Streptococcus gordonii: Pathogenesis and Host Response to Its Cell Wall Components
Abstract
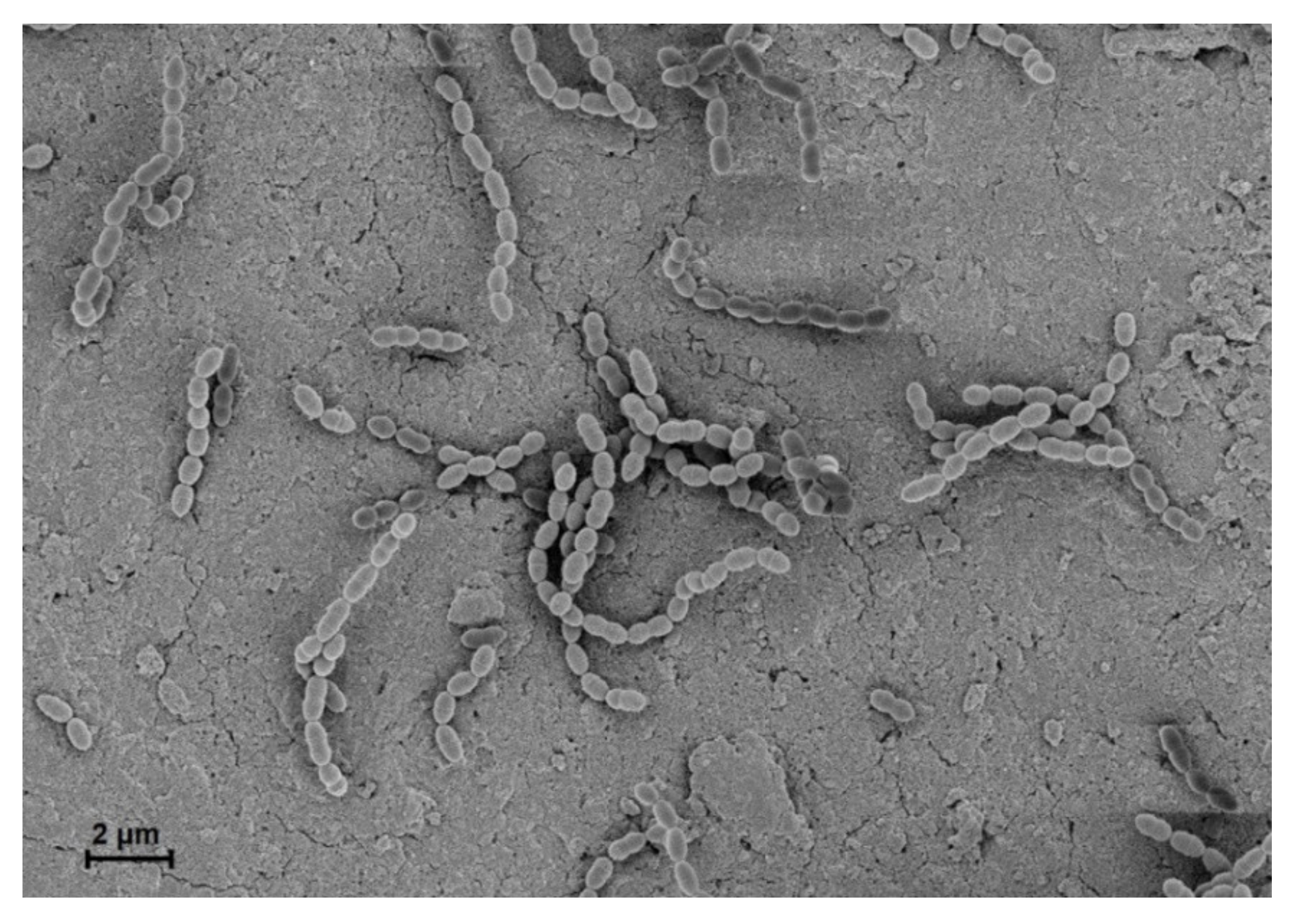
1. Overview of Streptococcus gordonii
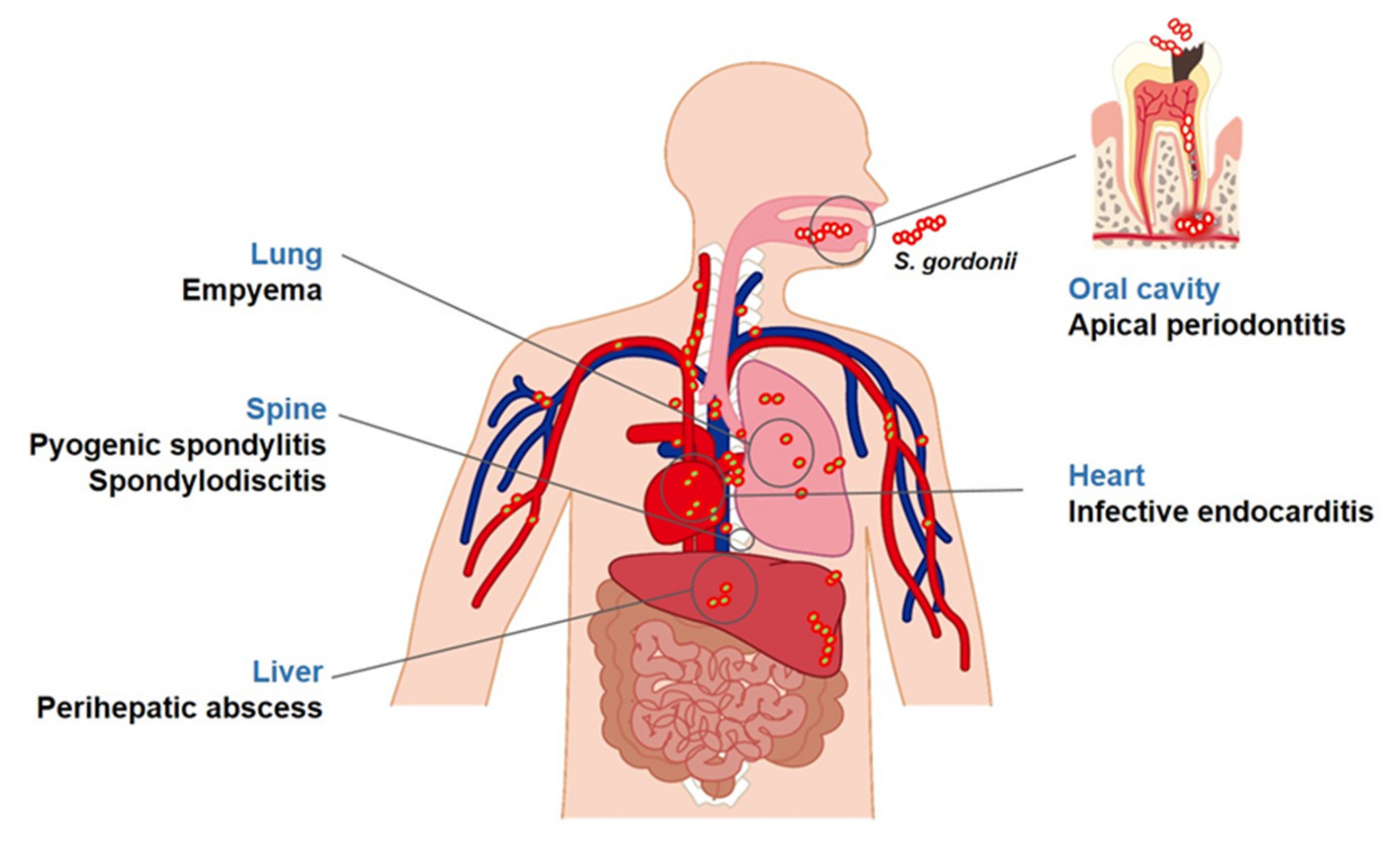
2. Diseases Associated with S. gordonii
2.1. Apical Periodontitis
2.2. Infective Endocarditis
2.3. Other Diseases
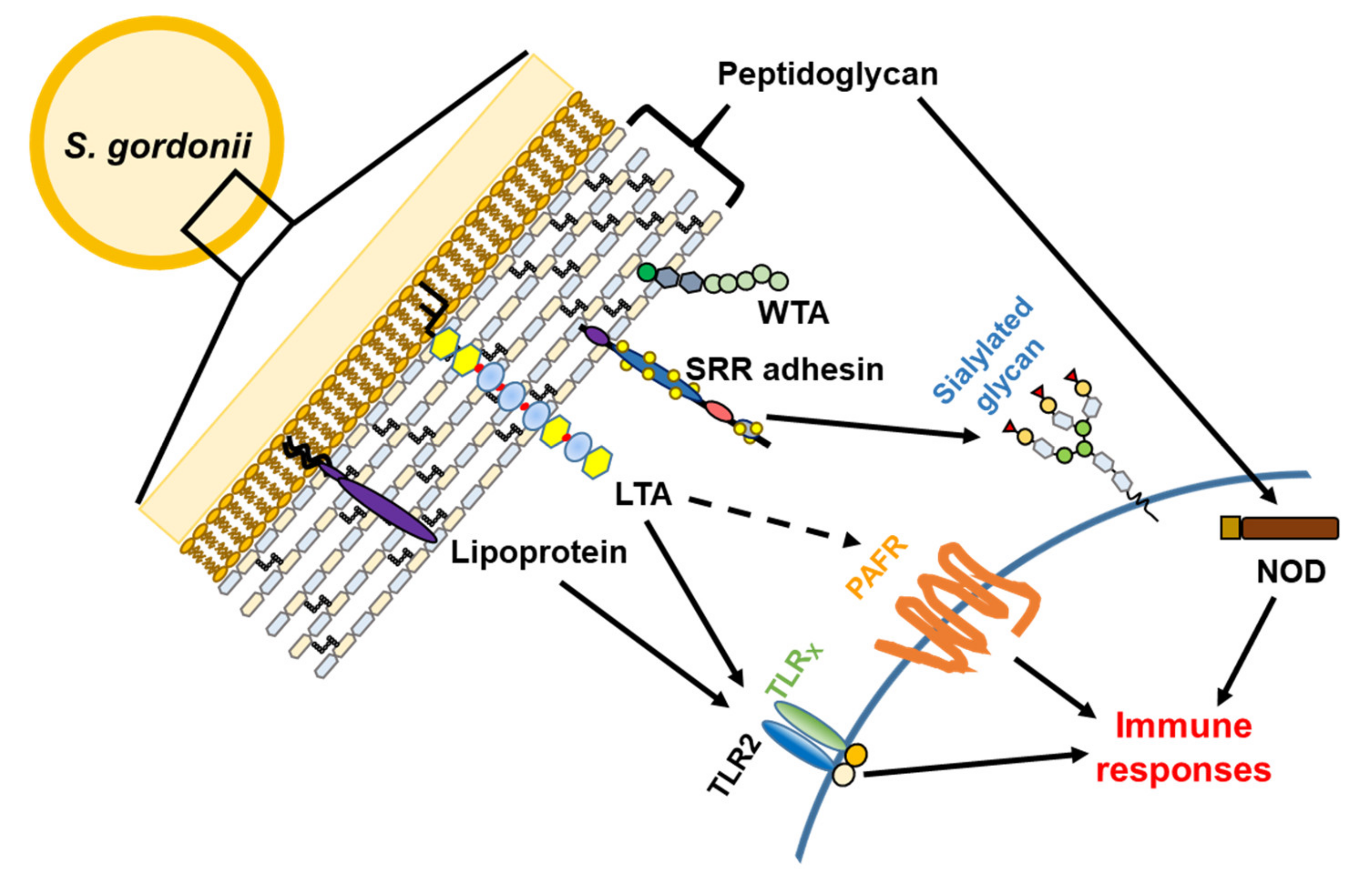
3. Virulence Factors
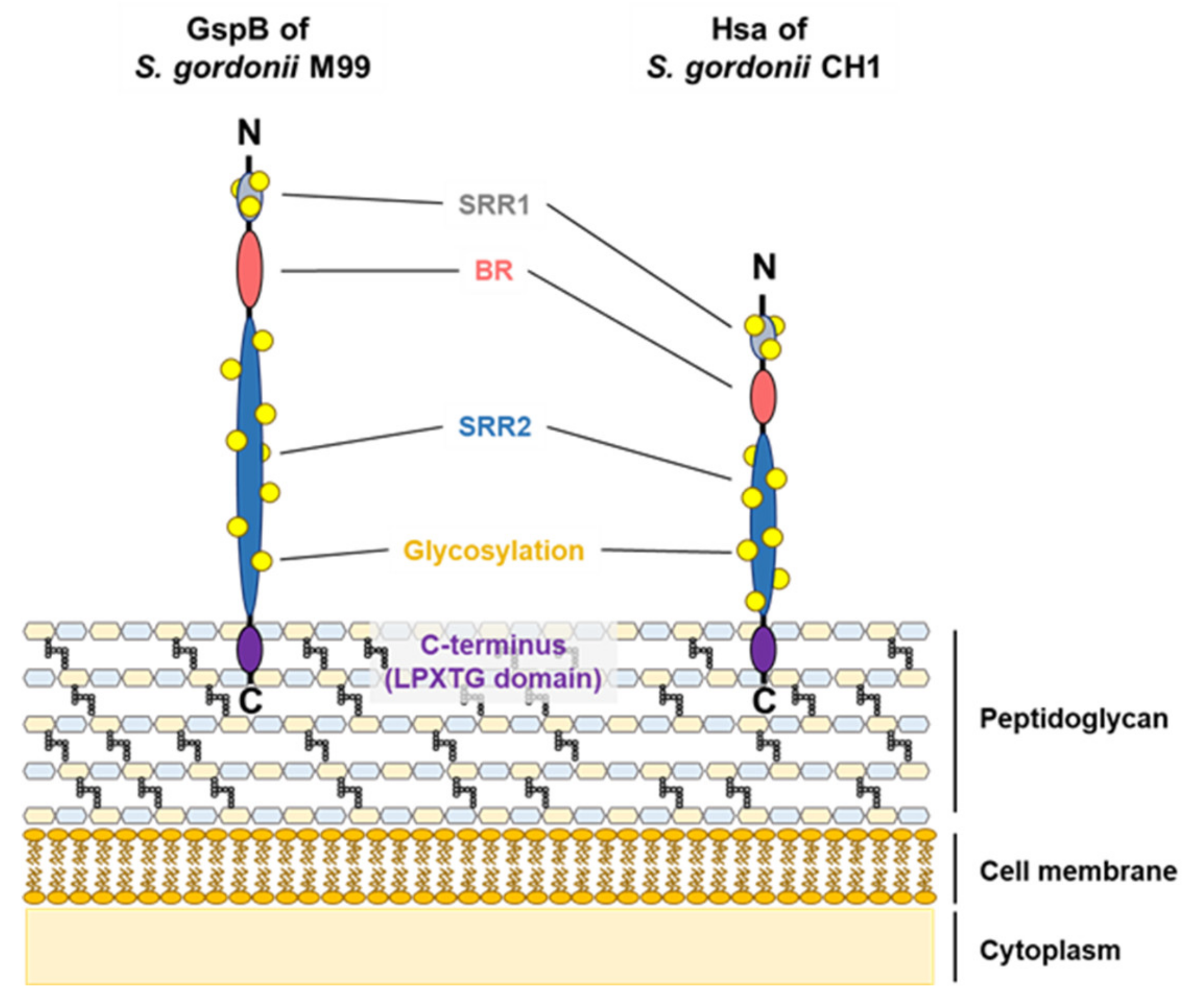
3.1. Serine-Rich Repeat Adhesins
3.2. Cell Wall Proteins
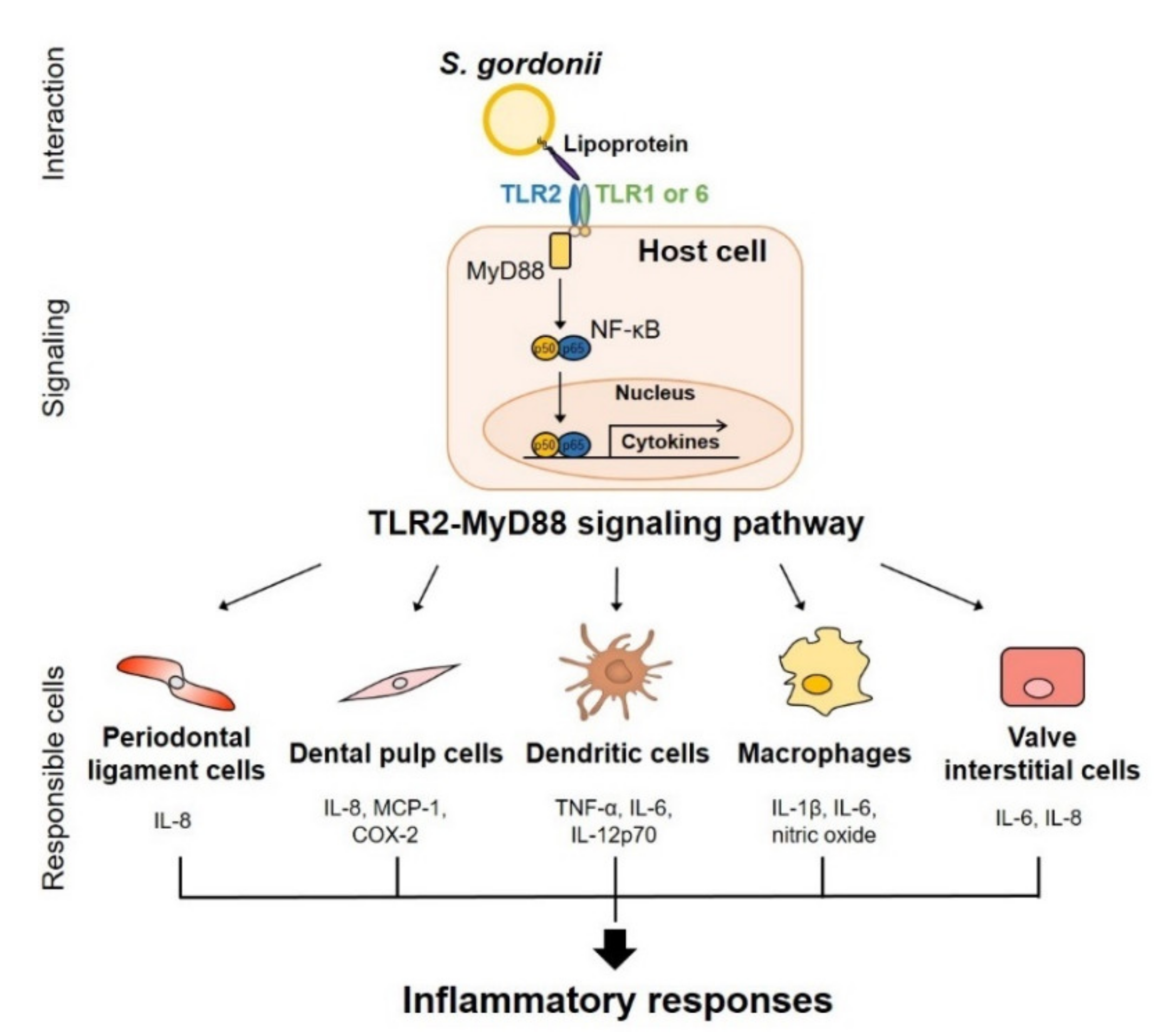
3.3. Lipoproteins
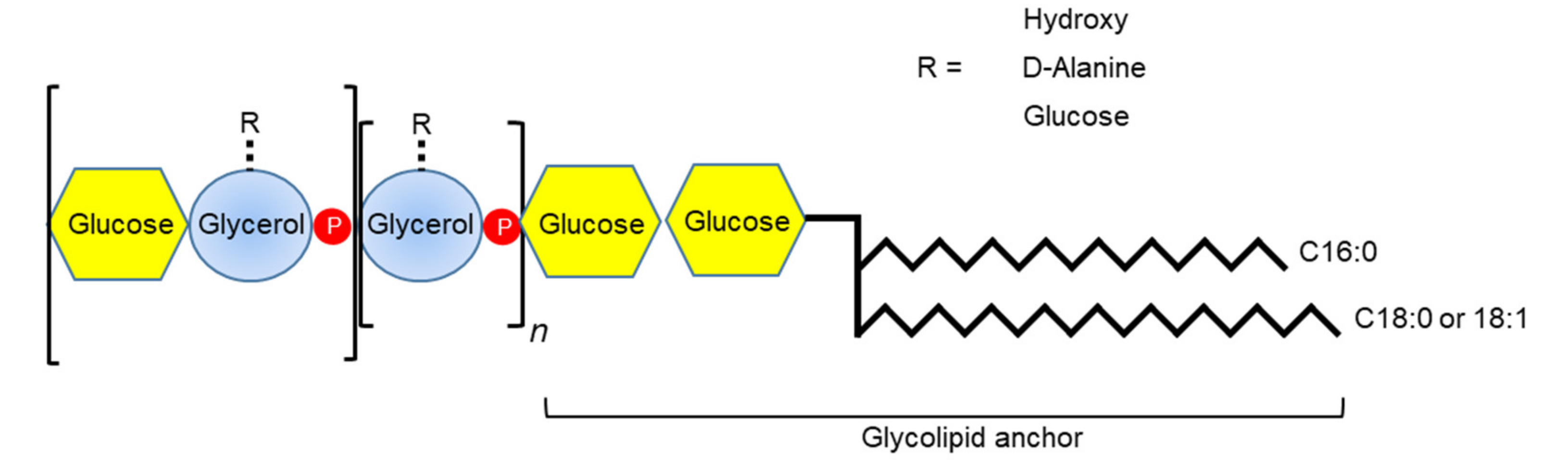
3.4. Teichoic Acids
3.5. Peptidoglycan
4. Therapeutic Strategies against S. gordonii Infections
4.1. Regulation of Biofilm
4.2. Inhibition of Bacterial Cell Wall Components
4.2.1. Lipoprotein
4.2.2. Lipoteichoic Acid/Wall Teichoic Acid
4.2.3. Peptidoglycan
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hardie, J.M.; Whiley, R.A. Classification and overview of the genera Streptococcus and Enterococcus. J. Appl. Microbiol. 1997, 83, 1S–11S. [Google Scholar] [CrossRef] [PubMed]
- Garnier, F.; Gerbaud, G.; Courvalin, P.; Galimand, M. Identification of clinically relevant viridans group streptococci to the species level by PCR. J. Clin. Microbiol. 1997, 35, 2337–2341. [Google Scholar] [CrossRef] [PubMed]
- Abranches, J.; Zeng, L.; Kajfasz, J.K.; Palmer, S.R.; Chakraborty, B.; Wen, Z.T.; Richards, V.P.; Brady, L.J.; Lemos, J.A. Biology of oral Streptococci. Microbiol. Spectr. 2018, 6. [Google Scholar] [CrossRef] [PubMed]
- Mundt, J.O. The ecology of the streptococci. Microb. Ecol. 1982, 8, 355–369. [Google Scholar] [CrossRef]
