Visual Detection of Clostridium perfringens Alpha Toxin by Combining Nanometer Microspheres with Smart Phones
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Plasmids
2.2. Animals and Preparation of Rabbit Polyclonal Antibody
2.3. Construction of Clostridium Alpha Toxin N-Terminal Prokaryotic Expression Vector
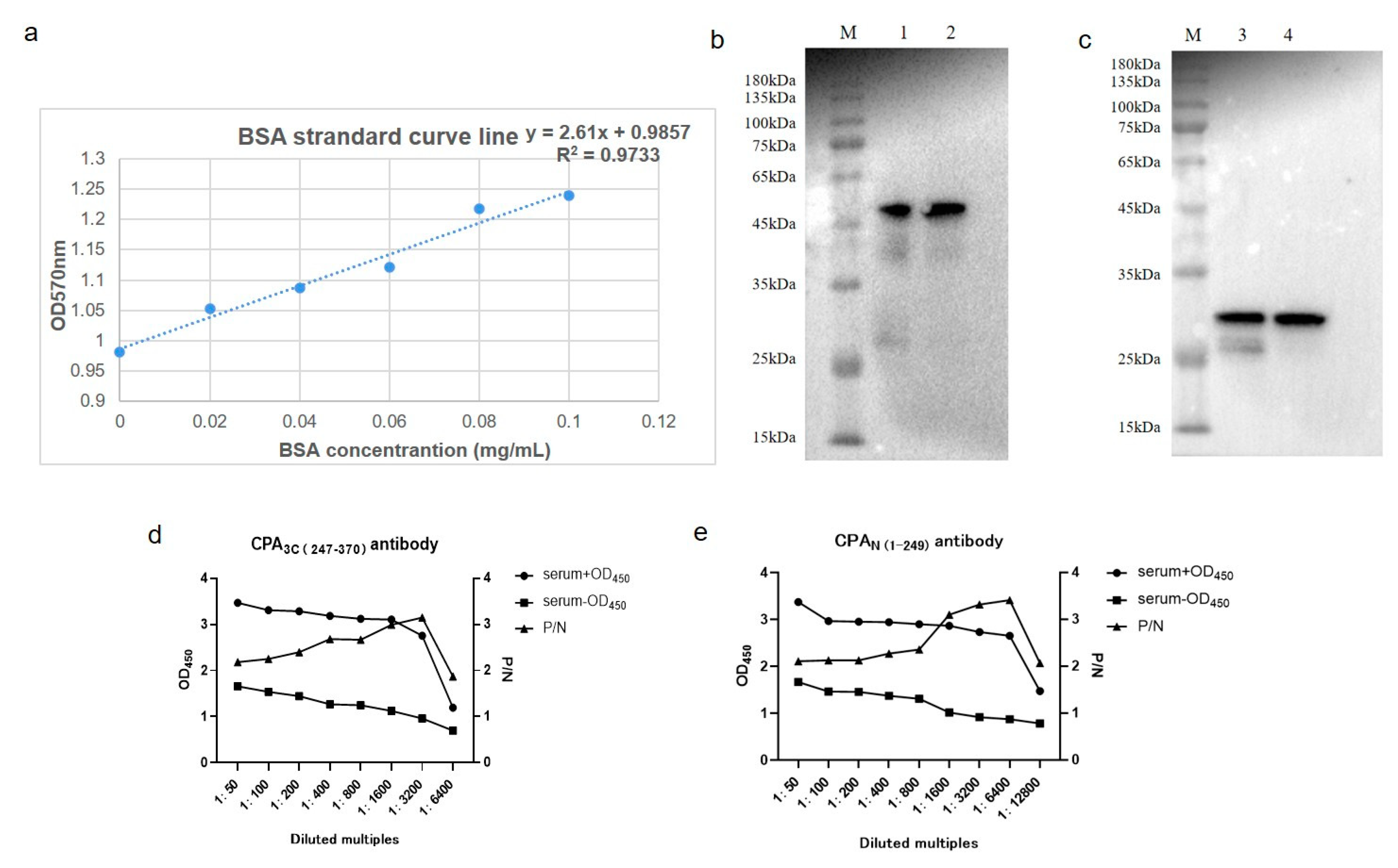
2.4. Purification of Recombinant Protein
2.5. Determination of Antibody Concentration and Antibody Titer by Bradford Method
2.6. Western-Blot Analysis
2.7. Indirect ELISA Analysis
2.8. Preparation of C. perfringens Alpha Toxin and Quantification of CPA Enzyme Activity
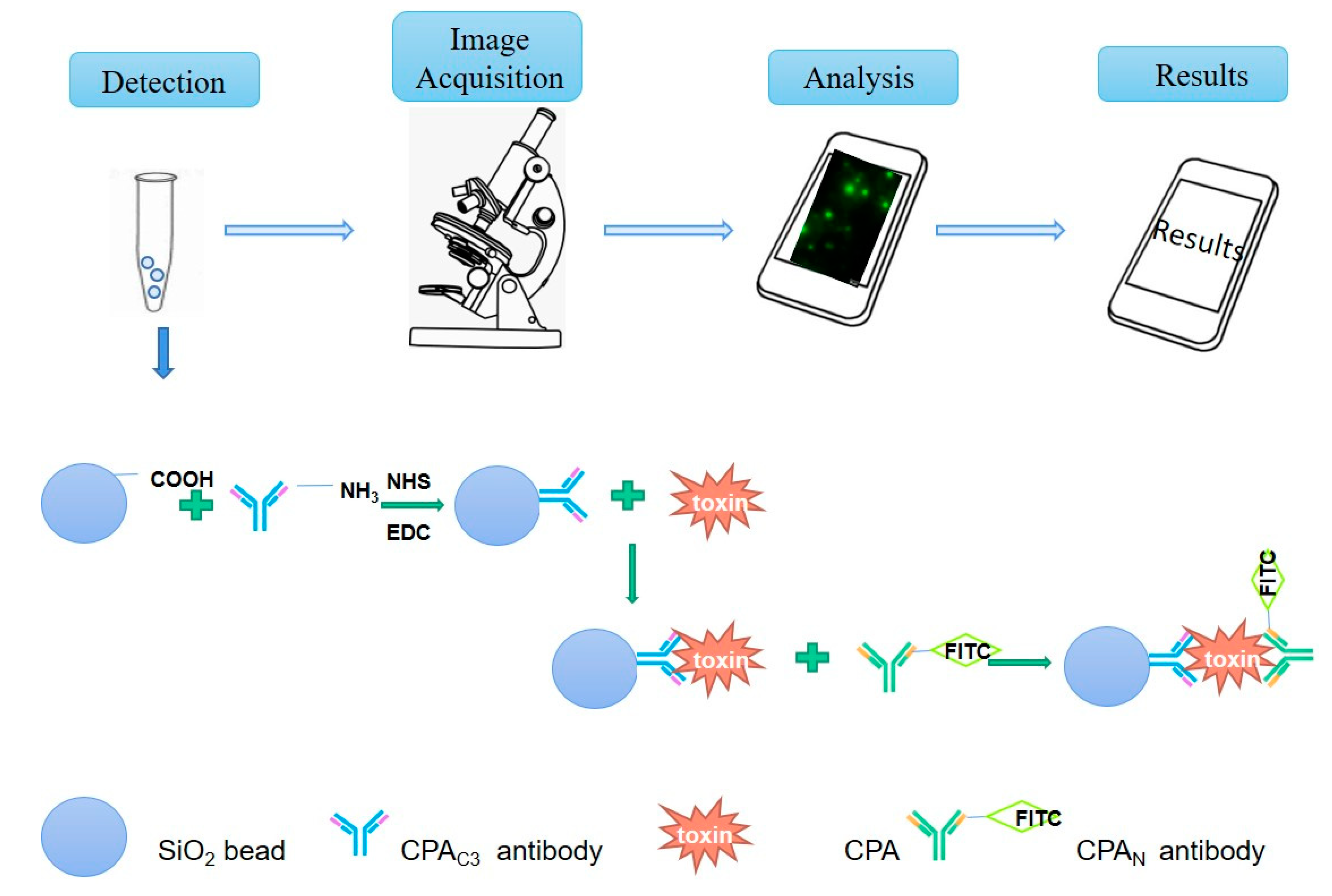
2.9. FITC-Labeled Antibody against C. perfringens Alpha Toxin
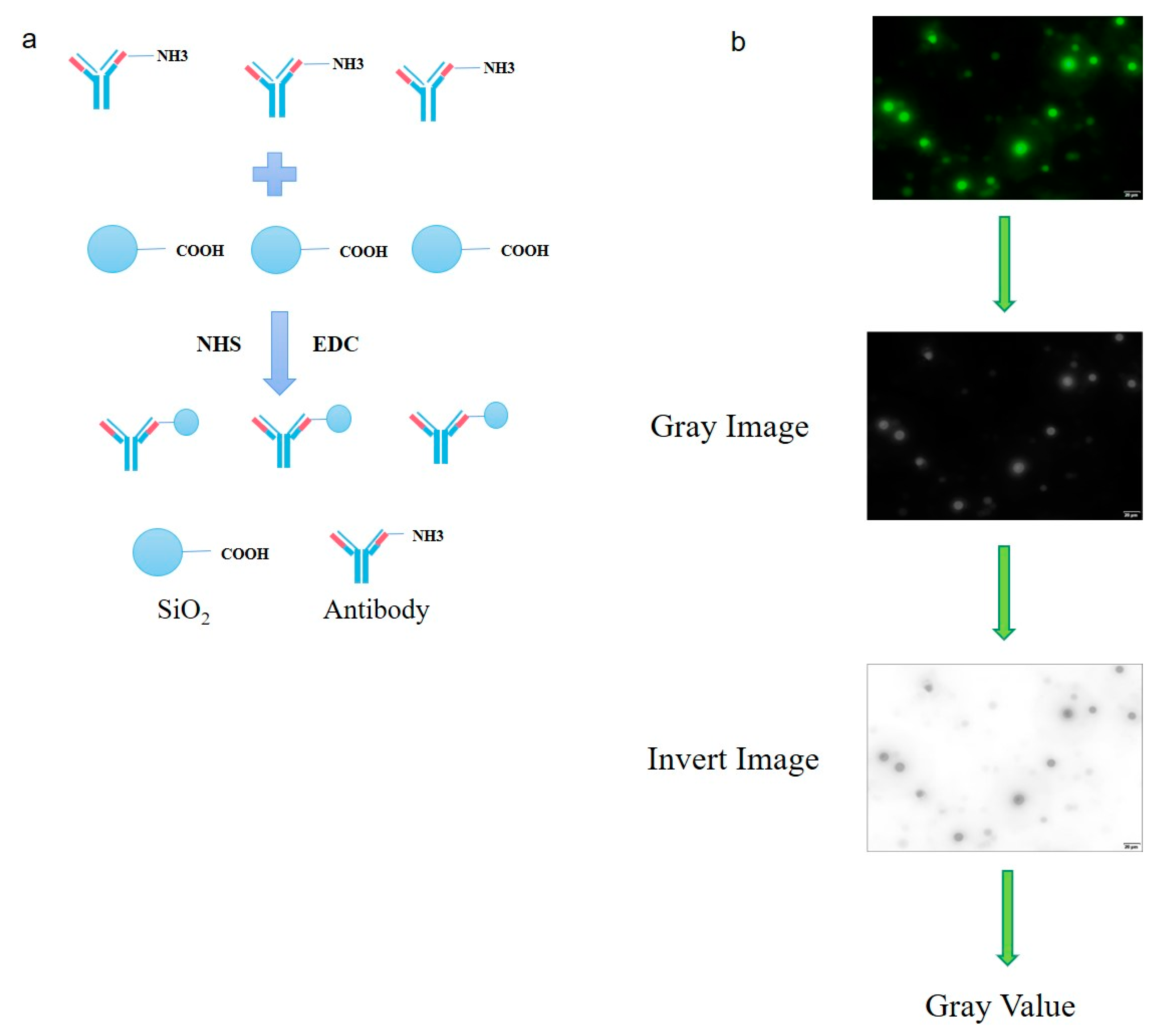
2.10. Coupling of Carboxyl-Modified Silica Microspheres with Antibody against CPAC3
2.11. Optimization of Detection Conditions
2.12. Statistical Analysis
3. Results
3.1. Construction of Clostridium Alpha Toxin (CPA) Domain Expression Vector and Preparation of Polyclonal Antibody
3.2. Quantification of Clostridium Alpha Toxin Concentration
3.3. Fluorescent Labeling of CPAN Antibody and Optimization of Silica Microsphere Detection Conditions
3.4. Establishment of Clostridium Toxin Fluorescence Detection System and Production and Application of Smartphone APP
4. Discussion
5. Patents
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lacey, J.A.; Johanesen, P.A.; Lyras, D.; Moore, R.J. In silico Identification of Novel Toxin Homologs and Associated Mobile Genetic Elements in Clostridium perfringens. Pathogens 2019, 8, 16. [Google Scholar] [CrossRef]
- Kumar, N.P.; Kumar, N.V.; Karthik, A. Molecular detection and characterization of Clostridium perfringens toxin genes causing necrotic enteritis in broiler chickens. Trop. Anim. Health Prod. 2019, 51, 1559–1569. [Google Scholar] [CrossRef] [PubMed]
