Filling the Void: An Optimized Polymicrobial Interkingdom Biofilm Model for Assessing Novel Antimicrobial Agents in Endodontic Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Ontological Analysis to Inform Biofilm Model Development
2.2. Biofilm Model Development
2.2.1. Microbial Standardization
2.2.2. Development of Single and Mixed-Species Biofilm in Microtiter Plates
2.3. Biofilm Characterization
2.3.1. Quantification of Biomass
2.3.2. Visualization
2.3.3. Quantitative Analysis of Biofilm Composition
2.4. In Vitro Biofilm Susceptibility Testing
2.5. In Vitro Biofilm Susceptibility Testing on Bovine Dentine Discs
2.6. Statistical Analysis
3. Results
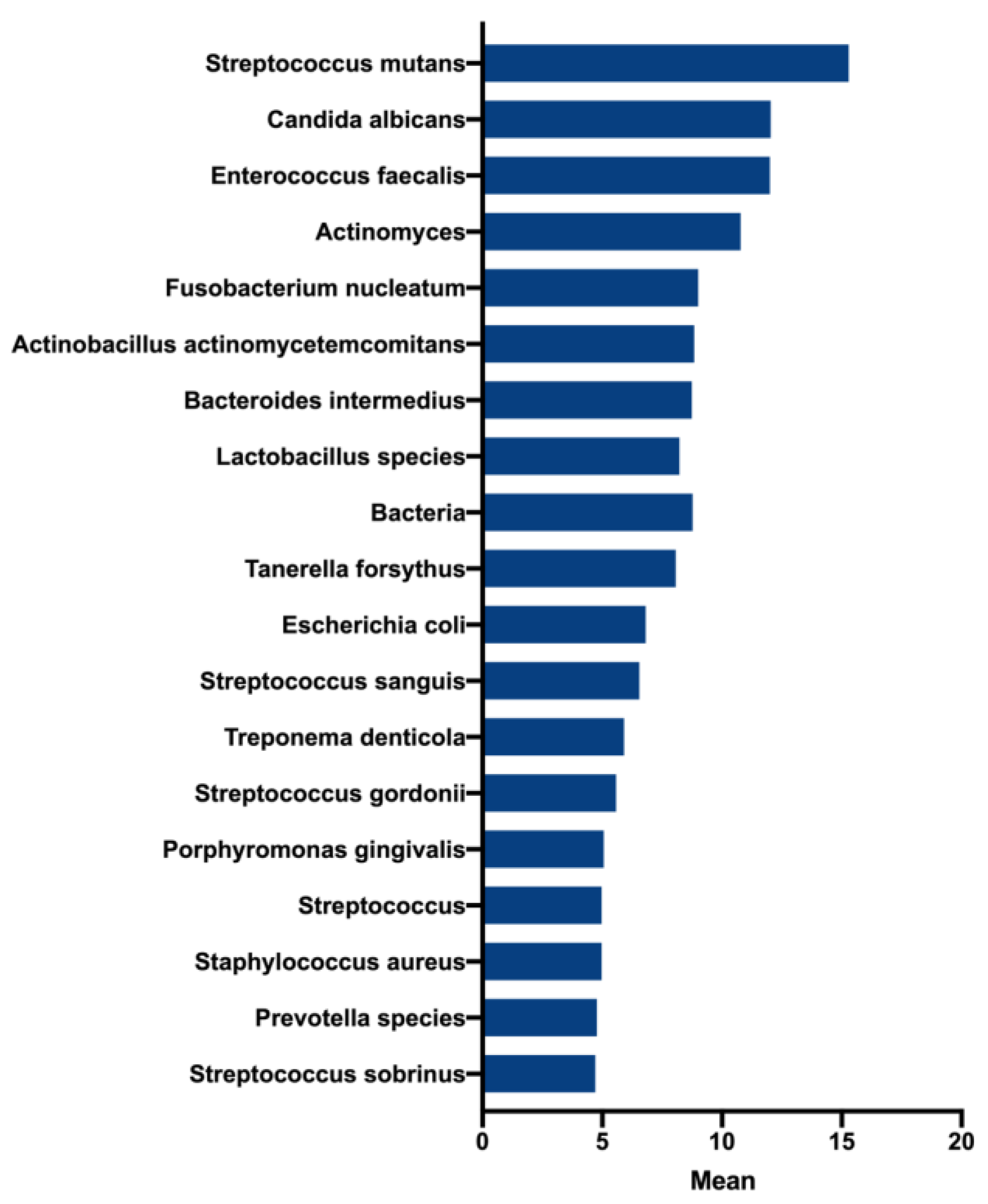
3.1. Conception of the Endodontic Biofilm Model
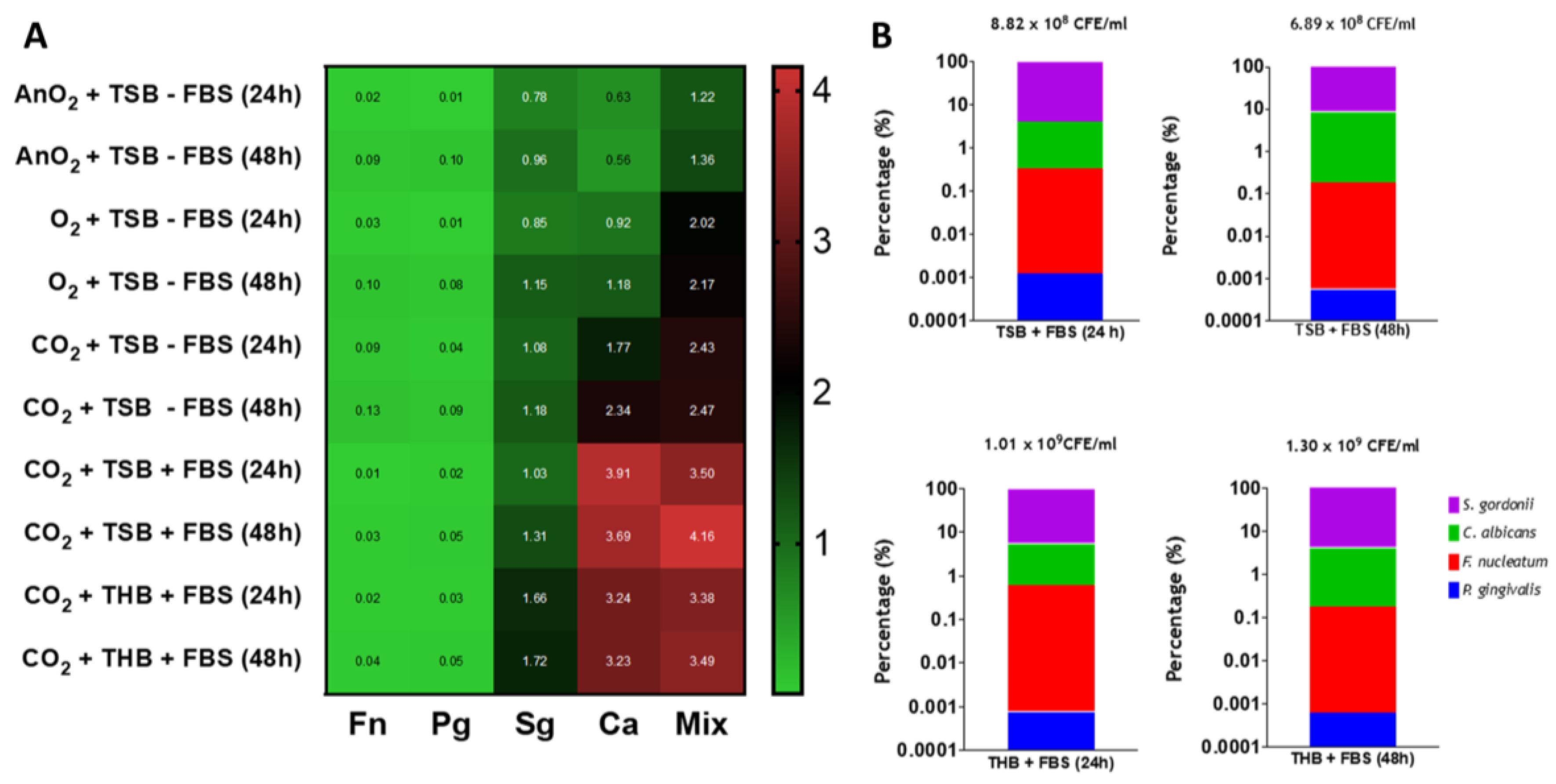
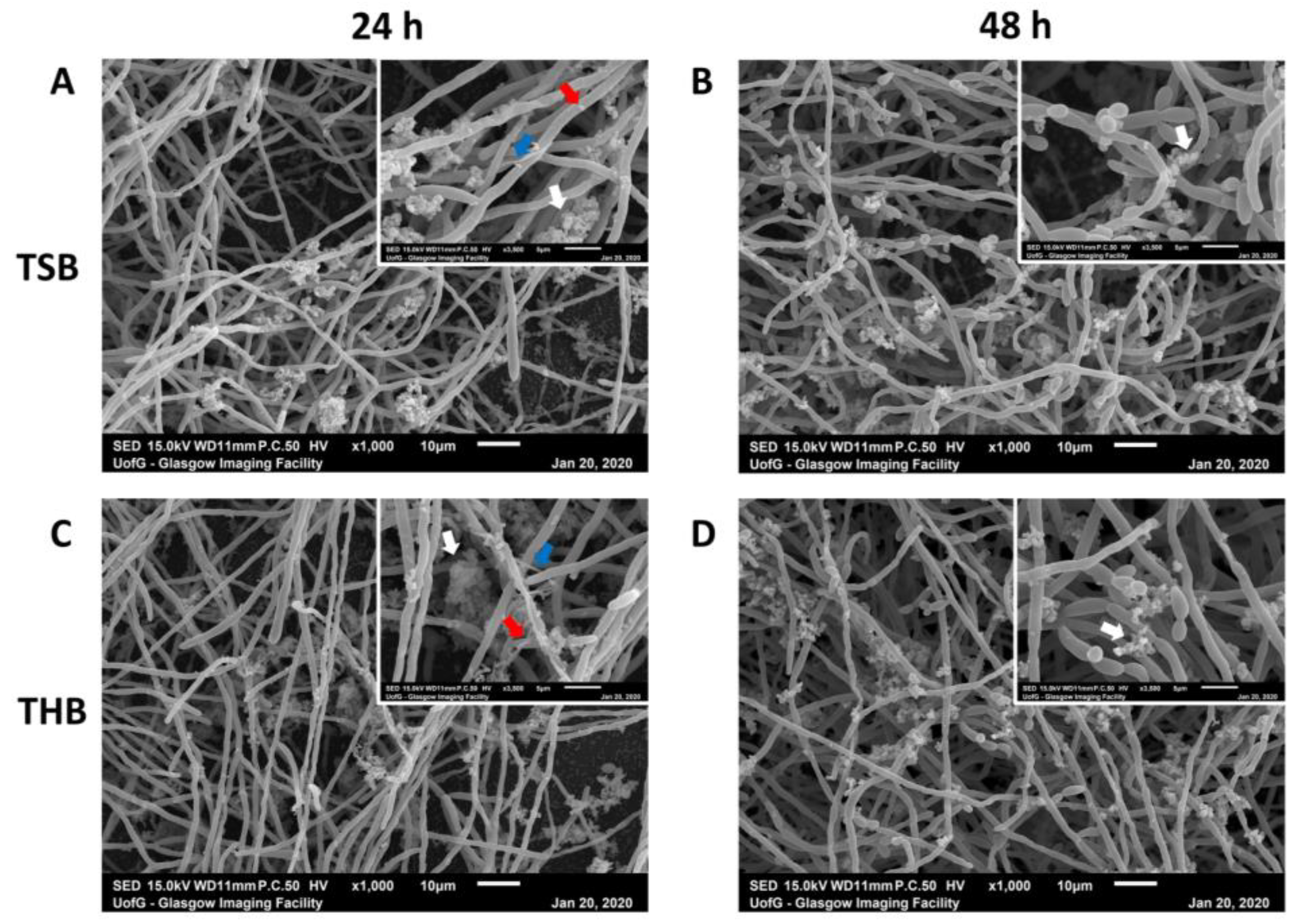
3.2. Endodontic Biofilm Model Optimisation
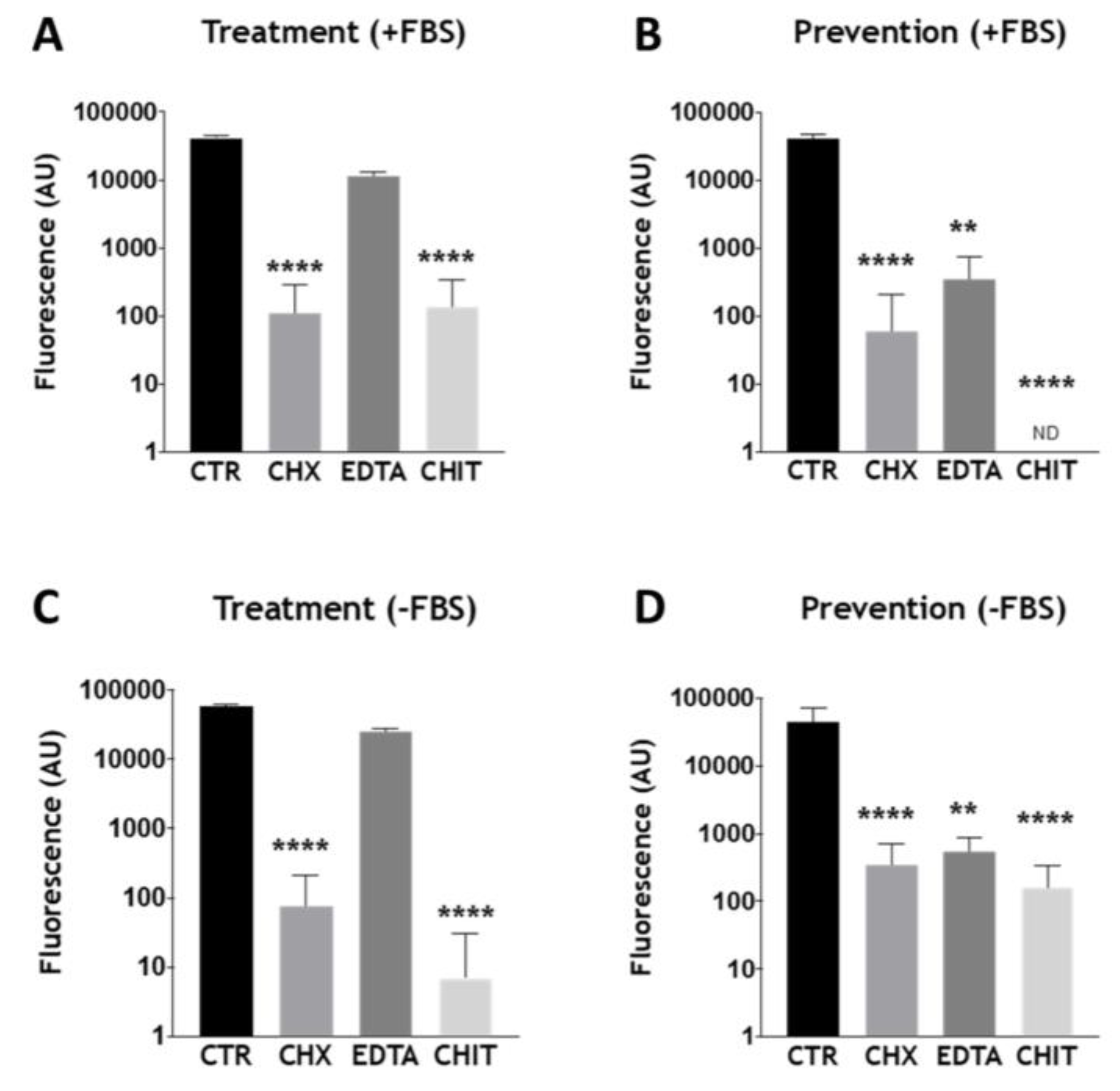
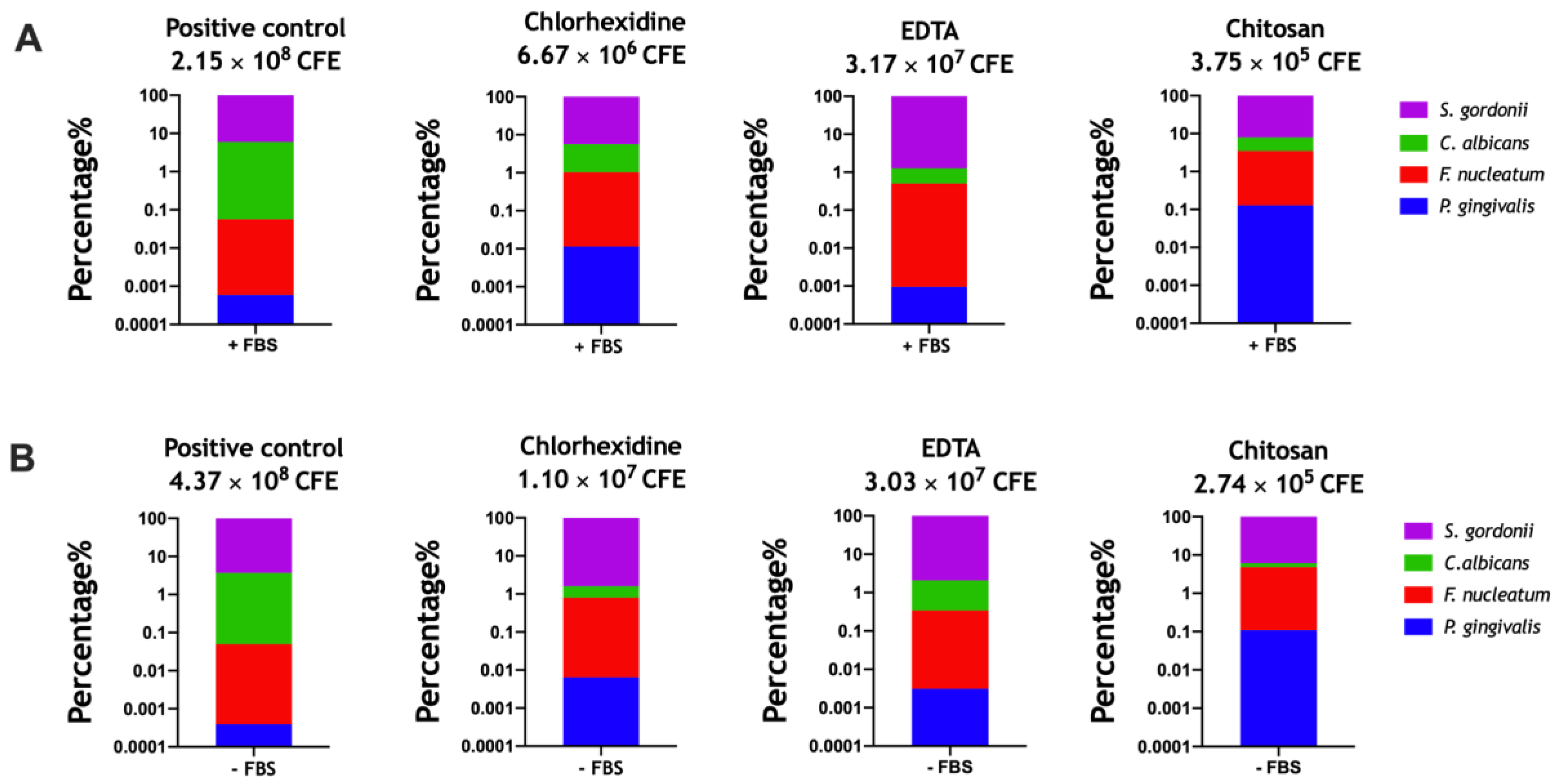
3.3. Assessing Antimicrobials within the Optimized Endodontic Biofilm Model
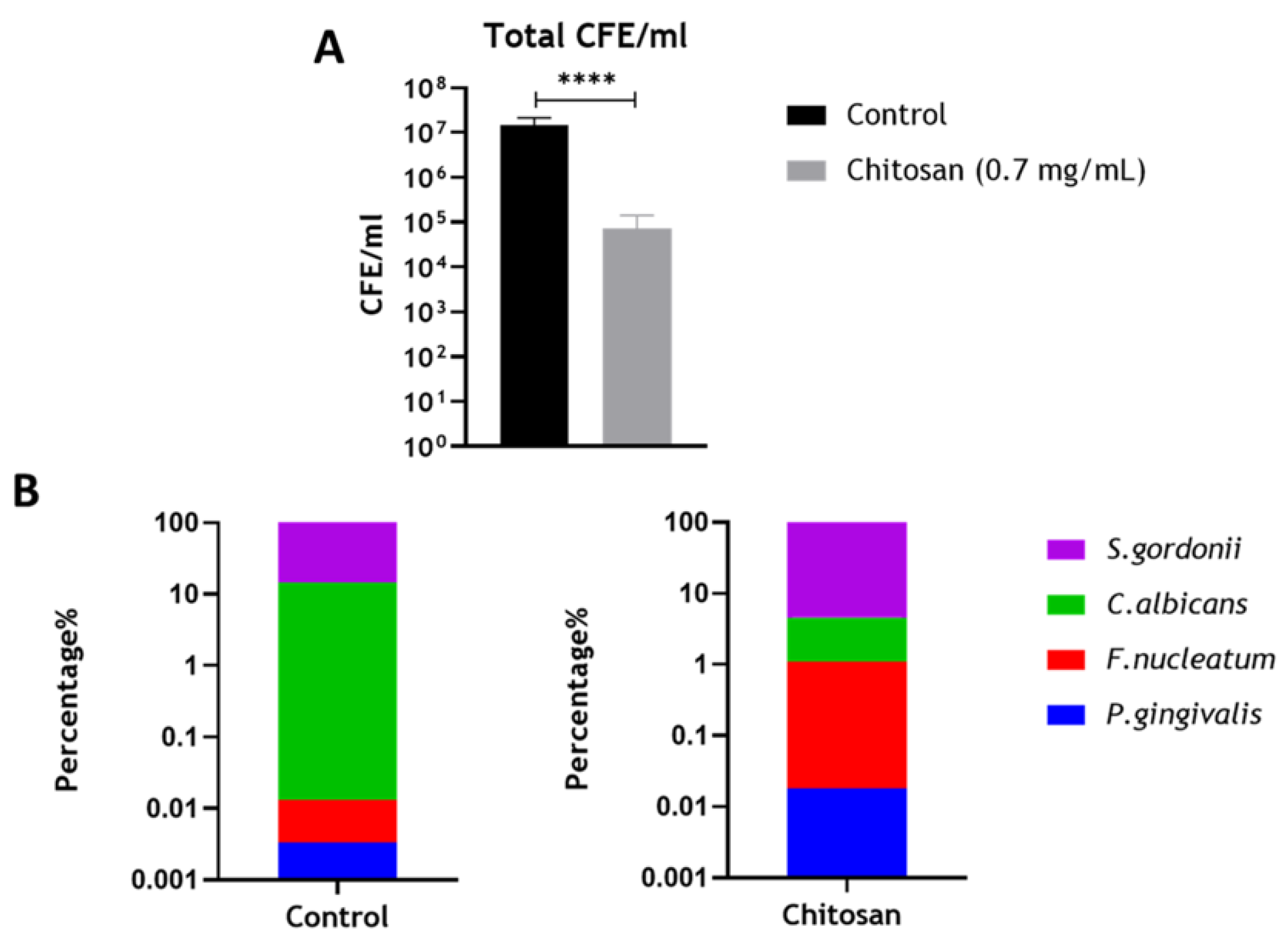
3.4. Assessing the Effect of Chitosan on the Optimized Endodontic Biofilm Model upon Dentine
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Swimberghe, R.; Coenye, T.; De Moor, R.; Meire, M. Biofilm model systems for root canal disinfection: A literature review. Int. Endod. J. 2019, 52, 604–628. [Google Scholar] [CrossRef] [PubMed]
- Abusrewil, S.; Alshanta, O.; Albashaireh, K.; Alqahtani, S.; Nile, C.; Scott, J.; McLean, W. Detection, treatment and prevention of endodontic biofilm infections: what’s new in 2020? Crit. Rev. Microbiol. 2020, 46, 194–212. [Google Scholar] [CrossRef] [PubMed]
- Delaney, C.; Kean, R.; Short, B.; Tumelty, M.; McLean, W.; Nile, C.J.; Ramage, G. Fungi at the Scene of the Crime: Innocent Bystanders or Accomplices in Oral Infections? Curr. Clin. Microbiol. Rep. 2018, 5, 190–200. [Google Scholar] [CrossRef]
- Freilich, S.; Zarecki, R.; Eilam, O.; Segal, E.S.; Henry, C.S.; Kupiec, M.; Gophna, U.; Sharan, R.; Ruppin, E. Competitive and cooperative metabolic interactions in bacterial communities. Nat. Commun. 2011, 2, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G.; Liang, S.; Payne, M.A.; Hashim, A.; Jotwani, R.; Eskan, M.A.; McIntosh, M.L.; Alsam, A.; Kirkwood, K.L.; Lambris, J.D. Low-abundance biofilm species orchestrates inflammatory periodontal disease through the commensal microbiota and complement. Cell Host Microbe 2011, 10, 497–506. [Google Scholar] [CrossRef]