- Pinto, B.; Pierotti, R.; Canale, G.; Reali, D. Characterization of ‘faecal streptococci’ as indicators of faecal pollution and distribution in the environment. Lett. Appl. Microbiol. 1999, 29, 258–263. [Google Scholar] [CrossRef]
- Mosailova, N.; Truong, J.; Dietrich, T.; Ashurst, J. Streptococcus gordonii: A rare cause of infective endocarditis. Case Rep. Infect. Dis. 2019, 2019, 7127848. [Google Scholar] [CrossRef]
- Cheng, J.; Hu, H.; Fang, W.; Shi, D.; Liang, C.; Sun, Y.; Gao, G.; Wang, H.; Zhang, Q.; Wang, L.; et al. Detection of pathogens from resected heart valves of patients with infective endocarditis by next-generation sequencing. Int. J. Infect. Dis. 2019, 83, 148–153. [Google Scholar] [CrossRef]
- Gilbert, K.; Joseph, R.; Vo, A.; Patel, T.; Chaudhry, S.; Nguyen, U.; Trevor, A.; Robinson, E.; Campbell, M.; McLennan, J.; et al. Children with severe early childhood caries: Streptococci genetic strains within carious and white spot lesions. J. Oral. Microbiol. 2014, 6. [Google Scholar] [CrossRef]
- Zandi, H.; Kristoffersen, A.K.; Orstavik, D.; Rocas, I.N.; Siqueira, J.F., Jr.; Enersen, M. Microbial analysis of endodontic infections in root-filled teeth with apical periodontitis before and after irrigation using pyrosequencing. J. Endod. 2018, 44, 372–378. [Google Scholar] [CrossRef]
- Chavez de Paz, L.; Svensater, G.; Dahlen, G.; Bergenholtz, G. Streptococci from root canals in teeth with apical periodontitis receiving endodontic treatment. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2005, 100, 232–241. [Google Scholar] [CrossRef]
- Back, C.R.; Sztukowska, M.N.; Till, M.; Lamont, R.J.; Jenkinson, H.F.; Nobbs, A.H.; Race, P.R. The Streptococcus gordonii adhesin CshA protein binds host fibronectin via a catch-clamp mechanism. J. Biol. Chem. 2017, 292, 1538–1549. [Google Scholar] [CrossRef] [PubMed]