- Oda, M.; Kihara, A.; Yoshioka, H.; Saito, Y.; Watanabe, N.; Uoo, K.; Higashihara, M.; Nagahama, M.; Koide, N.; Yokochi, T.; et al. Effect of erythromycin on biological activities induced by clostridium perfringens alpha-toxin. J. Pharm. Exp. Ther. 2008, 327, 934–940. [Google Scholar] [CrossRef] [PubMed]
- Takehara, M.; Seike, S.; Sonobe, Y.; Bandou, H.; Yokoyama, S.; Takagishi, T.; Miyamoto, K.; Kobayashi, K.; Nagahama, M. Clostridium perfringens alpha-toxin impairs granulocyte colony-stimulating factor receptor-mediated granulocyte production while triggering septic shock. Commun. Biol. 2019, 2, 45. [Google Scholar] [CrossRef] [PubMed]
- Takagishi, T.; Takehara, M.; Seike, S.; Miyamoto, K.; Kobayashi, K.; Nagahama, M. Clostridium perfringens alpha-toxin impairs erythropoiesis by inhibition of erythroid differentiation. Sci. Rep. 2017, 7, 5217. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.S.; Kim, D.H.; Bae, D.; Kim, S.H.; Kim, H.; Moon, J.S.; Song, K.Y.; Chon, J.W.; Seo, K.H. Prevalence, toxin-typing, and antimicrobial susceptibility of Clostridium perfringens from retail meats in Seoul, Korea. Anaerobe 2020, 102235. [Google Scholar] [CrossRef]
- Titball, R.W.; Hunter, S.E.; Martin, K.L.; Morris, B.C.; Shuttleworth, A.D.; Rubidge, T.; Anderson, D.W.; Kelly, D.C. Molecular cloning and nucleotide sequence of the alpha-toxin (phospholipase C) of Clostridium perfringens. Infect. Immun. 1989, 57, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Forti, K.; Cagiola, M.; Pellegrini, M.; Anzalone, L.; Di Paolo, A.; Corneli, S.; Severi, G.; De Giuseppe, A. Generation of recombinant baculovirus expressing atoxic C-terminal CPA toxin of Clostridium perfringens and production of specific antibodies. BMC Biotechnol. 2020, 20, 7. [Google Scholar] [CrossRef]
