Fluorescence Detection of Type III Secretion Using a Glu-CyFur Reporter System in Citrobacter rodentium
Abstract
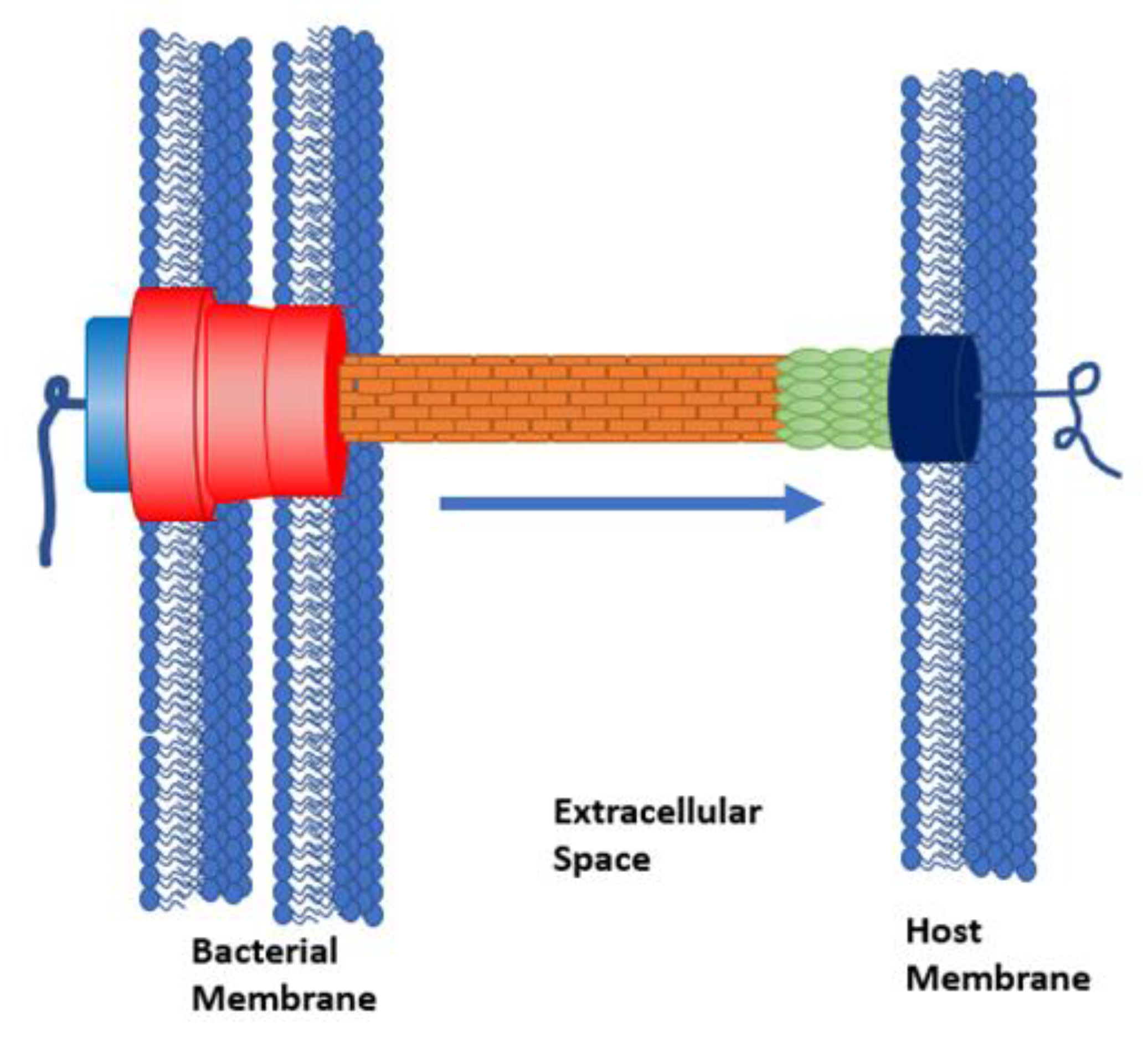
1. Introduction
2. Materials and Methods
2.1. Materials and Compounds
2.2. Cell Lines
2.3. Enzyme Expression and Lysate Production
2.4. Synthesis of Glu-CyFur
2.5. Compound Screening
2.6. Cytotoxicity Screening
2.7. CPG2 Direct Inhibition Analysis
2.8. Data Analysis
2.9. CPG2 Expressional Analysis
3. Results
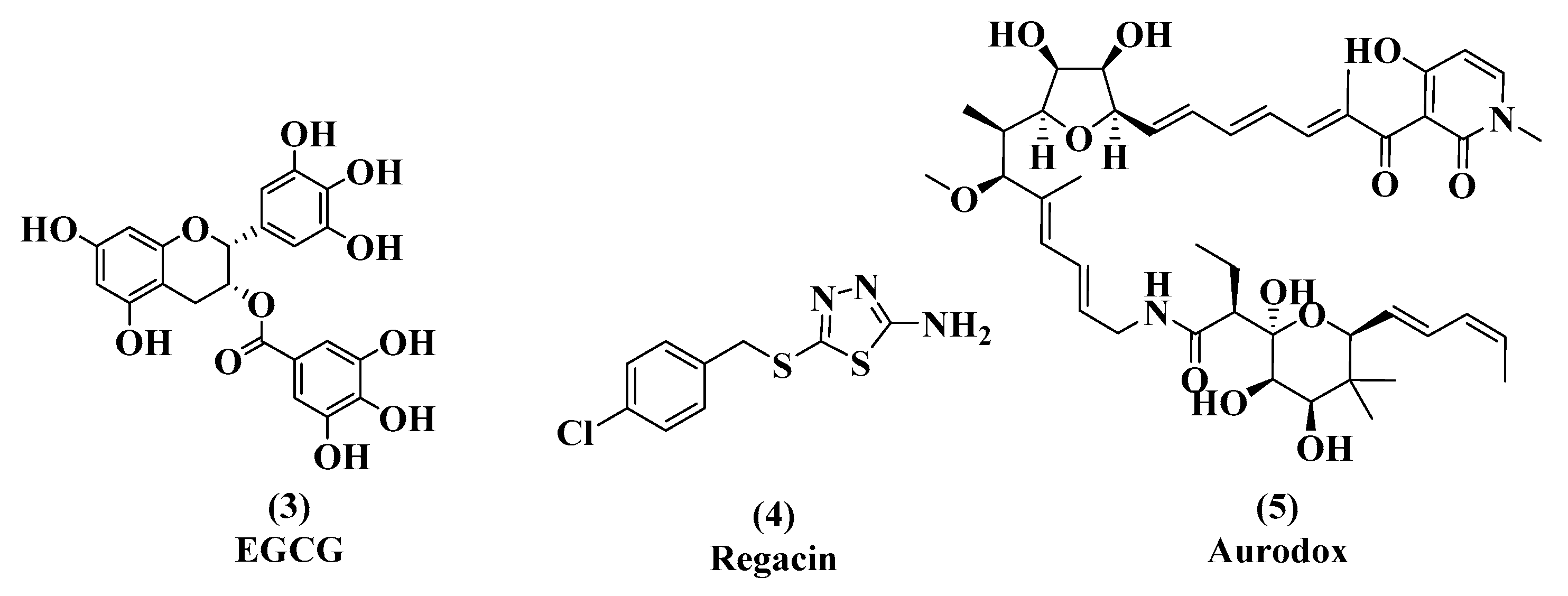
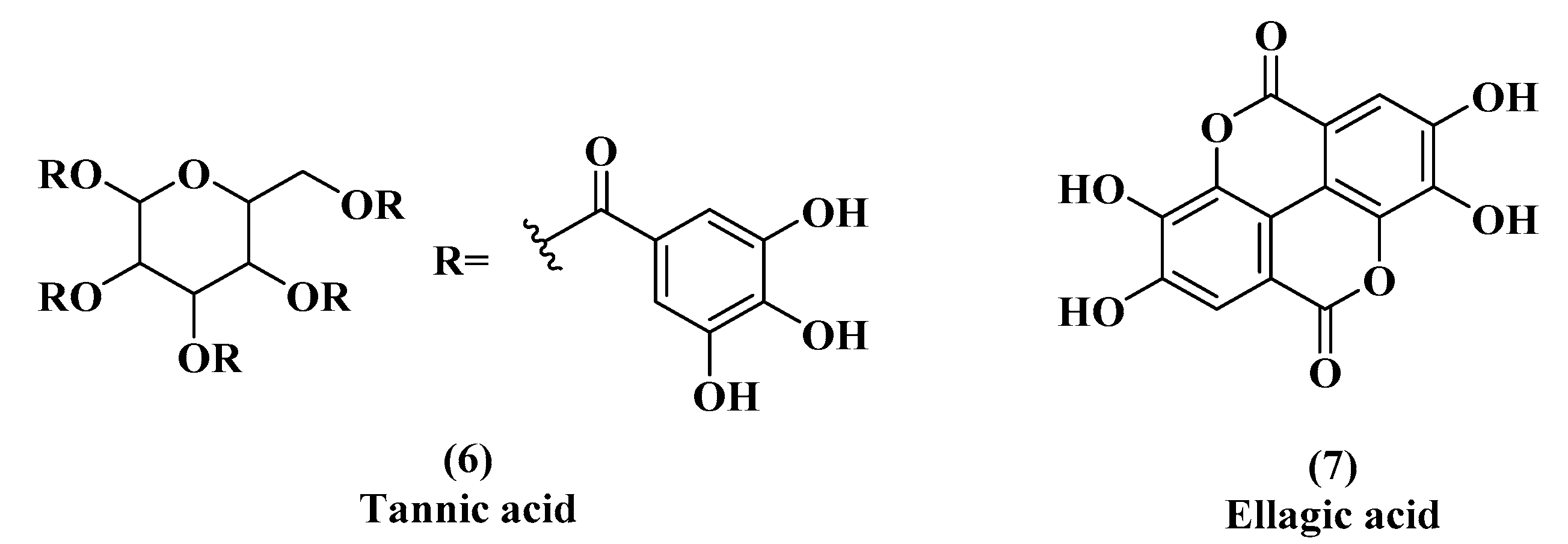
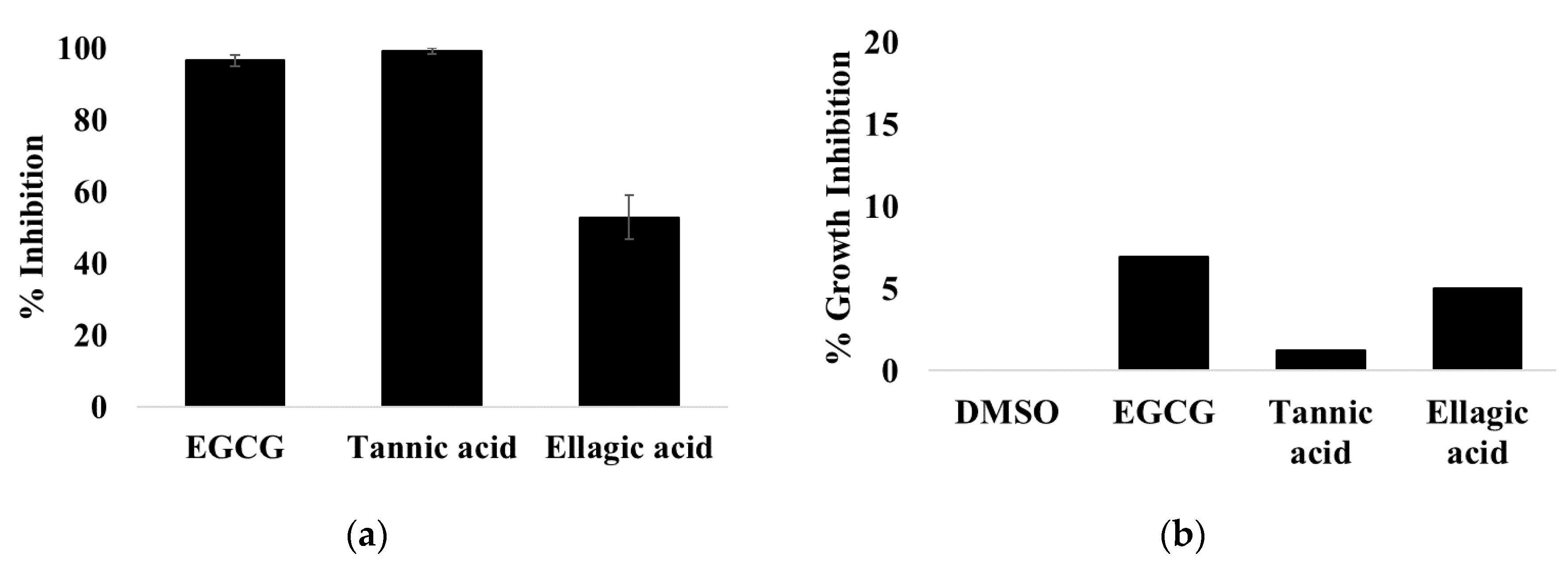
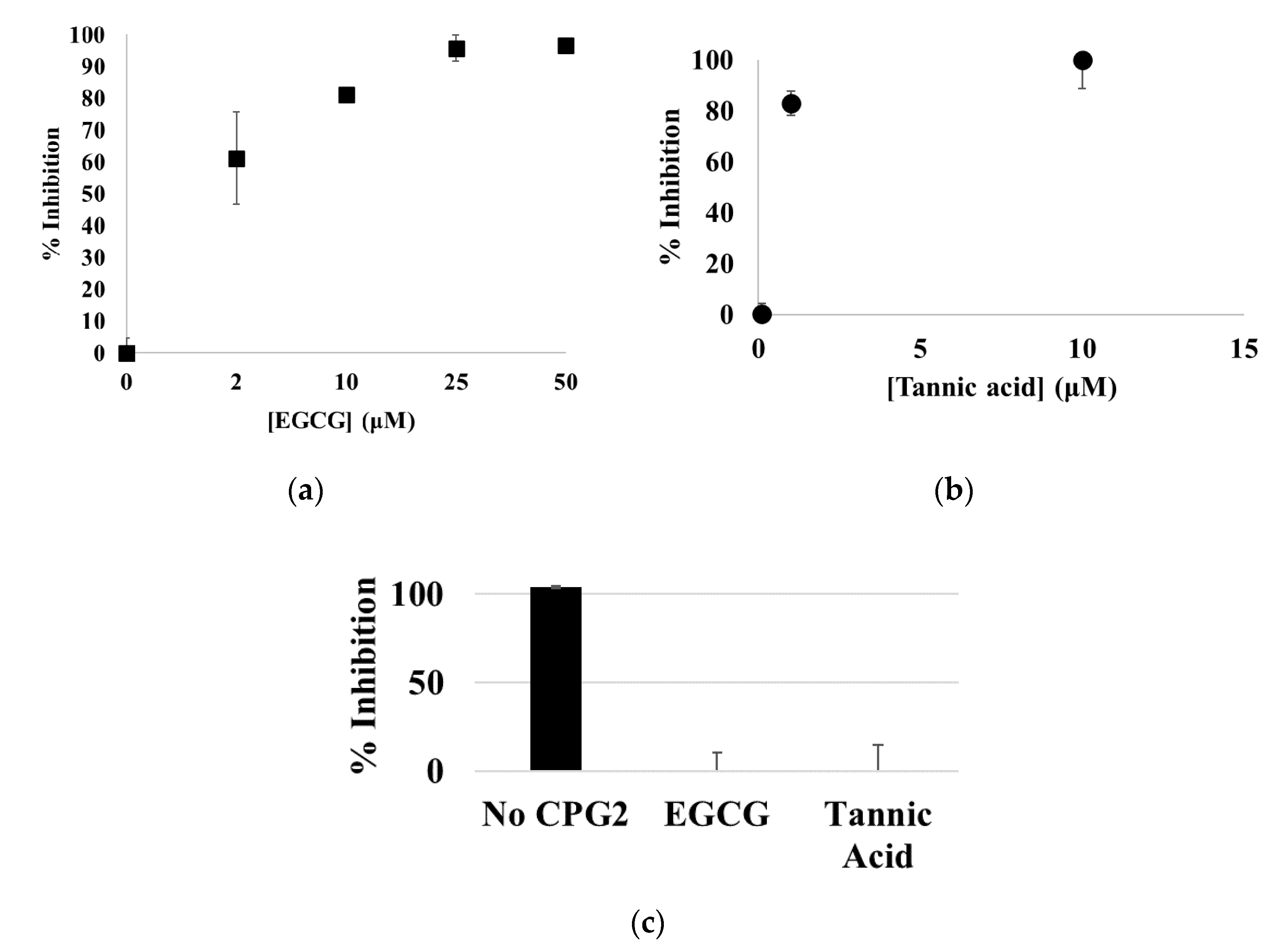
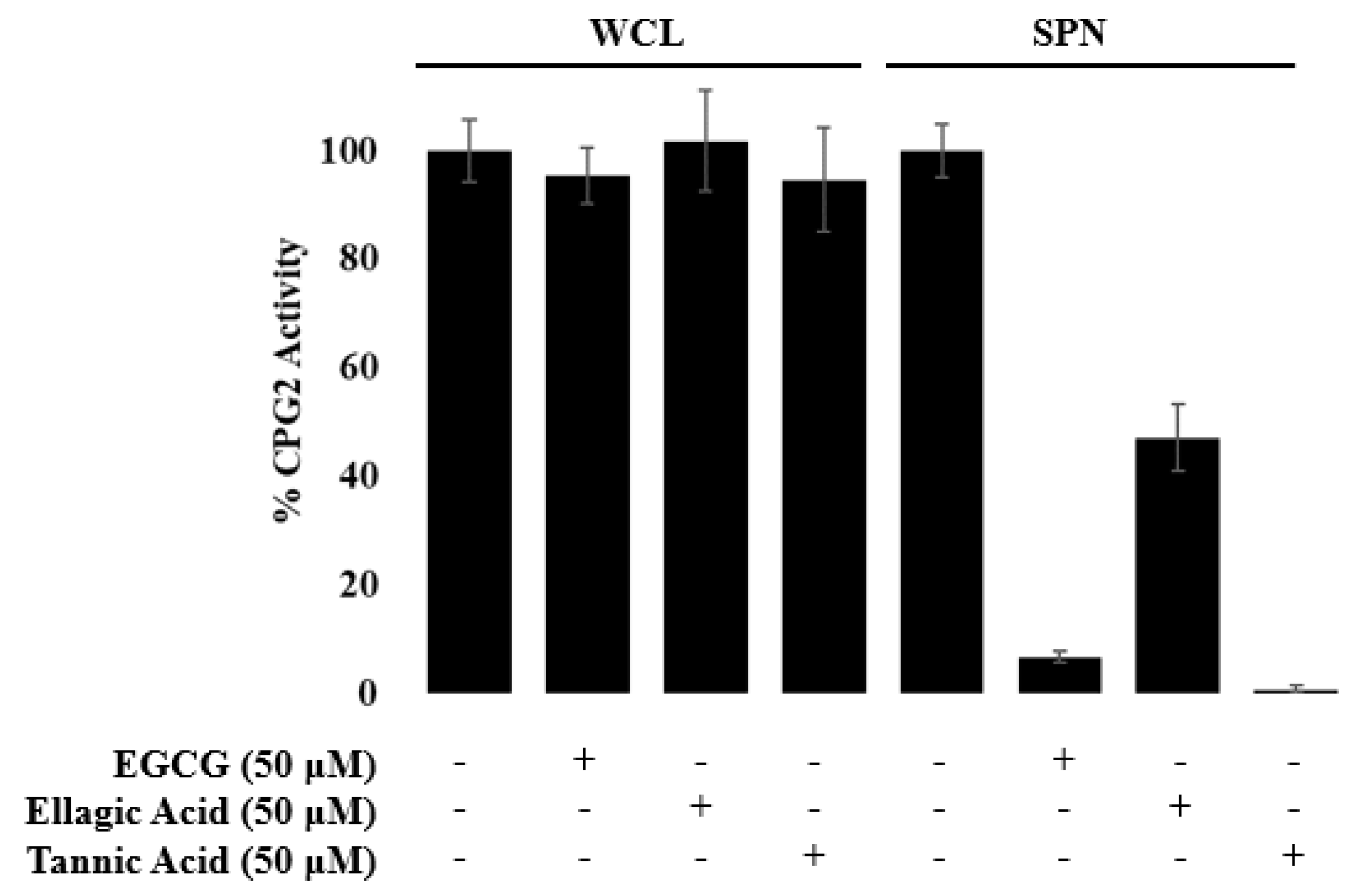
3.1. EGCG, Tannic Acid, and Ellagic Acid
3.2. Regacin
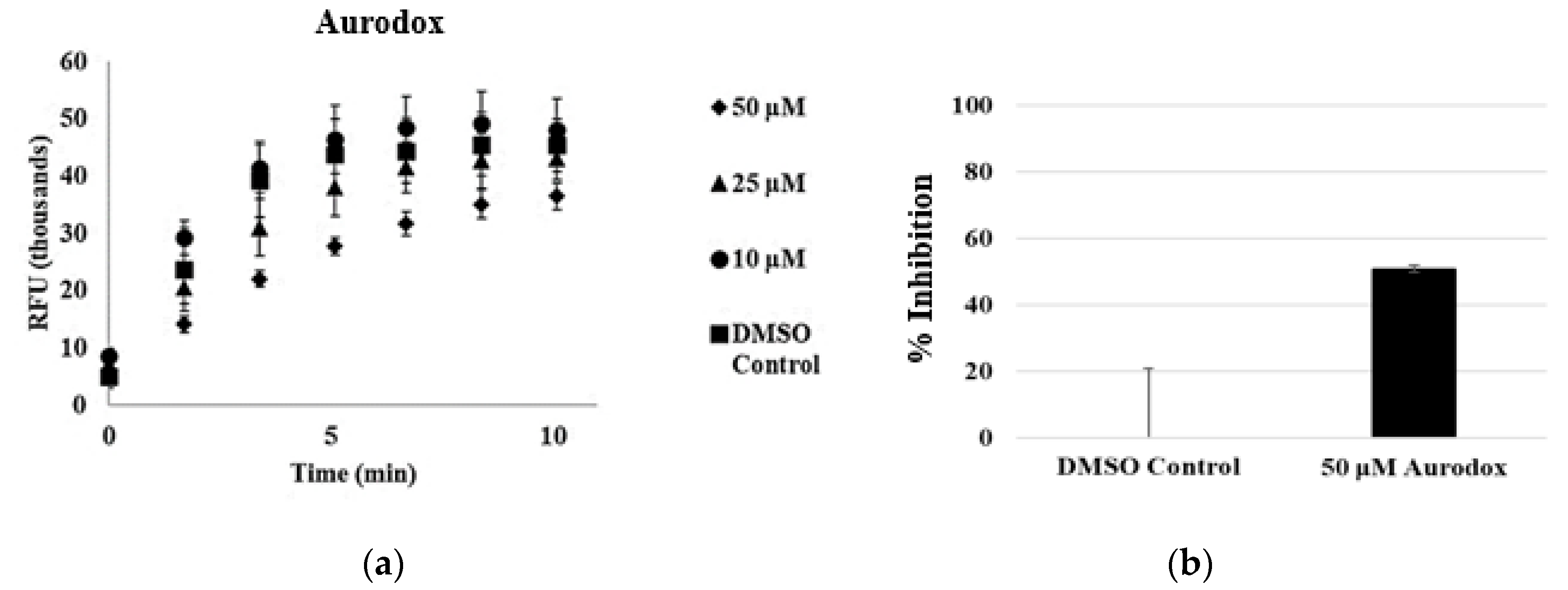
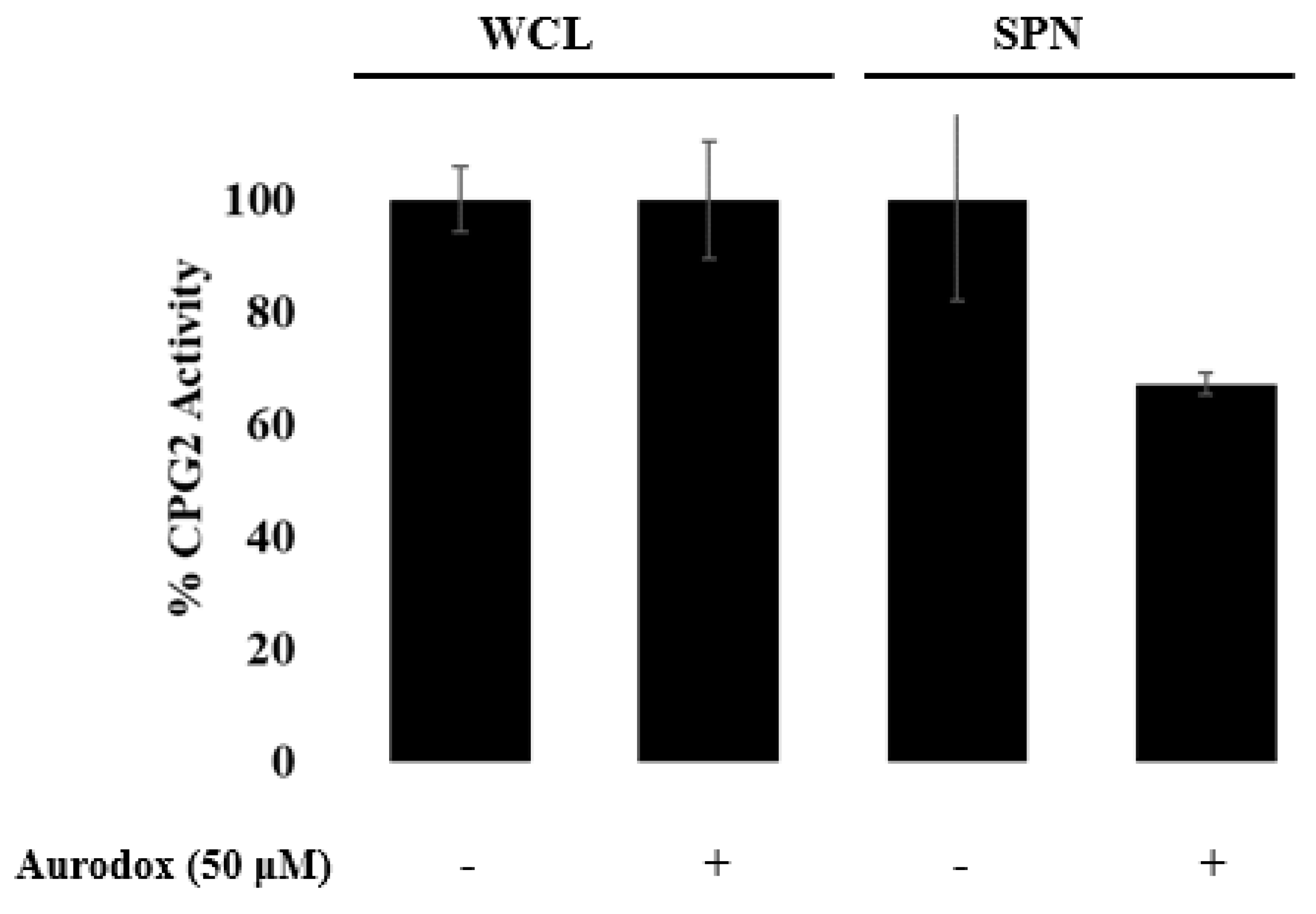
3.3. Aurodox
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fasciano, A.C.; Shaban, L.; Mecsas, J. Promises and challenges of the type three secretion system injectisome as an antivirulence target. EcoSal Plus 2019, 8. [Google Scholar] [CrossRef]
- Keyser, P.; Elofsson, M.; Rosell, S.; Wolf-Watz, H. Virulence blockers as alternatives to antibiotics: Type III secretion inhibitors against Gram-negative bacteria. J. Intern. Med. 2008, 264, 17–29. [Google Scholar] [CrossRef]
- Marshall, N.C.; Finlay, B.B. Targeting the type III secretion system to treat bacterial infections. Expert Opin. Ther. Targets 2014, 18, 137–152. [Google Scholar] [CrossRef] [PubMed]
- Tsou, L.K.; Dossa, P.D.; Hang, H.C. Small molecules aimed at type III secretion systems to inhibit bacterial virulence. Med. Chem. Comm. 2013, 4, 68–79. [Google Scholar] [CrossRef]