- Loo, C.Y.; Corliss, D.A.; Ganeshkumar, N. Streptococcus gordonii biofilm formation: Identification of genes that code for biofilm phenotypes. J. Bacteriol. 2000, 182, 1374–1382. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.K.; Espaillat, A.; Cava, F. Bacterial strategies to preserve cell wall integrity against environmental threats. Front. Microbiol. 2018, 9, 2064. [Google Scholar] [CrossRef] [PubMed]
- Chandler, C.E.; Ernst, R.K. Bacterial lipids: Powerful modifiers of the innate immune response. F1000Res 2017, 6. [Google Scholar] [CrossRef]
- Andrian, E.; Qi, G.; Wang, J.; Halperin, S.A.; Lee, S.F. Role of surface proteins SspA and SspB of Streptococcus gordonii in innate immunity. Microbiology (Reading) 2012, 158, 2099–2106. [Google Scholar] [CrossRef]
- Ko, E.B.; Kim, S.K.; Seo, H.S.; Yun, C.H.; Han, S.H. Serine-rich repeat adhesins contribute to Streptococcus gordonii-induced maturation of human dendritic cells. Front. Microbiol. 2017, 8, 523. [Google Scholar] [CrossRef][Green Version]
- Takamatsu, D.; Bensing, B.A.; Cheng, H.; Jarvis, G.A.; Siboo, I.R.; Lopez, J.A.; Griffiss, J.M.; Sullam, P.M. Binding of the Streptococcus gordonii surface glycoproteins GspB and Hsa to specific carbohydrate structures on platelet membrane glycoprotein Ibalpha. Mol. Microbiol. 2005, 58, 380–392. [Google Scholar] [CrossRef]
- Nair, P.N. Pathogenesis of apical periodontitis and the causes of endodontic failures. Crit Rev. Oral Biol Med. 2004, 15, 348–381. [Google Scholar] [CrossRef]
- Buckley, M.; Spangberg, L.S. The prevalence and technical quality of endodontic treatment in an American subpopulation. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1995, 79, 92–100. [Google Scholar] [CrossRef]