- Titball, R.W.; Leslie, D.L.; Harvey, S.; Kelly, D. Hemolytic and sphingomyelinase activities of Clostridium perfringens alpha-toxin are dependent on a domain homologous to that of an enzyme from the human arachidonic acid pathway. Infect. Immun. 1991, 59, 1872–1874. [Google Scholar] [CrossRef]
- Gao, X.; Ma, Y.; Wang, Z.; Bai, J.; Jia, S.; Feng, B.; Jiang, Y.; Cui, W.; Tang, L.; Li, Y.; et al. Oral immunization of mice with a probiotic Lactobacillus casei constitutively expressing the alpha-toxoid induces protective immunity against Clostridium perfringens alpha-toxin. Virulence 2019, 10, 166–179. [Google Scholar] [CrossRef]
- Jige, D.; Qi, X.; Zhen, Z.; Qihong, L.; Chunsheng, Y.; Wensheng, Y.; Kai, K.; Xiaoyun, C. Expression and Protective Efficacy of Clostridium perfringens α Toxin Derivative. China Anim. Husb. Vet. Med. 2019, 39, 265–270, 280. [Google Scholar]
- Mingfan, Y.; Yongjun, T.; Baoan, C.; Xuebin, W.; Shunyao, M. Analysis of SDS-PAGE Electrophoresis Patterns of Bacterial Proteins and Membrane Proteins of Different C. Chin. J. Vet. Med. 2000, 26, 12–14. [Google Scholar]
- Hale, M.L.; Stiles, B.G. Detection of Clostridium perfringens alpha toxin using a capture antibody ELISA. Toxicon 1999, 37, 471–484. [Google Scholar] [CrossRef]
- Cunn, L.; Jingyou, Z.; Hong, Z. Determination of alpha toxin of Clostridium perfringens by micro-method. J. Lanzhou Univ. 2000, 36, 101–104. [Google Scholar]
- Jin, X.; Li, Y.; Zhang, Y. Development of an indirect ELISA for diagnosis of bovine enterotoxemia resulted from Clostridium perfringens type D. Chin. Vet. Sci. 2008, 529, 358–364. [Google Scholar]
- Fach, P.; Guillou, J.P. Detection by in vitro amplification of the alpha-toxin (phospholipase C) gene from Clostridium perfringens. J. Appl. Bacteriol. 2010, 74, 61–66. [Google Scholar] [CrossRef]