- Ricucci, D.; Siqueira Jr, J.F.; Bate, A.L.; Ford, T.R.P. Histologic investigation of root canal–treated teeth with apical periodontitis: A retrospective study from twenty-four patients. J. Endod. 2009, 35, 493–502. [Google Scholar] [CrossRef]
- Ricucci, D.; Siqueira, J.F., Jr. Biofilms and apical periodontitis: Study of prevalence and association with clinical and histopathologic findings. J. Endod. 2010, 36, 1277–1288. [Google Scholar] [CrossRef]
- Brown, J.L.; Johnston, W.; Delaney, C.; Short, B.; Butcher, M.C.; Young, T.; Butcher, J.; Riggio, M.; Culshaw, S.; Ramage, G. Polymicrobial oral biofilm models: Simplifying the complex. J. Med. Microbiol. 2019, 68, 1573–1584. [Google Scholar] [CrossRef]
- Munson, M.A.; Pitt-Ford, T.; Chong, B.; Weightman, A.; Wade, W.G. Molecular and cultural analysis of the microflora associated with endodontic infections. J. Dent. Res. 2002, 81, 761–766. [Google Scholar] [CrossRef]
- Fabricius, L.; Dahlén, G.; Ohman, A.E.; Möller, A.J. Predominant indigenous oral bacteria isolated from infected root canals after varied times of closure. Scand. J. Dent. Res. 1982, 90, 134–144. [Google Scholar] [CrossRef]
- Shin, J.M.; Luo, T.; Lee, K.H.; Guerreiro, D.; Botero, T.M.; McDonald, N.J.; Rickard, A.H. Deciphering endodontic microbial communities by next-generation sequencing. J. Endod. 2018, 44, 1080–1087. [Google Scholar] [CrossRef] [PubMed]
- Manoil, D.; Al-Manei, K.; Belibasakis, G.N. A Systematic Review of the Root Canal Microbiota Associated with Apical Periodontitis: Lessons from Next-Generation Sequencing. PROTEOMICS–Clin. Appl. 2020, 14, 1900060. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.H.; Lee, K.W.K.; Burmølle, M.; Kjelleberg, S.; Rice, S.A. All together now: Experimental multispecies biofilm model systems. Environ. Microbiol. 2017, 19, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Marsh, P.D. The role of continuous culture in modelling the human microflora. J. Chem. Technol. Biotechnol. 1995, 64, 1–9. [Google Scholar] [CrossRef]
- Allkja, J.; Bjarnsholt, T.; Coenye, T.; Cos, P.; Fallarero, A.; Harrison, J.J.; Lopes, S.P.; Oliver, A.; Pereira, M.O.; Ramage, G.; et al. Minimum information guideline for spectrophotometric and fluorometric methods to assess biofilm formation in microplates. Biofilm 2020, 2, 100010. [Google Scholar] [CrossRef]