- Stauffacher, S.; Lussi, A.; Nietzsche, S.; Neuhaus, K.W.; Eick, S. Bacterial invasion into radicular dentine-an in vitro study. Clin. Oral Investig. 2017, 21, 1743–1752. [Google Scholar] [CrossRef]
- Zheng, L.; Chen, Z.; Itzek, A.; Herzberg, M.C.; Kreth, J. CcpA regulates biofilm formation and competence in Streptococcus gordonii. Mol. Oral Microbiol. 2012, 27, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.J.; Yeh, C.Y.; Shun, C.T.; Hsu, R.B.; Cheng, H.W.; Lin, C.S.; Chia, J.S. Platelets enhance biofilm formation and resistance of endocarditis-inducing streptococci on the injured heart valve. J. Infect. Dis. 2012, 205, 1066–1075. [Google Scholar] [CrossRef] [PubMed]
- Kanwar, I.; Sah, A.K.; Suresh, P.K. Biofilm-mediated antibiotic-resistant oral bacterial infections: Mechanism and combat strategies. Curr. Pharm. Des. 2017, 23, 2084–2095. [Google Scholar] [CrossRef] [PubMed]
- Love, R.M. Bacterial adhesins--their role in tubule invasion and endodontic disease. Aust Endod. J. 2002, 28, 25–28. [Google Scholar] [CrossRef]
- Kim, A.R.; Ahn, K.B.; Kim, H.Y.; Seo, H.S.; Kum, K.Y.; Yun, C.H.; Han, S.H. Streptococcus gordonii lipoproteins induce IL-8 in human periodontal ligament cells. Mol. Immunol. 2017, 91, 218–224. [Google Scholar] [CrossRef]
- Yoo, Y.J.; Perinpanayagam, H.; Lee, J.Y.; Oh, S.; Gu, Y.; Kim, A.R.; Chang, S.W.; Baek, S.H.; Kum, K.Y. Synthetic human beta defensin-3-C15 peptide in endodontics: Potential therapeutic agent in Streptococcus gordonii lipoprotein-stimulated human dental pulp-derived cells. Int. J. Mol. Sci. 2019, 21. [Google Scholar] [CrossRef]
