Exopolymeric Substances Control Microbial Community Structure and Function by Contributing to both C and Fe Nutrition in Fe-Limited Southern Ocean Provinces
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Setup
2.2. Chemical Parameters
2.3. Biological Parameters
2.4. Statistical Analysis
3. Results
3.1. Nutrients and dFe Concentrations
3.2. Plankton Community Structure
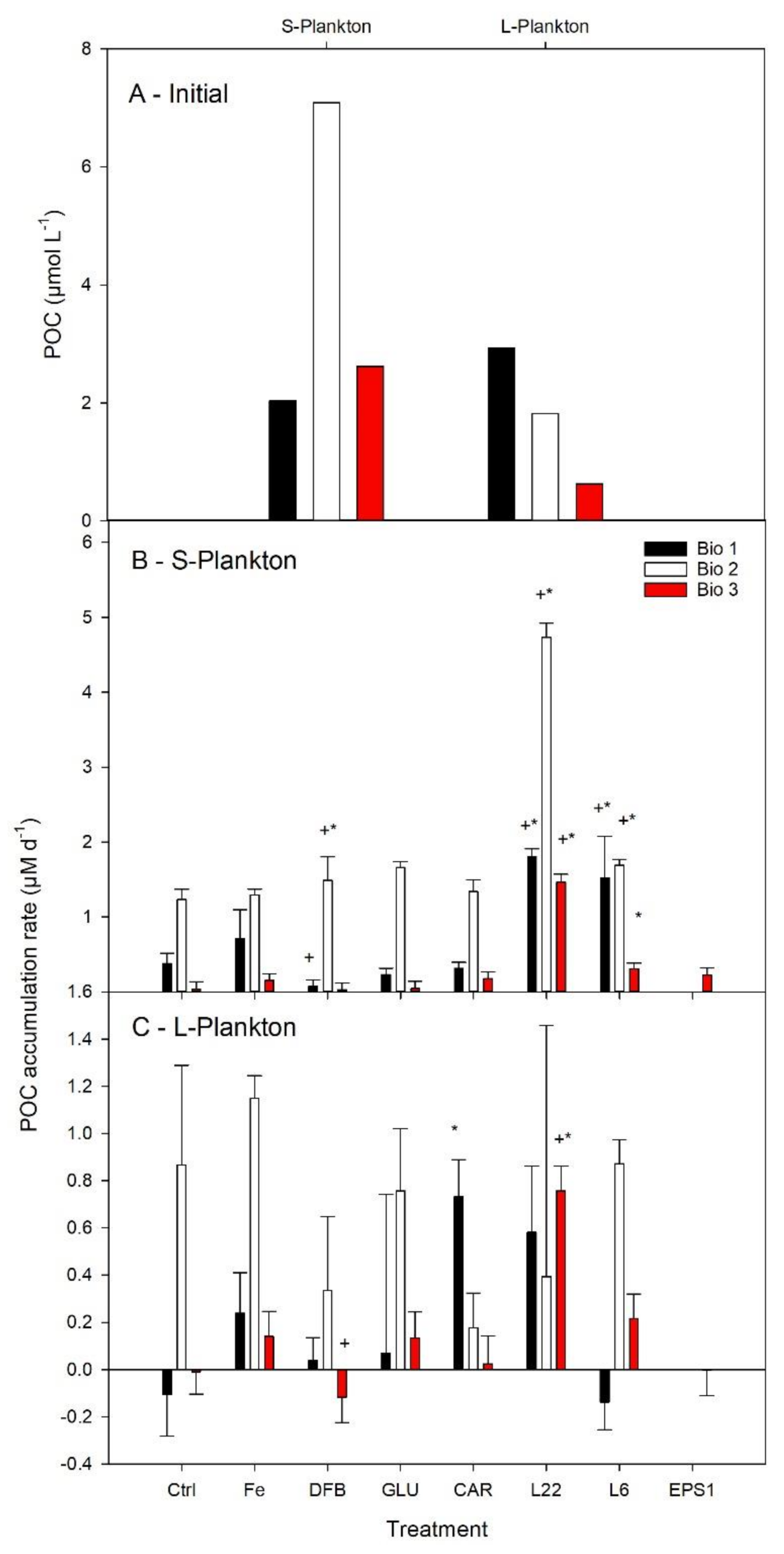
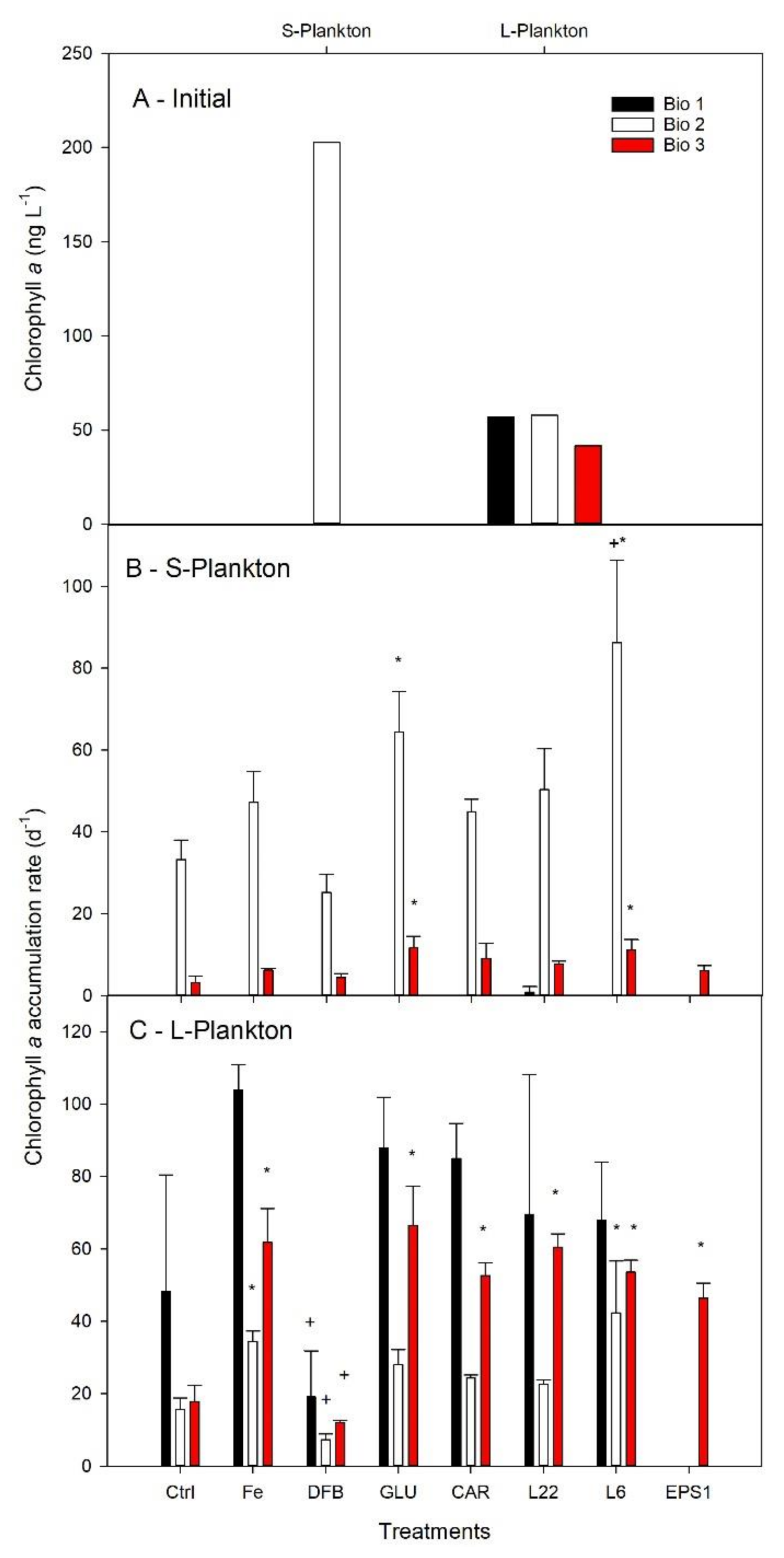
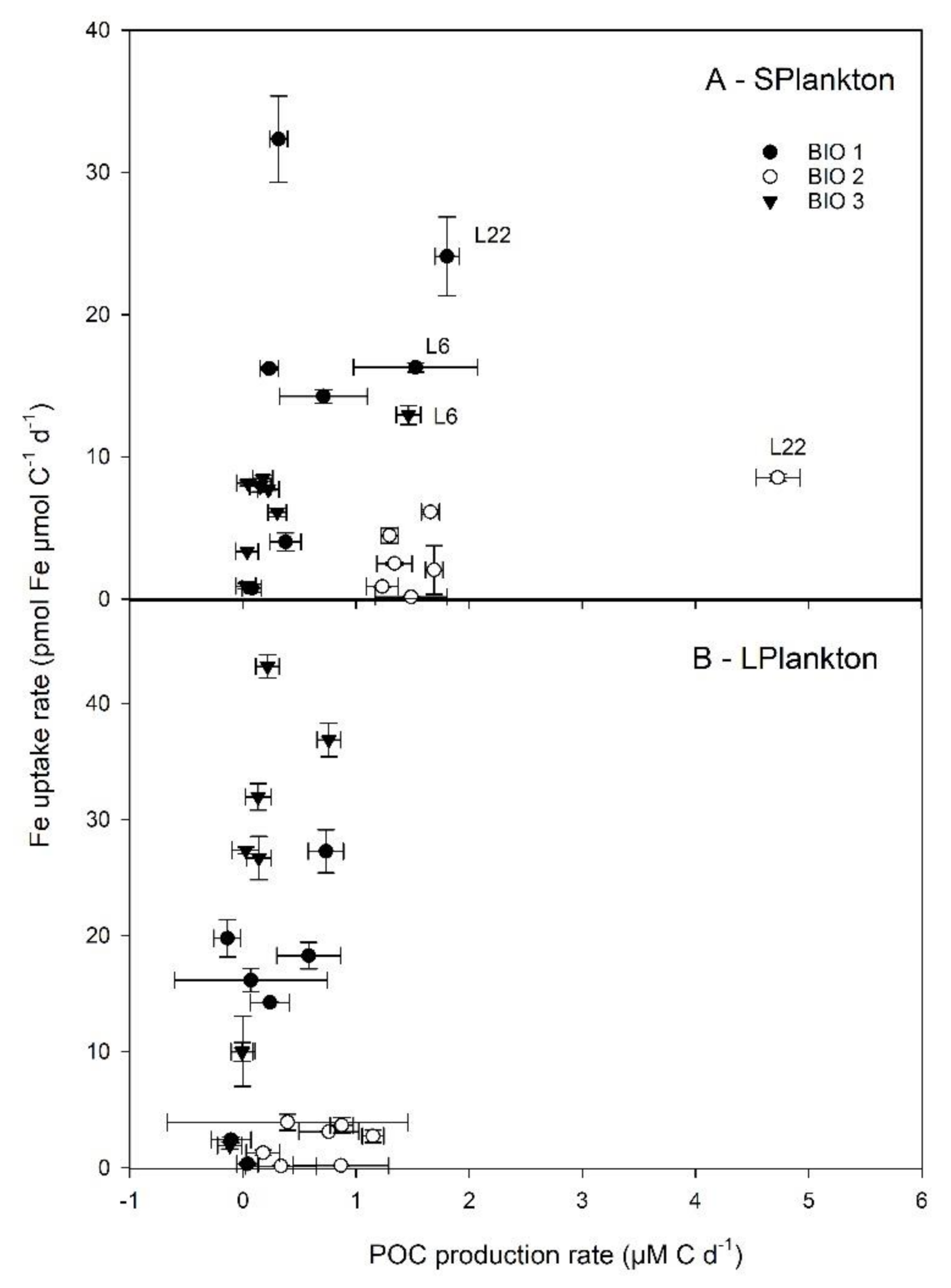
3.3. Plankton Biomass and Their Accumulation Rates
3.4. Photophysiological Responses to Fe Enrichment
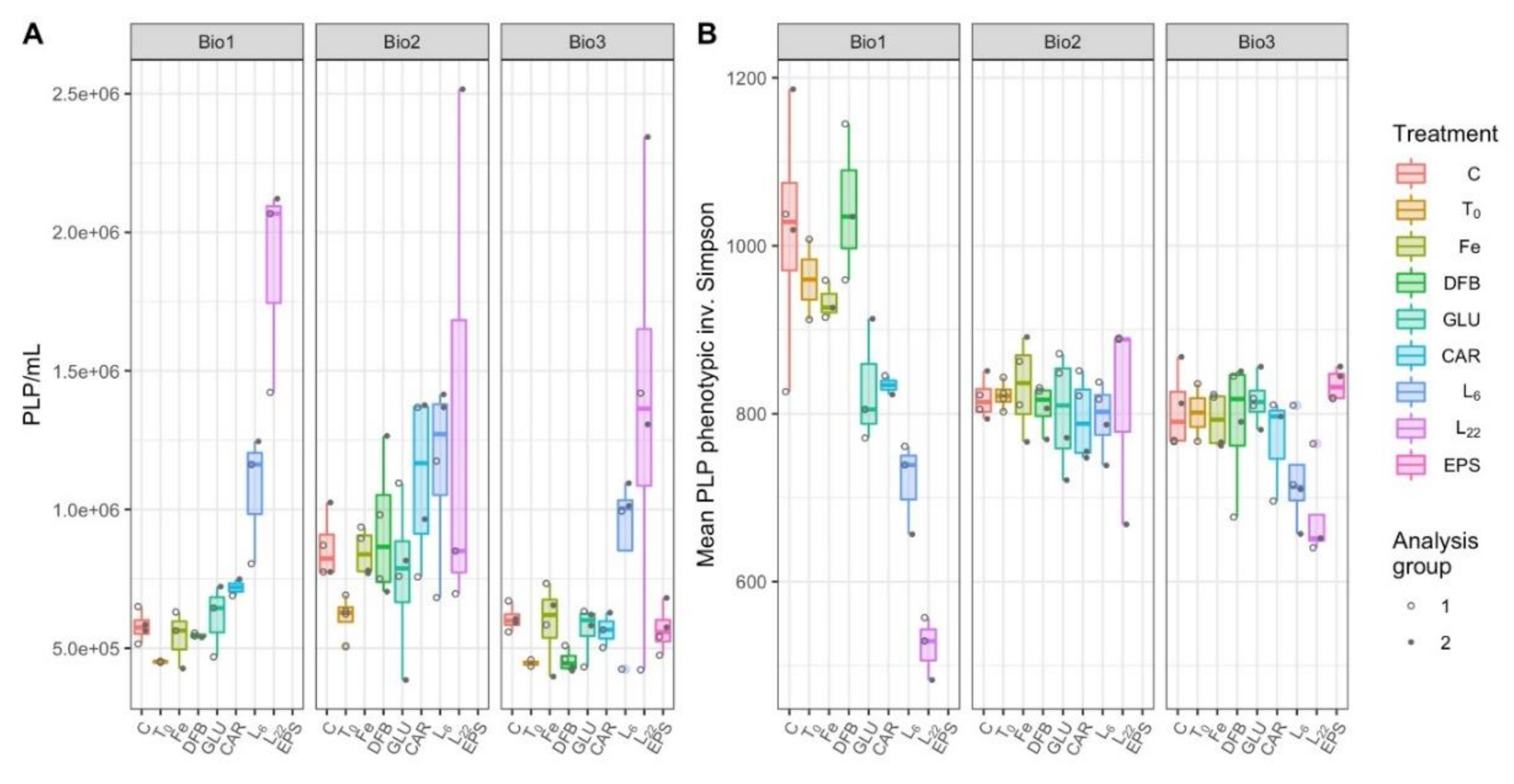
3.5. Prokaryote-Like Particles Concentration and Diversity
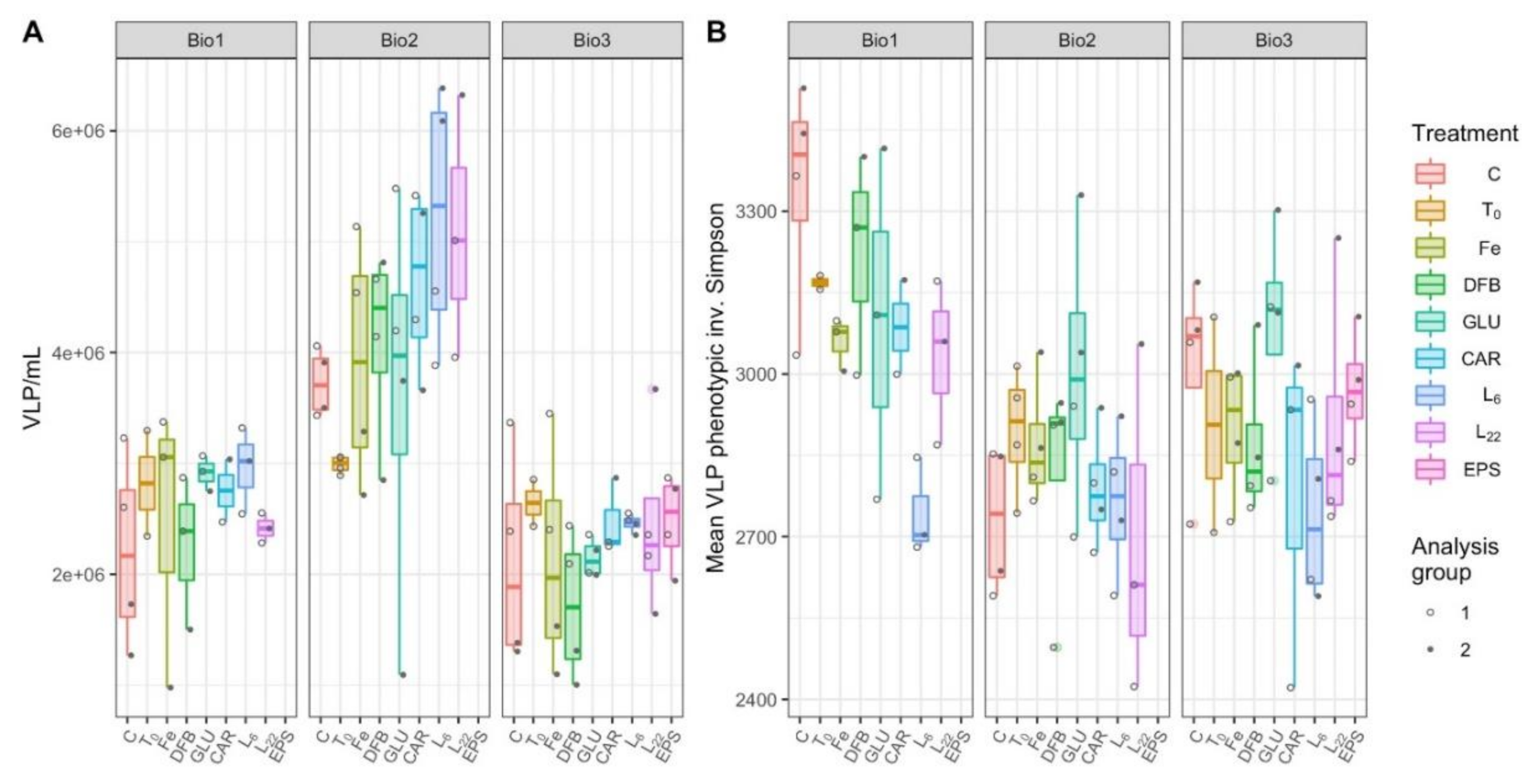
3.6. Virus-Like Particles Concentration and Diversity
4. Discussion
4.1. Evidence for Fe-Limitation at All Three Sampling Sites
4.2. Response to Fe and Organic Ligands Additions by Virus, Prokaryotes, and Plankton
4.3. The Response of the Plankton Community to the Different Additions
4.4. The Response by Prokaryotes and Virus Communities to the Different Additions
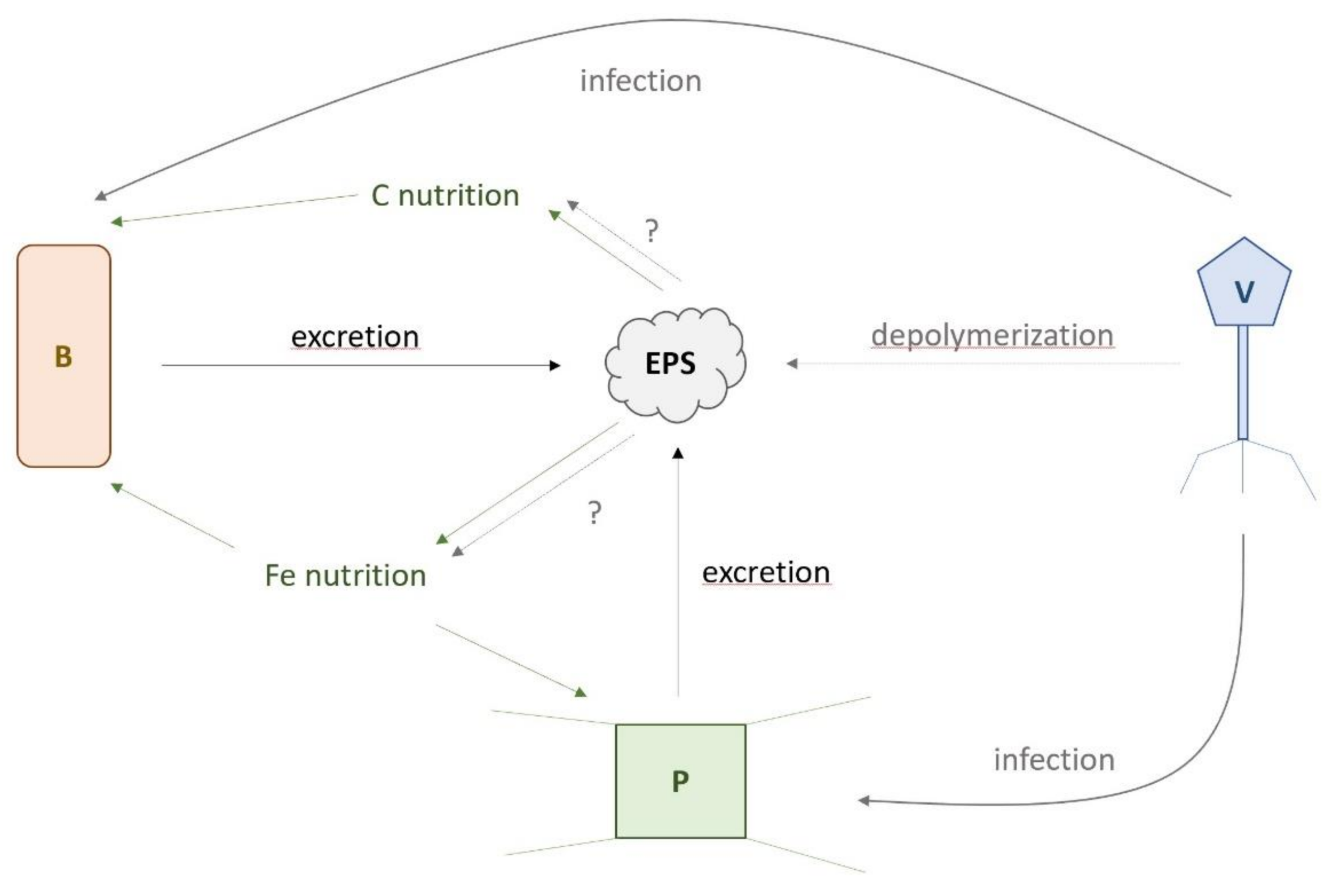
4.5. Conceptual Framework on How EPS Could Affect SO Microbial Community
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Suttle, C.A. Marine viruses-major players in the global ecosystem. Nat. Rev. Microbiol. 2007, 5, 801–812. [Google Scholar] [CrossRef] [PubMed]
- Breitbart, M.; Bonnain, C.; Malki, K.; Sawaya, N.A. Phage puppet masters of the marine microbial realm. Nat. Microbiol. 2018, 3, 754–766. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, S.W.; Suttle, C.A. Viruses and nutrient cycles in the sea: Viruses play critical roles in the structure and function of aquatic food webs. Bioscience 1999, 49, 781–788. [Google Scholar] [CrossRef] [Green Version]
- Fuhrman, J.A. Marine viruses and their biogeochemical and ecological effects. Nature 1999, 399, 541–548. [Google Scholar] [CrossRef]
- Chisholm, S.W. Oceanography: Stirring times in the Southern Ocean. Nature 2000, 407, 685–687. [Google Scholar] [CrossRef]
- Jiao, N.; Herndl, G.J.; Hansell, D.A.; Benner, R.; Kattner, G.; Wilhelm, S.W.; Kirchman, D.L.; Weinbauer, M.G.; Luo, T.; Chen, F.; et al. Microbial production of recalcitrant dissolved organic matter: Long-term carbon storage in the global ocean. Nat. Rev. Microbiol. 2010, 8, 593–599. [Google Scholar] [CrossRef] [Green Version]