- Koci, O.; Logan, M.; Svolos, V.; Russell, R.K.; Gerasimidis, K.; Ijaz, U.Z. An automated identification and analysis of ontological terms in gastrointestinal diseases and nutrition-related literature provides useful insights. PeerJ 2018, 6, e5047. [Google Scholar] [CrossRef]
- Pafilis, E.; Buttigieg, P.L.; Ferrell, B.; Pereira, E.; Schnetzer, J.; Arvanitidis, C.; Jensen, L.J. EXTRACT: Interactive extraction of environment metadata and term suggestion for metagenomic sample annotation. Database (Oxford) 2016, 2016, baw005. [Google Scholar] [CrossRef]
- Miles, A.A.; Misra, S.S.; Irwin, J.O. The estimation of the bactericidal power of the blood. J. Hyg. 1938, 38, 732–749. [Google Scholar] [CrossRef]
- Montelongo-Jauregui, D.; Srinivasan, A.; Ramasubramanian, A.K.; Lopez-Ribot, J.L. An In Vitro Model for Oral Mixed Biofilms of Candida albicans and Streptococcus gordonii in Synthetic Saliva. Front. Microbiol. 2016, 7, 686. [Google Scholar] [CrossRef]
- Erlandsen, S.L.; Kristich, C.J.; Dunny, G.M.; Wells, C.L. High-resolution visualization of the microbial glycocalyx with low-voltage scanning electron microscopy: Dependence on cationic dyes. J. Histochem. Cytochem. 2004, 52, 1427–1435. [Google Scholar] [CrossRef]
- Sherry, L.; Lappin, G.; O’Donnell, L.E.; Millhouse, E.; Millington, O.R.; Bradshaw, D.J.; Axe, A.S.; Williams, C.; Nile, C.J.; Ramage, G. Viable Compositional Analysis of an Eleven Species Oral Polymicrobial Biofilm. Front. Microbiol. 2016, 7, 912. [Google Scholar] [CrossRef] [PubMed]
- CLSI. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts. Clinical and Laboratory Standards Institute (CLSI), 3rd ed.; Document M27-A3; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008. [Google Scholar]
- Vieira, A.P.M.; Arias, L.S.; de Souza Neto, F.N.; Kubo, A.M.; Lima, B.H.R.; de Camargo, E.R.; Pessan, J.P.; Delbem, A.C.B.; Monteiro, D.R. Antibiofilm effect of chlorhexidine-carrier nanosystem based on iron oxide magnetic nanoparticles and chitosan. Colloids Surf. B Biointerfaces 2019, 174, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Persoon, I.F.; Buijs, M.J.; Ozok, A.R.; Crielaard, W.; Krom, B.P.; Zaura, E.; Brandt, B.W. The mycobiome of root canal infections is correlated to the bacteriome. Clin. Oral. Investig. 2017, 21, 1871–1881. [Google Scholar] [CrossRef] [PubMed]