- Vernier, A.; Diab, M.; Soell, M.; Haan-Archipoff, G.; Beretz, A.; Wachsmann, D.; Klein, J.P. Cytokine production by human epithelial and endothelial cells following exposure to oral viridans streptococci involves lectin interactions between bacteria and cell surface receptors. Infect. Immun. 1996, 64, 3016–3022. [Google Scholar] [CrossRef]
- Henkels, K.M.; Frondorf, K.; Gonzalez-Mejia, M.E.; Doseff, A.L.; Gomez-Cambronero, J. IL-8-induced neutrophil chemotaxis is mediated by Janus kinase 3 (JAK3). FEBS Lett. 2011, 585, 159–166. [Google Scholar] [CrossRef]
- Park, O.J.; Kim, J.; Kim, H.Y.; Kwon, Y.; Yun, C.H.; Han, S.H. Streptococcus gordonii induces bone resorption by increasing osteoclast differentiation and reducing osteoblast differentiation. Microb. Pathog. 2019, 126, 218–223. [Google Scholar] [CrossRef]
- Ataoglu, T.; Ungor, M.; Serpek, B.; Haliloglu, S.; Ataoglu, H.; Ari, H. Interleukin-1beta and tumour necrosis factor-alpha levels in periapical exudates. Int. Endod. J. 2002, 35, 181–185. [Google Scholar] [CrossRef]
- Safavi, K.E.; Rossomando, E.F. Tumor necrosis factor identified in periapical tissue exudates of teeth with apical periodontitis. J. Endod. 1991, 17, 12–14. [Google Scholar] [CrossRef]
- Graunaite, I.; Lodiene, G.; Maciulskiene, V. Pathogenesis of apical periodontitis: A literature review. J. Oral Maxillofac. Res. 2012, 2, e1. [Google Scholar] [CrossRef] [PubMed]
- Yombi, J.; Belkhir, L.; Jonckheere, S.; Wilmes, D.; Cornu, O.; Vandercam, B.; Rodriguez-Villalobos, H. Streptococcus gordonii septic arthritis: Two cases and review of literature. BMC Infect. Dis. 2012, 12, 215. [Google Scholar] [CrossRef] [PubMed]
- Bannay, A.; Hoen, B.; Duval, X.; Obadia, J.F.; Selton-Suty, C.; Le Moing, V.; Tattevin, P.; Iung, B.; Delahaye, F.; Alla, F.; et al. The impact of valve surgery on short- and long-term mortality in left-sided infective endocarditis: Do differences in methodological approaches explain previous conflicting results? Eur. Heart J. 2011, 32, 2003–2015. [Google Scholar] [CrossRef]