- Boyd, P.W.; Watson, A.J.; Law, C.S.; Abraham, E.R.; Trull, T.; Murdoch, R.; Bakker, D.C.E.; Bowie, A.R.; Buesseler, K.O.; Chang, H.; et al. A mesoscale phytoplankton bloom in the polar Southern Ocean stimulated by iron fertilization. Nature 2000, 407, 695–702. [Google Scholar] [CrossRef]
- Martin, J.H. Glacial-interglacial CO2 change: The Iron Hypothesis. Paleoceanography 1990, 5, 1–13. [Google Scholar] [CrossRef]
- Fourquez, M.; Bressac, M.; Deppeler, S.L.; Ellwood, M.; Obernosterer, I.; Trull, T.W.; Boyd, P.W. Microbial competition in the Subpolar Southern Ocean: An Fe-C co-limitation experiment. Front. Mar. Sci. 2020, 6, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Hutchins, D.; Bruland, K.W. Grazer-mediated regeneration and assimilation of Fe, Zn and Mn from planktonic prey. Mar. Ecol. Prog. Ser. 1994, 110, 259–269. [Google Scholar] [CrossRef]
- Poorvin, L.; Rinta-Kanto, J.M.; Hutchins, D.A.; Wilhelm, S.W. Viral release of iron and its bioavailability to marine plankton. Limnol. Oceanogr. 2004, 49, 1734–1741. [Google Scholar] [CrossRef] [Green Version]
- Bonnain, C.; Breitbart, M.; Buck, K.N. The ferrojan horse hypothesis: Iron-virus interactions in the Ocean. Front. Mar. Sci. 2016, 3, 82. [Google Scholar] [CrossRef] [Green Version]
- Obernosterer, I.; Fourquez, M.; Blain, S. Fe and C co-limitation of heterotrophic bacteria in the naturally fertilized region off the Kerguelen Islands. Biogeosciences 2015, 12, 1983–1992. [Google Scholar] [CrossRef] [Green Version]
- Hassler, C.; Cabanes, D.J.E.; Blanco-Ameijeiras, S.; Sanders, S.G.; Benner, R. The role of labile and refractory ligands in the global ocean iron cycle: Closing the loop. Mar. Fresh. Res. 2020, 71, 311–320. [Google Scholar] [CrossRef]
- Caprara, S.; Buck, K.N.; Gerringa, L.J.A.; Rijkenberg, M.J.A.; Monticelli, D.A. Compilation of iron speciation data for open oceanic waters. Front. Mar. Sci. 2016, 3, 221. [Google Scholar] [CrossRef] [Green Version]
- Raven, J.A.; Evans, M.C.W.; Korb, R.E. The role of trace metals in photosynthetic electron transport in O2-evolving organisms. Photosynth. Res. 1999, 60, 111–150. [Google Scholar] [CrossRef]