- Yamagishi, T.; Sugitani, K.; Tanishima, K.; Nakamura, S. Polymerase Chain Reaction Test for Differentiation of Five Toxin Types of Clostridium perfringens. Microbiol. Immunol. 2013, 41, 295–299. [Google Scholar] [CrossRef]
- Bhatnagar, J.; Deleon-Carnes, M.; Kellar, K.L.; Bandyopadhyay, K.; Antoniadou, Z.A.; Shieh, W.J.; Paddock, C.D.; Zaki, S.R. Rapid, simultaneous detection of Clostridium sordellii and Clostridium perfringens in archived tissues by a novel PCR-based microsphere assay: Diagnostic implications for pregnancy-associated toxic shock syndrome cases. Infect. Dis. Obs. Gynecol. 2012, 2012, 972845. [Google Scholar] [CrossRef]
- Labib, M.; Berezovski, M.V. Electrochemical aptasensors for microbial and viral pathogens. Adv. Biochem. Eng. Biotechnol. 2014, 140, 155–181. [Google Scholar] [CrossRef]
- Berezhnoy, A.; Castro, I.; Levay, A.; Malek, T.R.; Gilboa, E. Aptamer-targeted inhibition of mTOR in T cells enhances antitumor immunity. J. Clin. Investig. 2014, 124, 188–197. [Google Scholar] [CrossRef]
- Wu, C.; Wang, C.; Yan, L.; Yang, C.J. Pyrene excimer nucleic acid probes for biomolecule signaling. J. Biomed. Nanotechnol. 2009, 5, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Iliuk, A.B.; Hu, L.; Tao, W.A. Aptamer in bioanalytical applications. Anal. Chem. 2011, 83, 4440–4452. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Li, Y.; Zhang, L.; Dai, S.; Wang, J.; Li, Y.; Zhang, L.; Huang, J. Staphylococcus enterotoxin profile of China isolates and the superantigenicity of some novel enterotoxins. Arch. Microbiol. 2017, 199, 723–736. [Google Scholar] [CrossRef] [PubMed]
- Yamakawa, Y.; Ohsaka, A. Purification and some properties of phospholipase C (alpha-toxin) of Clostridium perfringens. J. Biochem. 1977, 81, 115. [Google Scholar] [CrossRef]
- Xue, J.; Chen, X.; Cai, J.; Xue, W.; Ke, H. A Substitute Experiment on Detecting the Virulence of α Toxin of Clostridium Perferingens Type A. Jiangsu J. Agric. Sci. 2002, 18, 52–55. [Google Scholar]