- Rabea, E.I.; Badawy, M.E.; Stevens, C.V.; Smagghe, G.; Steurbaut, W. Chitosan as antimicrobial agent: Applications and mode of action. Biomacromolecules 2003, 4, 1457–1465. [Google Scholar] [CrossRef] [PubMed]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef]
- Shih, P.Y.; Liao, Y.T.; Tseng, Y.K.; Deng, F.S.; Lin, C.H. A Potential Antifungal Effect of Chitosan Against Candida albicans Is Mediated via the Inhibition of SAGA Complex Component Expression and the Subsequent Alteration of Cell Surface Integrity. Front. Microbiol. 2019, 10, 602. [Google Scholar] [CrossRef]
- Pu, Y.; Liu, A.; Zheng, Y.; Ye, B. In vitro damage of Candida albicans biofilms by chitosan. Exp. Ther. Med. 2014, 8, 929–934. [Google Scholar] [CrossRef]
- Pena, A.; Sanchez, N.S.; Calahorra, M. Effects of chitosan on Candida albicans: Conditions for its antifungal activity. Biomed. Res. Int. 2013, 2013, 527549. [Google Scholar] [CrossRef]
- Ganan, M.; Lorentzen, S.B.; Agger, J.W.; Heyward, C.A.; Bakke, O.; Knutsen, S.H.; Aam, B.B.; Eijsink, V.G.H.; Gaustad, P.; Sorlie, M. Antifungal activity of well-defined chito-oligosaccharide preparations against medically relevant yeasts. PLoS ONE 2019, 14, e0210208. [Google Scholar] [CrossRef]
- Arias, L.S.; Butcher, M.C.; Short, B.; McKloud, E.; Delaney, C.; Kean, R.; Monteiro, D.R.; Williams, C.; Ramage, G.; Brown, J.L. Chitosan Ameliorates Candida auris Virulence in a Galleria mellonella Infection Model. Antimicrob. Agents Chemother. 2020, 64. [Google Scholar] [CrossRef]
- Alves, F.R.; Siqueira, J.F., Jr.; Carmo, F.L.; Santos, A.L.; Peixoto, R.S.; Rôças, I.N.; Rosado, A.S. Bacterial community profiling of cryogenically ground samples from the apical and coronal root segments of teeth with apical periodontitis. J. Endod. 2009, 35, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Chavez de Paz, L.E. Redefining the persistent infection in root canals: Possible role of biofilm communities. J. Endod. 2007, 33, 652–662. [Google Scholar] [CrossRef] [PubMed]