- Lis, H.; Shaked, Y.; Kranzler, C.; Keren, N.; Morel, F.M.M. Iron bioavailability to phytoplankton: An empirical approach. ISME J. 2015, 9, 1003–1013. [Google Scholar] [CrossRef] [Green Version]
- Velazquez, I.B.; Ibisanmi, E.; Maas, E.W.; Boyd, P.W.; Nodder, S.; Sander, S.G. Ferrioxamine siderophores detected amongst iron binding ligands produced during the remineralization of marine particles. Front. Mar. Sci. 2016, 3, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Decho, A.W. Microbial exopolymer secretions in ocean environments: Their role (s) in food webs and marine processes. Oceanogr. Mar. Biol. Annu. Rev. 1990, 28, 73–153. [Google Scholar]
- Norman, L.; Worms, I.A.M.; Angles, E.; Bowie, A.R.; Nichols, C.M.; Ninh Pham, A.; Slaveykova, V.; Townsend, A.T.; Waite, T.D.; Hassler, C.S. The role of bacterial and algal exopolymeric substances in iron chemistry. Mar. Chem. 2015, 173, 148–161. [Google Scholar] [CrossRef]
- Hassler, C.S.; Norman, L.; Mancuso Nichols, C.A.; Clementson, L.A.; Robinson, C.; Schoemann, V.; Watson, R.J.; Doblin, M.A. Iron associated with exopolymeric substances is highly bioavailable to oceanic phytoplankton. Mar. Chem. 2015, 173, 136–147. [Google Scholar] [CrossRef]
- Benner, R.; Pakulski, J.D.; McCarthy, M.; Hedges, J.I.; Hatcher, P.G. Bulk chemical characteristics of dissolved organic matter in the ocean. Science 1992, 255, 1561–1564. [Google Scholar] [CrossRef] [PubMed]
- Cabanes, D.J.E.; Blanco-Ameijeiras, S.; Bergin, K.; Trimborn, S.; Völkner, C.; Lelchat, F.; Hassler, C.S. Using Fe chemistry to predict its bioavailability for natural plankton assemblages from the SO. Mar. Chem. 2020, 225, 103853. [Google Scholar] [CrossRef]
- Lelchat, F.; Cérantola, S.; Brandily, C.; Colliec-Jouault, S.; Baudoux, A.-C.; Ojima, T.; Boisset, C. The marine bacteria Cobetia marina DSMZ 4741 synthesizes an unexpected K-antigen-like exopolysaccharide. Carbohydr. Polym. 2015, 124, 347–356. [Google Scholar] [CrossRef] [Green Version]
- Hathorne, E.C.; Haley, B.; Stichel, T.; Grasse, P.; Zieringer, M.; Frank, M. Online preconcentration ICP-MS analysis of rare earth elements in seawater. Geochem. Geophys. Geosystems 2012, 13, Q01020. [Google Scholar] [CrossRef] [Green Version]
- Trimborn, S.; Brenneis, T.; Hoppe, C.J.M.; Laglera, L.; Norman, L.; Santos-Echeandia, J.; Völkner, C.; Wolf-Gladrow, D.; Hassler, C.S. Iron sources alter the response of SO phytoplankton to ocean acidification. Mar. Ecol. Progr. Ser. 2017, 578, 35–50. [Google Scholar] [CrossRef] [Green Version]