- Donnenberg, M.S. Pathogenic strategies of enteric bacteria. Nature 2000, 406, 768–774. [Google Scholar] [CrossRef]
- Baron, C. Antivirulence drugs to target bacterial secretion systems. Curr. Opin. Microbiol. 2010, 13, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, K.G.; Girón, J.A.; Jerse, A.E.; McDaniel, T.K.; Donnenberg, M.S.; Kaper, J.B. Enteropathogenic Escherichia coli contains a putative type III secretion system necessary for the export of proteins involved in attaching and effacing lesion formation. Proc. Natl. Acad. Sci. USA 1995, 92, 7996–8000. [Google Scholar] [CrossRef] [PubMed]
- Franzin, F.M.; Sircili, M.P. Locus of enterocyte effacement: A pathogenicity island involved in the virulence of enteropathogenic and enterohemorragic Escherichia coli subjected to a complex network of gene regulation. BioMed Res. Int. 2015, 2015, 534738. [Google Scholar] [CrossRef]
- Croxen, M.A.; Finlay, B.B. Molecular mechanisms of Escherichia coli pathogenicity. Nat. Rev. Microbiol. 2010, 8, 26–38. [Google Scholar] [CrossRef]
- Salmond, G.P.C.; Reeves, P.J. Membrane traffic wardens and protein secretion in Gram-negative bacteria. Trends Biochem. Sci. 1993, 18, 7–12. [Google Scholar] [CrossRef]
- Dai, W.; Li, Z. Conserved type III secretion system exerts important roles in Chlamydia trachomatis. Int. J. Exp. Pathol. 2014, 7, 5404–5414. [Google Scholar]
- Cornelis, G.R.; Gijsegem, F.V. Assembly and function of type III secretory systems. Annu. Rev. Microbiol. 2000, 54, 735–774. [Google Scholar] [CrossRef] [PubMed]