- Urbina, P.; Flores-Diaz, M.; Alape-Giron, A.; Alonso, A.; Goni, F.M. Effects of bilayer composition and physical properties on the phospholipase C and sphingomyelinase activities of Clostridium perfringens alpha-toxin. Biochim. Biophys. Acta 2011, 1808, 279–286. [Google Scholar] [CrossRef]
- Urbina, P.; Collado, M.I.; Alonso, A.; Goni, F.M.; Flores-Diaz, M.; Alape-Giron, A.; Ruysschaert, J.M.; Lensink, M.F. Unexpected wide substrate specificity of C. perfringens alpha-toxin phospholipase C. Biochim. Biophys. Acta 2011, 1808, 2618–2627. [Google Scholar] [CrossRef]
- Rood, J.I.; Adams, V.; Lacey, J.; Lyras, D.; Mcclane, B.A.; Melville, S.B.; Moore, R.J.; Popoff, M.R.; Sarker, M.R.; Songer, J.G.; et al. Expansion of the Clostridium perfringens toxin-based typing scheme. Anaerobe 2018, 53, 5–10. [Google Scholar] [CrossRef]
- Saint-Joanis, B.; Garnier, T.; Cole, S.T. Gene cloning shows the alpha-toxin of Clostridium perfringens to contain both sphingomyelinase and lecithinase activities. Mol. Gen. Genet. 1989, 219, 453–460. [Google Scholar] [CrossRef]
- Nagahama, M.; Nakayama, T.; Michiue, K.; Sakurai, J. Site-specific mutagenesis of Clostridium perfringens alpha-toxin: Replacement of Asp-56, Asp-130, or Glu-152 causes loss of enzymatic and hemolytic activities. Infect. Immun. 1997, 65, 3489–3492. [Google Scholar] [CrossRef]
- Alape-Girón, A.; Flores-Díaz, M.; Guillouard, I.; Naylor, C.E.; Titball, R.W.; Rucavado, A.; Lomonte, B.; Basak, A.K.; Gutiérrez, J.M.; Cole, S.T. Identification of residues critical for toxicity in Clostridium perfringens phospholipase °C, the key toxin in gas gangrene. Eur. J. Biochem. 2000, 267, 5191–5197. [Google Scholar] [CrossRef] [PubMed]