- Barlow, R.G.; Cummings, D.G.; Gibb, S.W. Improved resolution of mono-and divinyl chlorophylls a and b and zeaxanthin and lutein in phytoplankton extracts using reverse phase C-8 HPLC. Mar. Ecol. Prog. Ser. 1997, 161, 303–307. [Google Scholar] [CrossRef]
- Taylor, B.B.; Torrecilla, E.; Bernhardt, A.; Taylor, M.H.; Peeken, I.; Röttgers, R.; Piera, J.; Bracher, A. Bio-optical provinces in the eastern Atlantic Ocean. Biogeosciences 2011, 8, 3609–3629. [Google Scholar] [CrossRef] [Green Version]
- Kolber, Z.S.; Prášil, O.; Falkowski, P.G. Measurements of variable chlorophyll fluorescence using fast repetition rate techniques: Defining methodology and experimental protocols. Biochim. Biophys. Acta BBA Bioenerg. 1998, 1367, 88–106. [Google Scholar] [CrossRef] [Green Version]
- Oxborough, K.; Moore, C.M.; Suggett, D.J.; Lawson, T.; Chan, H.G.; Geider, R.J. Direct estimation of functional PSII reaction center concentration and PSII electron flux on a volume basis: A new approach to the analysis of Fast Repetition Rate fluorometry (FRRf) data. Limnol. Oceanogr. Methods 2012, 10, 142–154. [Google Scholar] [CrossRef] [Green Version]
- Jacquet, S.; Dorigo, U.; Personnic, S. A few tests prior to flow cytometry and epifluorescence analyses of freshwater bacterio-and virioplankton communities; INRA UMR CARRTEL: Thonon, France, 2013; Volume 1, pp. 1–30. ISBN 978-1-62808-709-3. [Google Scholar]
- Props, R.; Monsieurs, P.; Mysara, M.; Clement, L.; Boon, N. Measuring the biodiversity of microbial communities by flow cytometry. Methods Ecol. Evol. 2016, 7, 1376–1385. [Google Scholar] [CrossRef]
- De Roy, K.; Clement, L.; Thas, O.; Wang, Y.; Boon, N. Flow cytometry for fast microbial community fingerprinting. Wat. Res. 2012, 46, 907–919. [Google Scholar] [CrossRef] [PubMed]
- Hahne, F.; LeMeur, N.; Brinkman, R.R.; Ellis, B.; Haaland, P.; Sarkar, D.; Spidlen, J.; Strain, E.; Gentleman, R. FlowCore: A Bioconductor pack- age for high throughput flow cytometry. BMC Bioinform. 2009, 10, 106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Props, R.; Schmidt, M.L.; Heyse, J.; Vanderploeg, H.A.; Boon, N.; Denef, V.J. Flow cytometric monitoring of bacterioplankton phenotypic diversity predicts high population-specific feeding rates by invasive dreissenid mussels. Env. Microbio. 