- Murdoch, D.R.; Corey, G.R.; Hoen, B.; Miro, J.M.; Fowler, V.G.; Bayer, A.S.; Karchmer, A.W.; Olaison, L.; Pappas, P.A.; Moreillon, P.; et al. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: The International Collaboration on Endocarditis-Prospective Cohort Study. Arch. Intern. Med. 2009, 169, 463–473. [Google Scholar] [CrossRef]
- Pant, S.; Patel, N.J.; Deshmukh, A.; Golwala, H.; Patel, N.; Badheka, A.; Hirsch, G.A.; Mehta, J.L. Trends in infective endocarditis incidence, microbiology, and valve replacement in the United States from 2000 to 2011. J. Am. Coll. Cardiol. 2015, 65, 2070–2076. [Google Scholar] [CrossRef]
- Chamat-Hedemand, S.; Dahl, A.; Ostergaard, L.; Arpi, M.; Fosbol, E.; Boel, J.; Oestergaard, L.B.; Lauridsen, T.K.; Gislason, G.; Torp-Pedersen, C.; et al. Prevalence of infective endocarditis in Streptococcal bloodstream infections is dependent on Streptococcal species. Circulation 2020, 142, 720–730. [Google Scholar] [CrossRef]
- Veloso, T.R.; Amiguet, M.; Rousson, V.; Giddey, M.; Vouillamoz, J.; Moreillon, P.; Entenza, J.M. Induction of experimental endocarditis by continuous low-grade bacteremia mimicking spontaneous bacteremia in humans. Infect. Immun. 2011, 79, 2006–2011. [Google Scholar] [CrossRef]
- Takahashi, Y.; Takashima, E.; Shimazu, K.; Yagishita, H.; Aoba, T.; Konishi, K. Contribution of sialic acid-binding adhesin to pathogenesis of experimental endocarditis caused by Streptococcus gordonii DL1. Infect. Immun. 2006, 74, 740–743. [Google Scholar] [CrossRef]