- Lovland, A.; Kaldhusdal, M.; Reitan, L.J. Diagnosing Clostridium perfringens-associated necrotic enteritis in broiler flocks by an immunoglobulin G anti-alpha-toxin enzyme-linked immunosorbent assay. Avian Pathol. 2003, 32, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Effat, M.M. Restriction enzyme analysis and DNA sequencing comparison for alpha-toxin gene among different types of Clostridium perfringens. Pak. J. Biol. Sci. 2008, 11, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Olsvik, Ø.; Fossum, K.; Berdal, B.P. Staphylococcal enterotoxin A, B, and C produced by coagulase-negative strains within the family Micrococcaceae. Apmis 2010, 90, 441–444. [Google Scholar] [CrossRef] [PubMed]
- Mcclane, B.A.; Strouse, R.J. Rapid detection of Clostridium perfringens type A enterotoxin by enzyme-linked immunosorbent assay. J. Clin. Microbiol. 1984, 19, 112–115. [Google Scholar] [CrossRef]
- Uzal, F.A.; Kelly, W.R. Enterotoxaemia in goats. Vet. Res. Commun. 1996, 20, 481–492. [Google Scholar] [CrossRef]
- Yoo, H.S.; Lee, S.U.; Park, K.; Park, Y.H. Molecular typing and epidemiological survey of prevalence of Clostridium perfringens types by multiplex PCR. J. Clin. Microbiol. 1997, 35, 228–232. [Google Scholar] [CrossRef]
- Gurjar, A.A.; Hegde, N.V.; Love, B.C.; Jayarao, B.M. Real-time multiplex PCR assay for rapid detection and toxintyping of Clostridium perfringens toxin producing strains in feces of dairy cattle. Mol. Cell Probes 2008, 22, 90–95. [Google Scholar] [CrossRef]
- Moro, A.J.; Schmidt, J.; Doussineau, T.; Lapresta-Fernandez, A.; Wegener, J.; Mohr, G.J. Surface-functionalized fluorescent silica nanoparticles for the detection of ATP. Chem. Commun. 2011, 47, 6066–6068. [Google Scholar] [CrossRef]
- Wan, J.; Meng, X.; Liu, E.; Chen, K. Incorporation of magnetite nanoparticle clusters in fluorescent silica nanoparticles for high-performance brain tumor delineation. Nanotechnology 2010, 21, 235104. [Google Scholar] [CrossRef]