2018, 20, 521–534. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.O. Diversity and evenness: A unifying notation and its consequences. Ecology 1973, 54, 427–432. [Google Scholar] [CrossRef] [Green Version]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. lmerTest Package:Tests in Linear Mixed Effects Models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Trimborn, S.; Hoppe, C.J.M.; Taylor, B.B.; Bracher, A.; Hassler, C. Physiological characteristics of open ocean and coastal phytoplankton communities of Western Antarctic Peninsula and Drake Passage waters. Deep Sea Res. I 2015, 98, 115–124. [Google Scholar] [CrossRef]
- Cheah, W.; Soppa, M.A.; Wiegmann, S.; Ossebaar, S.; Laglera, L.M.; Strass, V.H.; Santos-Echeandia, J.; Hoppema, M.; Wolf-Gladrow, D.; Bracher, A. Importance of deep mixing and silicic acid in regulating phytoplankton biomass and community in the iron-limited Antarctic Polar Front region in summer. Deep Sea Res. Part II Top. Stud. Oceanogr. 2017, 138, 74–85. [Google Scholar] [CrossRef] [Green Version]
- Hopkinson, B.M.; Mitchell, B.G.; Reynolds, R.A.; Wang, H.; Selph, K.E.; Measures, C.I.; Hewes, C.D.; Holm-Hansen, O.; Barbeau, K.A. Iron limitation across chlorophyll gradients in the Southern Drake Passage: Phytoplankton responses to iron addition and photosynthetic indicators of iron stress. Limnol. Oceanogr. 2007, 52, 2540–2554. [Google Scholar] [CrossRef]
- Klunder, M.B.; Laan, P.; De Baar, H.J.W.; Middag, R.; Neven, I.; Van Ooijen, J. Dissolved Fe across the Weddell Sea and Drake Passage: Impact of DFe on nutrient uptake. Biogeosciences 2014, 11, 651–669. [Google Scholar] [CrossRef] [Green Version]
- Blain, S.; Sedwick, P.N.; Griffiths, F.B.; Quéguiner, B.; Bucciarelli, E.; Fiala, M.; Pondaven, P.; Tréguer, P. Quantification of algal iron requirements in the Subantarctic Southern Ocean (Indian sector). Deep Sea Res. 2002, 49, 3255–3273. [Google Scholar] [CrossRef]
- Coale, K.H.; Fitzwater, S.E.; Gordon, R.M.; Tanner, S.A.; Johnson, K.S. Iron limitation of phytoplankton growth affects nutrient drawdown ratios in the Southern Ocean. In Proceedings of the AGU Ocean Sciences Meeting, San Antonio, TX, USA, 24–28 January 2000. [Google Scholar]