- Bartra, S.S.; Lorica, C.; Qian, L.; Gong, X.; Bahnan, W.; Barreras, H.; Hernandes, R.; Lei, Z.; Plano, G.V.; Schesser, K. Chromosomally-encoded Yersinia pestis type III secretion effector proteins promote infection in cells and in mice. Front. Cell. Infect. Microbiol. 2019, 9, 23. [Google Scholar] [CrossRef] [PubMed]
- Marketon, M.M.; DePaolo, R.W.; DeBord, K.L.; Jabri, B.; Scheewind, O. Plague bacteria target immune cells during infection. Science 2005, 309, 1739–1742. [Google Scholar] [CrossRef]
- Hauser, A.R. The type III secretion system of Pseudomonas aeruginosa: Infection by injection. Nat. Rev. Microbiol. 2009, 7, 654–666. [Google Scholar] [CrossRef]
- Shaw, R.K.; Cleary, J.; Murphy, M.S.; Frankel, G.; Knutton, S. Interaction of enteropathogenic Escherichia coli with human intestinal mucosa: Role of effector proteins in brush border remodeling and formation of attaching and effacing lesions. Infect. Immun. 2005, 73, 1243–1251. [Google Scholar] [CrossRef]
- Pendergrass, H.A.; May, A.E. Natural product type III secretion system inhibitors. Antibiotics 2019, 8, 162. [Google Scholar] [CrossRef]
- Tao, H.; Fan, S.-S.; Jiang, S.; Xiang, X.; Yan, X.; Zhang, L.-H.; Cui, Z.-N. Small molecule inhibitors specifically targeting the type III secretion system of Xanthomonas oryzae on rice. Int. J. Mol. Sci. 2019, 20, 971. [Google Scholar] [CrossRef]
- Gu, L.; Zhou, S.; Zhu, L.; Liang, C.; Chen, X. Small-molecule inhibitors of the type III secretion system. Molecules 2015, 20, 17659–17674. [Google Scholar] [CrossRef]