| Capture Antibody | Blocking Condition | Detection Antibody | |
|---|---|---|---|
| Optimal dilution | 0.1 mg/mL | 5% BSA | 0.2 mg/mL |
| Reaction conditions | 25 °C, 2 h | 4 °C, 12 h | 37 °C, 1 h |
| Sample Group (3/group) | Average MN ± SD | Coefficient of Variation CV (%) |
|---|---|---|
| 1 | 0.195 ± 0.0048 | 2.46 |
| 2 | 0.095 ± 0.0098 | 10.3 |
| 3 | 0.053 ± 0.0018 | 3.40 |
| 4 | 0.027 ± 0.0022 | 8.14 |
| 5 | 0.014 ± 0.0017 | 12.1 |
| Sample | Toxins | Add Concentration (×LD50) | Detected Concentration | Recovery (%) |
|---|---|---|---|---|
| 1 | CPA | 0.625 | 0.50 | 80 |
| 2 | CPA | 2.5 | 2.72 | 108.8 |
| 3 | CPA | 10 | 10.24 | 102.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, A.; Chi, H.; Shi, J.; Sun, R.; Du, K.; Song, Y.; Zhu, M.; Zhang, L.; Huang, J. Visual Detection of Clostridium perfringens Alpha Toxin by Combining Nanometer Microspheres with Smart Phones. Microorganisms 2020, 8, 1865. https://doi.org/10.3390/microorganisms8121865
Cao A, Chi H, Shi J, Sun R, Du K, Song Y, Zhu M, Zhang L, Huang J. Visual Detection of Clostridium perfringens Alpha Toxin by Combining Nanometer Microspheres with Smart Phones. Microorganisms. 2020; 8(12):1865. https://doi.org/10.3390/microorganisms8121865
Chicago/Turabian StyleCao, Aiping, Heng Chi, Jingxuan Shi, Ruiqi Sun, Kang Du, Yinna Song, Min Zhu, Lilin Zhang, and Jinhai Huang. 2020. "Visual Detection of Clostridium perfringens Alpha Toxin by Combining Nanometer Microspheres with Smart Phones" Microorganisms 8, no. 12: 1865. https://doi.org/10.3390/microorganisms8121865
APA StyleCao, A., Chi, H., Shi, J., Sun, R., Du, K., Song, Y., Zhu, M., Zhang, L., & Huang, J. (2020). Visual Detection of Clostridium perfringens Alpha Toxin by Combining Nanometer Microspheres with Smart Phones. Microorganisms, 8(12), 1865. https://doi.org/10.3390/microorganisms8121865