- Maldonado, M.T.; Strzepek, R.F.; Sander, S.; Boyd, P.W. Acquisition of iron bound to strong organic complexes, with different Fe binding groups and photochemical reactivities, by plankton communities in Fe-limited subantarctic waters. Glob. Biogeochem. Cycles 2005, 19, GB4S23. [Google Scholar] [CrossRef] [Green Version]
- Tortell, P.D.; Maldonado, M.T.; Granger, J.; Price, N.M. Marine bacteria and biogeochemical cycling of iron in the oceans. FEMS Microbiol. Ecol. 1999, 29, 1–11. [Google Scholar] [CrossRef]
- Hassler, C.S.; Schoemann, V.; Boye, M.; Tagliabue, A.; Rozmarynowycz, M.; McKay, R.M.L. Iron bioavailability in the SO. Oceanogr. Mar. Biol. Annu. Rev. 2012, 50, 1–50. [Google Scholar]
- Thingstad, T.F.; Lignell, R. Theoretical models for the control of bacterial growth rate, abundance, diversity and carbon demand. Aquat. Microb. Ecol. 1997, 13, 19–27. [Google Scholar] [CrossRef] [Green Version]
- Knowles, B.; Silveira, C.B.; Bailey, B.A.; Barott, K.; Cantu, V.A.; Cobián-Güemes, A.G.; Coutinho, F.H.; Felts, D.B.; Furby, K.A.; George, E.E.; et al. Lytic to temperate switching of viral communities. Nature 2016, 531, 466–470. [Google Scholar] [CrossRef]
- Sutherland, I.W. Polysaccharases for microbial exopolysaccharides. Carbohydr. Polym. 1999, 38, 319–328. [Google Scholar] [CrossRef]
- Lelchat, F.; Mocaer, P.Y.; Ojima, T.; Michel, G.; Sarthou, G.; Bucciarelli, E.; Cérantola, S.; Colliec-Jouault, S.; Boisset, C.; Baudoux, A.-C. Viral degradation of marine bacterial exopolysaccharides. FEMS Microbiol. Ecol. 2019, 95, fiz079. [Google Scholar] [CrossRef]
- Zimmerman, A.E.; Howard-Varona, C.; Needham, D.M.; John, S.G.; Worden, A.Z.; Sullivan, M.B.; Waldbauer, J.R.; Coleman, M.L. Metabolic and biogeochemical consequences of viral infection in aquatic ecosystems. Nat. Rev. Microbiol. 2020, 18, 21–34. [Google Scholar] [CrossRef]
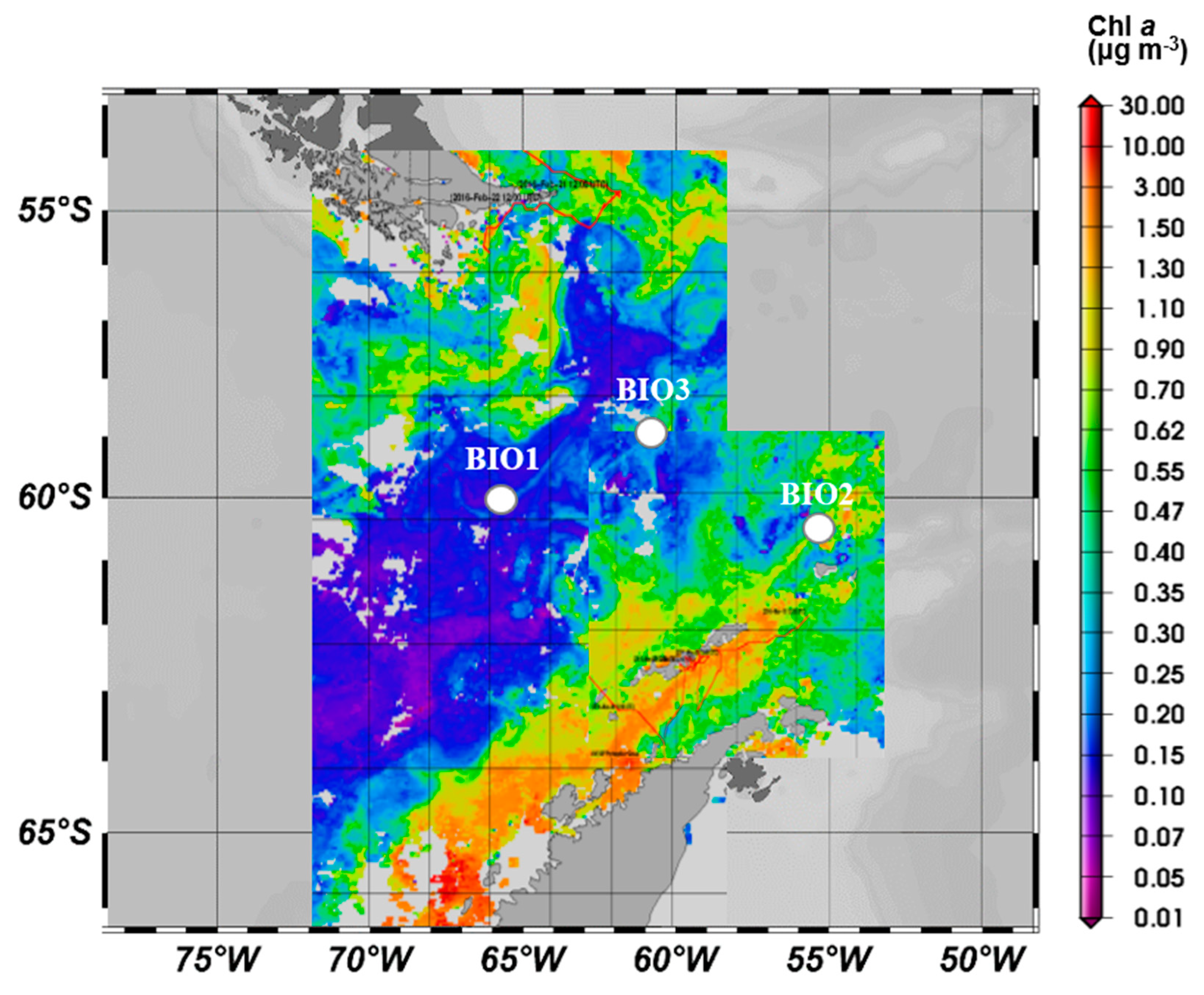
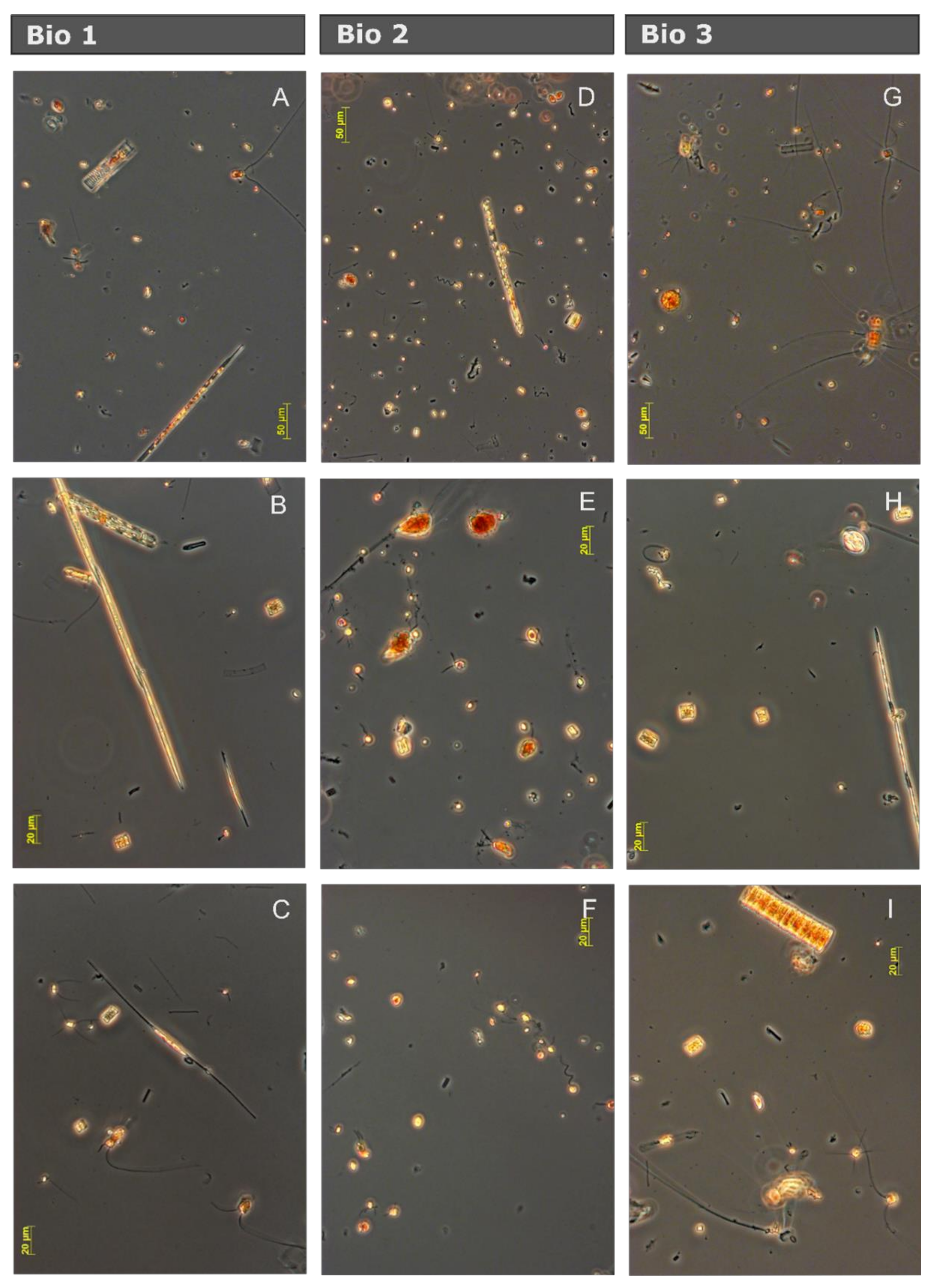
| Station | Bio 1 | Bio 2 | Bio 3 |
|---|---|---|---|
| Region | DP | WAP | DP |
| Latitude | 60°24.78′S | 60°29.94′S | 58°52.17′S |
| Longitude | 66°21.85′W | 55°29.70′W | 60°51.92′W |
| Depth (m) | 25 | 33 | 32 |
| Date (dd.mm.yy) | 03 March 2016 | 13 March 2016 | 17 March 2016 |
| Station | Treatment | NO3 (µM) | NO2 (µM) | NH4 (µM) | PO4 (µM) | SiO3 (µM) | dFe (nM) |
|---|---|---|---|---|---|---|---|