- Duncan, M.C.; Wong, W.R.; Dupzyk, A.J.; Bray, W.M.; Linington, R.G.; Auerbuch, V. An NF-κB-based high-throughput screen identifies piericidins as inhibitors of the Yersinia pseudotuberculosis type III secretion system. Antimicrob. Agents Chemother. 2014, 58, 1118–1126. [Google Scholar] [CrossRef]
- Figueira, R.; Holden, D.W. Functions of the Salmonella pathogenicity island 2 (SPI-2) type III secretion system effectors. Microbiology 2012, 2, 1147–1161. [Google Scholar] [CrossRef] [PubMed]
- Cornelis, G.R. The type III secretion injectisome. Nat. Rev. Microbiol. 2006, 4, 811–825. [Google Scholar] [CrossRef] [PubMed]
- Sekiya, K.; Ohishi, M.; Ogino, T.; Tamano, K.; Sasakawa, C.; Abe, A. Supermolecular structure of the enteropathogenic Escherichia coli type III secretion system and its direct interaction with the EspA-sheath-like structure. Proc. Natl. Acad. Sci. USA 2001, 98, 11638–11643. [Google Scholar] [CrossRef]
- Bzdzion, L.; Krezel, H.; Wrzeszcz, K.; Grzegorek, I.; Nowinska, K.; Chodaczek, G.; Swietnicki, W. Design of small molecule inhibitors of type III secretion system ATPase EscN from enteropathogenic Escherichia coli. Acta Biochim Pol. 2017, 64, 49–63. [Google Scholar] [CrossRef]
- Andrade, A.; Pardo, P.J.; Espinosa, N.; Pérez-Hernández, G.; González-Pedrajo, B. Enzymatic characterization of the enteropathogenic Escherichia coli type III secretion ATPase EscN. Arch. Biochem. Biophys. 2007, 468, 121–127. [Google Scholar] [CrossRef]
- Creasey, E.A.; Delahay, R.M.; Bishop, A.A.; Shaw, R.K.; Kenny, B.; Knutton, S.; Frankel, G. CesT is a bivalent enteropathogenic Escherichia coli chaperone required for translocation of both Tir and Map. Molec. Microbiol. 2003, 47, 209–221. [Google Scholar] [CrossRef]
