 1203–1213. [Google Scholar] [CrossRef] [PubMed]
- Michalsen, A.; Riegert, M.; Ludtke, R.; Backer, M.; Langhorst, J.; Schwickert, M.; Dobos, J.B. Mediterranean diet or extended fasting’s influence on changing the intestinal microflora, immunoglobulin A secretion and clinical outcome in patients with rheumatoid arthritis and fibromyalgia: An observational study. BMC Complementary Altern. Med. 2005, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- De Filippis, F.; Pellegrini, N.; Vannini, L.; Jeffery, I.B.; La Storia, A.; Laghi, L.; Serrazanetti, D.I.; Di Cagno, R.; Ferrocino, I.; Lazzi, C.; et al. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut 2016, 1812–1821. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Mantrana, I.; Selma-Royo, M.; Alcantara, C.; Collado, M.C. Shifts on Gut Microbiota Associated to Mediterranean Diet Adherence and Specific Dietary Intakes on General Adult Population. Front Microbiol. 2018, 9, 890. [Google Scholar] [CrossRef]
- Muñiz Pedrogo, D.A.; Chen, J.; Hillmann, B.; Jeraldo, P.; Al-Ghalith, G.; Taneja, V.; Davis, J.M.; Knights, D.; Nelson, H.; Faubion, W.A.; et al. An Increased Abundance of Clostridiaceae Characterizes Arthritis in Inflammatory Bowel Disease and Rheumatoid Arthritis: A Cross-sectional Study. Inflamm Bowel Dis. 2019, 25, 902–913. [Google Scholar] [CrossRef]
- Liu, X.; Zeng, B.; Zhang, J.; Li, W.; Mou, F.; Wang, H.; Zou, Q.; Zhong, B.; Wu, L.; Wei, H.; et al. Role of the Gut Microbiome in Modulating Arthritis Progression in Mice. Sci. Rep. 2016, 6, 30594. [Google Scholar] [CrossRef]
- Piant, A.; Arvikar, S.; Strle, K.; Drouin, E.E.; Wang, Q.; Costello, C.E.; Steere, A.C. Evidence of the Immune Relevance of Prevotella copri, a Gut Microbe, in Patients With Rheumatoid Arthritis. Arthritis Rheumatol. 2017, 69, 964–975. [Google Scholar] [CrossRef]
- Round, J.L.; Mazmanian, S.K. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc. Natl. Acad. Sci. USA 2010, 107, 12204–12212. [Google Scholar] [CrossRef]

| Age, years | 60.50 (53.00–69.5) |
| F (%) | 88 (33) |
| BMI | 24.21 (21.15–26.15) |
| Smoke (%) | 18 (33) |
| Disease duration, months | 13.00 (8.50–17.50) |
| DAS28 | 3.70 (2.98–4.80) |
| RF positive (%) | 65.00 |
| ACPA positive (%) | 63.33 |
| CRP (mg/dl) | 3.60 (2.00–8.75) |
| ESR (mm/hg) | 20.00 (11.50–33.00) |
| Low/Moderate Adherence (n = 40) | High Adherence (n = 20) | p Value | |
|---|---|---|---|
| Age, years | 59.50 (52.00–65.50) | 66.00 (56.50–70.00) | ns |
| F (%) | 30.00 (75.00) | 20.00 (100.00) | 0.023 |
| BMI | 24.45 (21.10–27.45) | 23.40 (21.20–26.02) | ns |
| Smoke (%) | 8.00 (20.00) | 3.00 (15.00) | ns |
| Disease duration, months | 11.50 (6.00–16.50) | 15.00 (12.50–19.50) | 0.033 |
| DAS28 | 3.95 (3.10–5.06) | 3.30 (2.87–3.75) | 0.034 |
| RF positive (%) | 27.00 (67.50) | 12.00 (60.00) | ns |
| ACPA positive (%) | 27.00 (67.50) | 11.00 (55.00) | ns |
| CRP (mg/l) | 4.86 (2.35–10.15) | 2.47 (1.00–5.50) | 0.037 |
| ESR (mm/hg) | 20.00 (10.50–34.50) | 20.00 (13.50–21) | ns |
| Univariate | Multivariate | |||
|---|---|---|---|---|
| B Coeff. | p-Value | B Coeff. | p-Value | |
| F (%) | −1.400 | ns | - | - |
| Disease duration, months | −0.002 | ns | - | - |
| CRP (mg/dL) | 0.0250 | ns | - | - |
| Class of adherence to Mediterranean diet | −1.021 | 0.047 | −1.130 | 0.042 |
| Therapy | 0.055 | ns | - | - |
| Age, years | 0.033 | ns | - | - |
| BMI | −0.008 | 0.042 | −0.060 | ns |
| Smoke (%) | −0.164 | 0.049 | −0.264 | ns |
| RF positive (%) | 0.66 | ns | - | - |
| ESR (mm/hg) | 0.040 | 0.0165 | 0.040 | 0.017 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Picchianti Diamanti, A.; Panebianco, C.; Salerno, G.; Di Rosa, R.; Salemi, S.; Sorgi, M.L.; Meneguzzi, G.; Mariani, M.B.; Rai, A.; Iacono, D.; et al. Impact of Mediterranean Diet on Disease Activity and Gut Microbiota Composition of Rheumatoid Arthritis Patients. Microorganisms 2020, 8, 1989. https://doi.org/10.3390/microorganisms8121989
Picchianti Diamanti A, Panebianco C, Salerno G, Di Rosa R, Salemi S, Sorgi ML, Meneguzzi G, Mariani MB, Rai A, Iacono D, et al. Impact of Mediterranean Diet on Disease Activity and Gut Microbiota Composition of Rheumatoid Arthritis Patients. Microorganisms. 2020; 8(12):1989. https://doi.org/10.3390/microorganisms8121989
Chicago/Turabian StylePicchianti Diamanti, Andrea, Concetta Panebianco, Gerardo Salerno, Roberta Di Rosa, Simonetta Salemi, Maria Laura Sorgi, Giorgia Meneguzzi, Maria Benedetta Mariani, Alessandra Rai, Dalila Iacono, and et al. 2020. "Impact of Mediterranean Diet on Disease Activity and Gut Microbiota Composition of Rheumatoid Arthritis Patients" Microorganisms 8, no. 12: 1989. https://doi.org/10.3390/microorganisms8121989
APA StylePicchianti Diamanti, A., Panebianco, C., Salerno, G., Di Rosa, R., Salemi, S., Sorgi, M. L., Meneguzzi, G., Mariani, M. B., Rai, A., Iacono, D., Sesti, G., Pazienza, V., & Laganà, B. (2020). Impact of Mediterranean Diet on Disease Activity and Gut Microbiota Composition of Rheumatoid Arthritis Patients. Microorganisms, 8(12), 1989. https://doi.org/10.3390/microorganisms8121989