- Sztukowska, M.N.; Dutton, L.C.; Delaney, C.; Ramsdale, M.; Ramage, G.; Jenkinson, H.F.; Nobbs, A.H.; Lamont, R.J. Community Development between Porphyromonas gingivalis and Candida albicans Mediated by InlJ and Als3. mBio 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Arzmi, M.H.; Dashper, S.; Catmull, D.; Cirillo, N.; Reynolds, E.C.; McCullough, M. Coaggregation of Candida albicans, Actinomyces naeslundii and Streptococcus mutans is Candida albicans strain dependent. FEMS Yeast Res. 2015, 15, fov038. [Google Scholar] [CrossRef]
- Leonhard, M.; Zatorska, B.; Moser, D.; Schneider-Stickler, B. Growth Media for Mixed Multispecies Oropharyngeal Biofilm Compositions on Silicone. Biomed. Res. Int. 2019, 2019, 8051270. [Google Scholar] [CrossRef]
- Bradshaw, D.J.; Marsh, P.D.; Watson, G.K.; Allison, C. Role of Fusobacterium nucleatum and coaggregation in anaerobe survival in planktonic and biofilm oral microbial communities during aeration. Infect Immun. 1998, 66, 4729–4732. [Google Scholar] [CrossRef]
- Bradshaw, D.J.; Marsh, P.D.; Watson, G.K.; Allison, C. Oral anaerobes cannot survive oxygen stress without interacting with facuItative/aerobic species as a microbial commmunity. Lett. Appl. Microbiol. 1997, 25, 385–387. [Google Scholar] [CrossRef]
- Diaz, P.I.; Rogers, A.H. The effect of oxygen on the growth and physiology of Porphyromonas gingivalis. Oral Microbiol. Immunol. 2004, 19, 88–94. [Google Scholar] [CrossRef]
- Diaz, P.I.; Zilm, P.S.; Rogers, A.H. Fusobacterium nucleatum supports the growth of Porphyromonas gingivalis in oxygenated and carbon-dioxide-depleted environments. Microbiology 2002, 148, 467–472. [Google Scholar] [CrossRef]
- Fox, E.P.; Cowley, E.S.; Nobile, C.J.; Hartooni, N.; Newman, D.K.; Johnson, A.D. Anaerobic bacteria grow within Candida albicans biofilms and induce biofilm formation in suspension cultures. Curr. Biol. 2014, 24, 2411–2416. [Google Scholar] [CrossRef]
- Zhou, Y.; Millhouse, E.; Shaw, T.; Lappin, D.F.; Rajendran, R.; Bagg, J.; Lin, H.; Ramage, G. Evaluating Streptococcus mutans Strain Dependent Characteristics in a Polymicrobial Biofilm Community. Front. Microbiol. 2018, 9, 1498. [Google Scholar] [CrossRef] [PubMed]
- Ramage, G.; O’Donnell, L.; Sherry, L.; Culshaw, S.; Bagg, J.; Czesnikiewicz-Guzik, M.; Brown, C.; McKenzie, D.; Cross, L.; MacInnes, A.; et al. Impact of frequency of denture cleaning on microbial and clinical parameters—A bench to chairside approach. J. Oral. Microbiol. 2019, 11, 1538437. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.L.; Johnston, W.; Delaney, C.; Rajendran, R.; Butcher, J.; Khan, S.; Bradshaw, D.; Ramage, G.; Culshaw, S. Biofilm-stimulated epithelium modulates the inflammatory responses in co-cultured immune cells. Sci. Rep. 2019, 9, 15779. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Darveau, R.P.; Curtis, M.A. The keystone-pathogen hypothesis. Nat. Rev. Microbiol. 2012, 10, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Hagi, T.T.; Klemensberger, S.; Bereiter, R.; Nietzsche, S.; Cosgarea, R.; Flury, S.; Lussi, A.; Sculean, A.; Eick, S. A Biofilm Pocket Model to Evaluate Different Non-Surgical Periodontal Treatment Modalities in Terms of Biofilm Removal and Reformation, Surface Alterations and Attachment of Periodontal Ligament Fibroblasts. PLoS ONE 2015, 10, e0131056. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Stojicic, S.; Qian, W.; Olsen, I.; Haapasalo, M. The synergistic antimicrobial effect by mechanical agitation and two chlorhexidine preparations on biofilm bacteria. J. Endod. 2010, 36, 100–104. [Google Scholar] [CrossRef]
- Sena, N.T.; Gomes, B.P.; Vianna, M.E.; Berber, V.B.; Zaia, A.A.; Ferraz, C.C.; Souza-Filho, F.J. In vitro antimicrobial activity of sodium hypochlorite and chlorhexidine against selected single-species biofilms. Int. Endod. J. 2006, 39, 878–885. [Google Scholar] [CrossRef]
- Alshanta, O.A.; Alqahtani, S.; Shaban, S.; Albashaireh, K.; McLean, W.; Ramage, G. Comparison of Three Endodontic Irrigant Regimens against Dual-Species Interkingdom Biofilms: Considerations for Maintaining the Status Quo. Antibiotics 2020, 9, 634. [Google Scholar] [CrossRef]
- Alshanta, O.A.; Shaban, S.; Nile, C.J.; McLean, W.; Ramage, G. Candida albicans Biofilm Heterogeneity and Tolerance of Clinical Isolates: Implications for Secondary Endodontic Infections. Antibiotics 2019, 8, 204. [Google Scholar] [CrossRef]
- Tamara, F.R.; Lin, C.; Mi, F.L.; Ho, Y.C. Antibacterial Effects of Chitosan/Cationic Peptide Nanoparticles. Nanomaterials 2018, 8, 88. [Google Scholar] [CrossRef]
- Arias, L.S.; Pessan, J.P.; de Souza Neto, F.N.; Lima, B.H.R.; de Camargo, E.R.; Ramage, G.; Delbem, A.C.B.; Monteiro, D.R. Novel nanocarrier of miconazole based on chitosan-coated iron oxide nanoparticles as a nanotherapy to fight Candida biofilms. Colloids Surf. B Biointerfaces 2020, 192, 111080. [Google Scholar] [CrossRef] [PubMed]