- Crawford, J.A.; Kaper, J.B. The N-terminus of enteropathogenic Escherichia coli (EPEC) Tir mediates transport across bacterial and eukaryotic cell membranes. Mol. Microbiol. 2002, 46, 855–868. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Li, Y.; Vallance, B.A.; Finlay, B.B. Locus of enterocyte effacement from Citrobacter rodentium: Sequence analysis and evidence for horizontal transfer among attaching and effacing pathogens. Infect. Immun. 2001, 69, 6323–6335. [Google Scholar] [CrossRef]
- Yount, J.S.; Tsou, L.K.; Dossa, P.D.; Kullas, A.L.; van der Velden, A.W.M.; Hang, H.C. Visible fluorescence detection of type III protein secretion from bacterial pathogens. J. Am. Chem. Soc. 2010, 132, 8244–8245. [Google Scholar] [CrossRef] [PubMed]
- Pendergrass, H.A.; May, A.E. Delivery of heterologous proteins, enzymes, and antigens via the bacterial type III secretion system. Microorganisms 2020, 8, 777. [Google Scholar] [CrossRef]
- Munera, D.; Crepin, V.F.; Marches, O.; Frankel, G. N-terminal type III secretion signal of enteropathogenic Escherichia coli translocator proteins. J. Bacteriol. 2010, 192, 3534–3539. [Google Scholar] [CrossRef] [PubMed]
- Afzal, M.; Safer, A.M.; Menon, M. Green tea polyphenols and their potential role in health and disease. Inflammopharmacology 2015, 23, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Han, Y.; Chen, C.; Sun, H.; He, D.; Guo, J.; Jiang, B.; Zhou, L.; Zeng, C. EGCG attenuates high glucose-induced endothelial cell inflammation by suppression of PKC and NF-κB signaling in human umbilical vein endothelial cells. Life Sci. 2013, 92, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Shankar, S.; Marsh, L.; Srivastava, R.K. EGCG inhibits growth of human pancreatic tumors orthotopically implanted in Balb C nude mice through modulation of FKHRL1/FOXO3a and neuropilin. Mol. Cell. Biochem. 2013, 372, 83–94. [Google Scholar] [CrossRef]
- Nandakumar, V.; Vaid, M.; Katiyar, S.K. (-)-Epigallocatechin-3-gallate reactivates silenced tumor suppressor genes, Cip1/p21 and p16 INK4a, by reducing DNA methylation and increasing histones acetylation in human skin cancer cells. Carcinogenesis 2011, 32, 537–544. [Google Scholar] [CrossRef]
- Rajendran, P.; Ho, E.; Williams, D.E.; Dashwood, R.H. Dietary phytochemicals, HDAC inhibition, and DNA damage/repair defects in cancer cells. Clin. Epigenetics 2011, 3, 4. [Google Scholar] [CrossRef]
- Khan, M.A.; Hussain, A.; Sundaram, M.K.; Alalami, U.; Gunasekera, D.; Ramesh, L.; Hamza, A.; Quraishi, U. (-)-Epigallocatechin-3-gallate reverses the expression of various tumor-suppressor genes by inhibiting DNA methyltransferases and histone deacetylases in human cervical cancer cells. Oncol. Rep. 2015, 33, 1976–1984. [Google Scholar] [CrossRef]
- Chu, C.; Deng, J.; Man, Y.; Qu, Y. Green tea extracts epigallocatechin-3-gallate for different treatments. Biomed. Res. Int. 2017, 2017, 5615647. [Google Scholar] [CrossRef]
- Nakasone, N.; Higa, N.; Toma, C.; Ogura, Y.; Suzuki, T.; Yamashiro, T. Epigallocatechin gallate inhibits the type III secretion system of Gram-negative enteropathogenic bacteria under model conditions. FEMS Microbiol. Lett. 2017, 364, fnx111. [Google Scholar] [CrossRef]