- Deng, L.; Bensing, B.A.; Thamadilok, S.; Yu, H.; Lau, K.; Chen, X.; Ruhl, S.; Sullam, P.M.; Varki, A. Oral streptococci utilize a Siglec-like domain of serine-rich repeat adhesins to preferentially target platelet sialoglycans in human blood. PLoS Pathog. 2014, 10, e1004540. [Google Scholar] [CrossRef]
- Segawa, T.; Saeki, A.; Hasebe, A.; Arimoto, T.; Kataoka, H.; Yokoyama, A.; Kawanami, M.; Shibata, K. Differences in recognition of wild-type and lipoprotein-deficient strains of oral Streptococci in vitro and in vivo. Pathog. Dis. 2013, 68, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Shun, C.T.; Yeh, C.Y.; Chang, C.J.; Wu, S.H.; Lien, H.T.; Chen, J.Y.; Wang, S.S.; Chia, J.S. Activation of human valve interstitial cells by a viridians streptococci modulin induces chemotaxis of mononuclear cells. J. Infect. Dis. 2009, 199, 1488–1496. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Baik, J.E.; Ahn, K.B.; Seo, H.S.; Yun, C.H.; Han, S.H. Streptococcus gordonii induces nitric oxide production through its lipoproteins stimulating Toll-like receptor 2 in murine macrophages. Mol. Immunol. 2017, 82, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Ciabattini, A.; Cuppone, A.M.; Pulimeno, R.; Iannelli, F.; Pozzi, G.; Medaglini, D. Stimulation of human monocytes with the gram-positive vaccine vector Streptococcus gordonii. Clin. Vaccine Immunol. 2006, 13, 1037–1043. [Google Scholar] [CrossRef]
- Corinti, S.; Medaglini, D.; Cavani, A.; Rescigno, M.; Pozzi, G.; Ricciardi-Castagnoli, P.; Girolomoni, G. Human dendritic cells very efficiently present a heterologous antigen expressed on the surface of recombinant gram-positive bacteria to CD4+ T lymphocytes. J. Immunol. 1999, 163, 3029–3036. [Google Scholar]