- Del Carpio-Perochena, A.; Kishen, A.; Felitti, R.; Bhagirath, A.Y.; Medapati, M.R.; Lai, C.; Cunha, R.S. Antibacterial Properties of Chitosan Nanoparticles and Propolis Associated with Calcium Hydroxide against Single- and Multispecies Biofilms: An In Vitro and In Situ Study. J. Endod. 2017, 43, 1332–1336. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.S.; Cai, X.; Guo, J.M.; Pashley, D.H.; Breschi, L.; Xu, H.H.K.; Wang, X.Y.; Tay, F.R.; Niu, L.N. Chitosan-Based Extrafibrillar Demineralization for Dentin Bonding. J. Dent. Res. 2019, 98, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Arias, L.S.; Brown, J.L.; Butcher, M.C.; Delaney, C.; Monteiro, D.R.; Ramage, G. A nanocarrier system that potentiates the effect of miconazole within different interkingdom biofilms. J. Oral Microbiol. 2020, 12, 1771071. [Google Scholar] [CrossRef] [PubMed]
- Teruel Jde, D.; Alcolea, A.; Hernandez, A.; Ruiz, A.J. Comparison of chemical composition of enamel and dentine in human, bovine, porcine and ovine teeth. Arch. Oral Biol. 2015, 60, 768–775. [Google Scholar] [CrossRef] [PubMed]
- Ururahy, M.S.; Curylofo-Zotti, F.A.; Galo, R.; Nogueira, L.F.; Ramos, A.P.; Corona, S.A. Wettability and surface morphology of eroded dentin treated with chitosan. Arch. Oral Biol. 2017, 75, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Mohammed Hassan, A.; Ali Goda, A.; Baroudi, K. The effect of different disinfecting agents on bond strength of resin composites. Int. J. Dent. 2014, 2014, 231235. [Google Scholar] [CrossRef]
- Gandolfi, M.G.; Taddei, P.; Pondrelli, A.; Zamparini, F.; Prati, C.; Spagnuolo, G. Demineralization, Collagen Modification and Remineralization Degree of Human Dentin after EDTA and Citric Acid Treatments. Materials 2018, 12, 25. [Google Scholar] [CrossRef]
- Janus, M.M.; Willems, H.M.; Krom, B.P. Candida albicans in Multispecies Oral Communities; A Keystone Commensal? Adv. Exp. Med. Biol. 2016, 931, 13–20. [Google Scholar] [CrossRef]
| Organism | Forward Primer 5’-3’ | Reverse Primer 5’-3’ |
|---|---|---|
| S. gordonii | GATACATAGCCGACCTGAG | TCCATTGCCGAAGATTCC |
| F. nucleatum | GGATTTATTGGGCGTAAAGC | GGCATTCCTACAAATATCTACGAA |
| P. gingivalis | GGAAGAGAAGACCGTAGCACAAGGA | GAGTAGGCGAAACGTCCATCAGGTC |
| C. albicans | CTCGTAGTTGAACCTTGGGC | GGCCTGCTTTGAACACTCTA |
| Percentage Composition (%) * | ||||
|---|---|---|---|---|
| S. gordonii | C. albicans | F. nucleatum | P. gingivalis | |