| Bio 1 | Ctrl | 22.55 | 0.17 | 0.25 | 1.14 | 16.47 | 0.74 |
| Fe | 21.56 | 0.17 | 0.18 | 1.07 | 14.63 | 1.57 | |
| DFB | 22.55 | 0.18 | 0.25 | 1.12 | 16.29 | 1.64 | |
| GLU | 22.29 | 0.17 | 0.13 | 1.12 | 15.98 | 1.59 | |
| CAR | 21.66 | 0.18 | 0.24 | 1.07 | 15.78 | 1.55 | |
| L6 | 22.67 | 0.18 | 0.22 | 1.20 | 16.67 | 1.54 | |
| L22 | 25.89 | 0.18 | 0.25 | 1.23 | 18.54 | 2.25 | |
| Bio 2 | Ctrl | 25.07 | 0.18 | 0.99 | 1.71 | 60.04 | 0.34 |
| Fe | 25.19 | 0.17 | 0.90 | 1.67 | 56.15 | 1.27 | |
| DFB | 25.31 | 0.17 | 0.87 | 1.67 | 57.23 | 1.37 | |
| GLU | 25.25 | 0.18 | 0.86 | 1.70 | 54.61 | 1.42 | |
| CAR | 25.14 | 0.18 | 0.89 | 1.76 | 57.85 | 1.28 | |
| L6 | 25.01 | 0.17 | 0.88 | 1.64 | 57.11 | 1.33 | |
| L22 | 25.28 | 0.17 | 0.93 | 1.71 | 57.34 | 1.73 | |
| Bio 3 | Ctrl | 25.81 | 0.25 | 0.28 | 1.34 | 21.31 | 0.59 |
| Fe | 25.72 | 0.25 | 0.22 | 1.34 | 21.38 | 1.57 | |
| DFB | 25.56 | 0.25 | 0.23 | 1.30 | 21.21 | 1.26 | |
| GLU | 26.77 | 0.26 | 0.25 | 1.54 | 21.75 | 1.48 | |
| CAR | 25.51 | 0.24 | 0.22 | 1.28 | 21.15 | 1.44 | |
| L6 | 25.92 | 0.25 | 0.24 | 1.42 | 21.54 | 1.24 | |
| L22 | 29.50 | 0.29 | 0.27 | 1.38 | 23.27 | 1.98 | |
| EPS1 | 25.20 | 0.25 | 0.23 | 1.31 | 21.34 | 1.04 |
| Station | Treatment | Fv/Fm (rel. Unit) | σPSII (nm2 PS−1) | p (rel. Unit) | τQa (µs) |
|---|---|---|---|---|---|
| Bio 1 | T0 | 0.25 ± 0.02 | 8.47 ± 0.87 | 0.23 ± 0.05 | 597 ± 42 |
| Ctrl | 0.29 ± 0.01 | 7.76 ± 0.06 | 0.21 ± 0.05 | 566 ± 10 | |
| Fe | 0.50 ± 0.03 *** | 3.97 ± 0.18 *** | 0.37 ± 0.02 *** | 624 ± 19 | |
| DFB | 0.34 ± 0.04 * | 5.88 ± 0.16 *** | 0.21 ± 0.04 | 655 ± 12 * | |
| GLU | 0.49 ± 0.01 *** | 4.69 ± 0.15 *** | 0.38 ± 0.02 *** | 640 ± 15 | |
| CAR | 0.46 ± 0.07 *** | 3.96 ± 0.35 *** | 0.35 ± 0.02 *** | 633 ± 27 | |
| L6 | 0.52 ± 0.03 *** | 4.52 ± 0.23 *** | 0.38 ± 0.01 *** | 624 ± 20 | |
| L22 | 0.51 ± 0.01 *** | 3.81 ± 0.08 *** | 0.36 ± 0.01 *** | 661 ± 19 * | |
| Bio 2 | T0 | 0.47 ± 0.01 | 4.12 ± 0.33 | 0.29 ± 0.05 | 662 ± 32 |
| Ctrl | 0.47 ± 0.01 | 4.90 ± 0.82 | 0.26 ± 0.02 | 591 ± 19 *** | |
| Fe | 0.53 ± 0.00 *** | 6.27 ± 0.05 *** | 0.37 ± 0.02 * | 625 ± 9 | |
| DFB | 0.40 ± 0.00 *** | 6.00 ± 0.09 ** | 0.15 ± 0.02 *** | 669 ± 7 | |
| GLU | 0.49 ± 0.02 | 4.77 ± 0.10 | 0.25 ± 0.06 | 678 ± 18 | |
| CAR | 0.50 ± 0.01 * | 4.00 ± 0.14 | 0.22 ± 0.05 | 762 ± 16 *** | |
| L6 | 0.53 ± 0.02 *** | 4.30 ± 0.08 | 0.32 ± 0.01 | 677 ± 4 | |