- Yang, J.; Hocking, D.M.; Cheng, C.; Dogovski, C.; Perugini, M.A.; Holien, J.K.; Parker, M.W.; Hartland, E.L.; Tauschek, M.; Robins-Browne, R.M. Disarming bacterial virulence through chemical inhibition of the DNA binding domain of an AraC-like transcriptional activator protein. J. Biol. Chem. 2013, 288, 31115–31126. [Google Scholar] [CrossRef]
- Berger, J.; Lehr, H.H.; Teitel, S.; Maehr, H.; Grunberg, E. A new antibiotic X-5108 of Streptomyces origin I. Production, isolation and properties. J. Antibiot. 1972, 26, 15–22. [Google Scholar] [CrossRef]
- Kimura, K.; Iwatsuki, M.; Nagai, T.; Matsumoto, A.; Takahashi, Y.; Shiomi, K.; Ōmura, S.; Abe, A. A small-molecule inhibitor of the bacterial type III secretion system protects against in vivo infection with Citrobacter rodentium. J. Antibiot. 2011, 64, 197–203. [Google Scholar] [CrossRef] [PubMed]
- McHugh, R.E.; O’Boyle, N.; Connoly, J.P.R.; Hoskisson, P.A.; Roe, A.J. Characterization of the mode of action of aurodox, a type III secretion system inhibitor from Streptomyces goldiniensis. Infect. Immun. 2019, 87, e00595-18. [Google Scholar]
- Huber, B.; Eberl, L.; Feucht, W.; Polster, J. Influence of polyphenols on bacterial biofilm formation and quorum-sensing. Z. Naturforsch. 2003, 58, 879–884. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Guo, K.P.; Qiu, L.; Shen, Y. Magnesium ethoxide as an effective catalyst in the synthesis of dicayanomethylendihydrofurans. Synth. Commun. 2006, 36, 1367–1372. [Google Scholar] [CrossRef]
- Byun, J.H.; Kim, H.Y.; Kim, Y.S.; Mook-Jung, I.; Kim, D.J.; Lee, W.K.; Yoo, K.H. Aminostyrylbenzofuran derivatives as potent inhibitors for Aβ fibril formation. Bioorganic Med. Chem. Lett. 2008, 18, 5591–5593. [Google Scholar] [CrossRef]