| TSB (24 h) | 95.79 | 3.86 | 0.34 | 0.001 |
| TSB (48 h) | 91.23 | 8.57 | 0.19 | 0.001 |
| THB (24 h) | 94.37 | 5.00 | 0.63 | 0.001 |
| TSB (48 h) | 95.82 | 4.00 | 0.18 | 0.001 |
| Percentage Composition (%) * | ||||||||
|---|---|---|---|---|---|---|---|---|
| S. gordonii | C. albicans | F. nucleatum | P. gingivalis (%) | |||||
| −FBS | +FBS | −FBS | +FBS | −FBS | +FBS | −FBS | +FBS | |
| +CONT | 96.30 | 93.98 | 3.65 | 5.96 | 0.05 | 0.06 | 0.0004 | 0.001 |
| CHX | 98.41 | 94.36 | 0.78 | 4.61 | 0.79 | 1.02 | 0.006 | 0.011 |
| EDTA | 97.91 | 98.73 | 1.74 | 0.76 | 0.38 | 0.51 | 0.003 | 0.001 |
| CHITOSAN | 93.85 | 92.10 | 1.35 | 4.42 | 4.68 | 3.36 | 0.110 | 0.130 |
| Percentage Composition (%) * | ||||
|---|---|---|---|---|
| S. gordonii | C. albicans | F. nucleatum | P. gingivalis | |
| + CONT | 85.54 | 14.51 | 0.01 | 0.003 |
| CHITOSAN | 95.42 | 3.48 | 1.09 | 0.018 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abusrewil, S.; Brown, J.L.; Delaney, C.D.; Butcher, M.C.; Kean, R.; Gamal, D.; Scott, J.A.; McLean, W.; Ramage, G. Filling the Void: An Optimized Polymicrobial Interkingdom Biofilm Model for Assessing Novel Antimicrobial Agents in Endodontic Infection. Microorganisms 2020, 8, 1988. https://doi.org/10.3390/microorganisms8121988
Abusrewil S, Brown JL, Delaney CD, Butcher MC, Kean R, Gamal D, Scott JA, McLean W, Ramage G. Filling the Void: An Optimized Polymicrobial Interkingdom Biofilm Model for Assessing Novel Antimicrobial Agents in Endodontic Infection. Microorganisms. 2020; 8(12):1988. https://doi.org/10.3390/microorganisms8121988
Chicago/Turabian StyleAbusrewil, Sumaya, Jason L. Brown, Christopher D. Delaney, Mark C. Butcher, Ryan Kean, Dalia Gamal, J. Alun Scott, William McLean, and Gordon Ramage. 2020. "Filling the Void: An Optimized Polymicrobial Interkingdom Biofilm Model for Assessing Novel Antimicrobial Agents in Endodontic Infection" Microorganisms 8, no. 12: 1988. https://doi.org/10.3390/microorganisms8121988
APA StyleAbusrewil, S., Brown, J. L., Delaney, C. D., Butcher, M. C., Kean, R., Gamal, D., Scott, J. A., McLean, W., & Ramage, G. (2020). Filling the Void: An Optimized Polymicrobial Interkingdom Biofilm Model for Assessing Novel Antimicrobial Agents in Endodontic Infection. Microorganisms, 8(12), 1988. https://doi.org/10.3390/microorganisms8121988