| L22 | 0.52 ± 0.00 *** | 5.23 ± 0.16 | 0.33 ± 0.00 | 681 ± 14 | |
| Bio 3 | T0 | 0.23 ± 0.03 | 4.67 ± 0.60 | 0.15 ± 0.02 | 477 ± 57 |
| Ctrl | 0.36 ± 0.07 * | 4.85 ± 0.91 | nd | 622 ± 72 | |
| Fe | 0.50 ± 0.01 *** | 3.60 ± 0.14 * | nd | 624 ± 2 | |
| DFB | 0.33 ± 0.03 * | 5.18 ± 0.32 | nd | 609 ± 32 | |
| GLU | 0.53 ± 0.00 *** | 2.67 ± 0.08 *** | nd | 615 ± 5 | |
| CAR | 0.48 ± 0.02 *** | 3.50 ± 0.05 ** | nd | 657 ± 13 | |
| L6 | 0.51 ± 0.03 *** | 2.80 ± 0.12 *** | nd | 618 ± 25 | |
| L22 | 0.52 ± 0.06 *** | 2.69 ± 0.04 *** | nd | 609 ± 17 | |
| EPS1 | 0.50 ± 0.02 *** | 2.92 ± 0.10 *** | nd | 596 ± 29 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blanco-Ameijeiras, S.; Cabanes, D.J.E.; Cable, R.N.; Trimborn, S.; Jacquet, S.; Wiegmann, S.; Völkner, C.; Lelchat, F.; Bracher, A.; Duhaime, M.B.; et al. Exopolymeric Substances Control Microbial Community Structure and Function by Contributing to both C and Fe Nutrition in Fe-Limited Southern Ocean Provinces. Microorganisms 2020, 8, 1980. https://doi.org/10.3390/microorganisms8121980
Blanco-Ameijeiras S, Cabanes DJE, Cable RN, Trimborn S, Jacquet S, Wiegmann S, Völkner C, Lelchat F, Bracher A, Duhaime MB, et al. Exopolymeric Substances Control Microbial Community Structure and Function by Contributing to both C and Fe Nutrition in Fe-Limited Southern Ocean Provinces. Microorganisms. 2020; 8(12):1980. https://doi.org/10.3390/microorganisms8121980
Chicago/Turabian StyleBlanco-Ameijeiras, Sonia, Damien J. E. Cabanes, Rachel N. Cable, Scarlett Trimborn, Stéphan Jacquet, Sonja Wiegmann, Christian Völkner, Florian Lelchat, Astrid Bracher, Melissa B. Duhaime, and et al. 2020. "Exopolymeric Substances Control Microbial Community Structure and Function by Contributing to both C and Fe Nutrition in Fe-Limited Southern Ocean Provinces" Microorganisms 8, no. 12: 1980. https://doi.org/10.3390/microorganisms8121980
APA StyleBlanco-Ameijeiras, S., Cabanes, D. J. E., Cable, R. N., Trimborn, S., Jacquet, S., Wiegmann, S., Völkner, C., Lelchat, F., Bracher, A., Duhaime, M. B., & Hassler, C. S. (2020). Exopolymeric Substances Control Microbial Community Structure and Function by Contributing to both C and Fe Nutrition in Fe-Limited Southern Ocean Provinces. Microorganisms, 8(12), 1980. https://doi.org/10.3390/microorganisms8121980