- Tsou, L.K.; Zhang, M.M.; Hang, H.C. Clickable fluorescent dyes for multimodal bioorthogonal imaging. Org. Biomol. Chem. 2009, 7, 5055–5058. [Google Scholar] [CrossRef] [PubMed]
- Sivasankar, C.; Jha, N.K.; Ghosh, R.; Shetty, P.H. Anti-quorum sensing and anti-virulence activity of tannic acid and it’s potential to breach resistance in Salmonella enterica Typhi/Paratyphi A clinical isolates. Microb. Pathog. 2020, 138, 103813. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pendergrass, H.A.; Johnson, A.L.; Hotinger, J.A.; May, A.E. Fluorescence Detection of Type III Secretion Using a Glu-CyFur Reporter System in Citrobacter rodentium. Microorganisms 2020, 8, 1953. https://doi.org/10.3390/microorganisms8121953
Pendergrass HA, Johnson AL, Hotinger JA, May AE. Fluorescence Detection of Type III Secretion Using a Glu-CyFur Reporter System in Citrobacter rodentium. Microorganisms. 2020; 8(12):1953. https://doi.org/10.3390/microorganisms8121953
Chicago/Turabian StylePendergrass, Heather A., Adam L. Johnson, Julia A. Hotinger, and Aaron E. May. 2020. "Fluorescence Detection of Type III Secretion Using a Glu-CyFur Reporter System in Citrobacter rodentium" Microorganisms 8, no. 12: 1953. https://doi.org/10.3390/microorganisms8121953
APA StylePendergrass, H. A., Johnson, A. L., Hotinger, J. A., & May, A. E. (2020). Fluorescence Detection of Type III Secretion Using a Glu-CyFur Reporter System in Citrobacter rodentium. Microorganisms, 8(12), 1953. https://doi.org/10.3390/microorganisms8121953