- Xiong, Y.Q.; Bensing, B.A.; Bayer, A.S.; Chambers, H.F.; Sullam, P.M. Role of the serine-rich surface glycoprotein GspB of Streptococcus gordonii in the pathogenesis of infective endocarditis. Microb. Pathog. 2008, 45, 297–301. [Google Scholar] [CrossRef]
- Krantz, A.M.; Ratnaraj, F.; Velagapudi, M.; Krishnan, M.; Gujjula, N.R.; Foral, P.A.; Preheim, L. Streptococcus Gordonii Empyema: A Case Report and Review of Empyema. Cureus 2017, 9, e1159. [Google Scholar] [CrossRef]
- Wu, P.; Chung, E.; Marzella, N.; Preis, J. Streptococcus gordonii Perihepatic Abscess: A Case Report. Am. J. Clin. Microbiol. Antimicrob. 2019, 2, 1038. [Google Scholar]
- Dadon, Z.; Cohen, A.; Szterenlicht, Y.M.; Assous, M.V.; Barzilay, Y.; Raveh-Brawer, D.; Yinnon, A.M.; Munter, G. Spondylodiskitis and endocarditis due to Streptococcus gordonii. Ann. Clin. Microbiol. Antimicrob. 2017, 16, 68. [Google Scholar] [CrossRef]
- Nakamura, D.; Kondo, R.; Makiuchi, A.; Isobe, K. Empyema and pyogenic spondylitis caused by direct Streptococcus gordonii infection after a compression fracture: A case report. Surg. Case Rep. 2019, 5, 52. [Google Scholar] [CrossRef]
- Lizcano, A.; Sanchez, C.J.; Orihuela, C.J. A role for glycosylated serine-rich repeat proteins in Gram-positive bacterial pathogenesis. Mol. Oral Microbiol. 2012, 27, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Bensing, B.A.; Sullam, P.M. An accessory sec locus of Streptococcus gordonii is required for export of the surface protein GspB and for normal levels of binding to human platelets. Mol. Microbiol. 2002, 44, 1081–1094. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Konishi, K.; Cisar, J.O.; Yoshikawa, M. Identification and characterization of hsa, the gene encoding the sialic acid-binding adhesin of Streptococcus gordonii DL1. Infect. Immun. 2002, 70, 1209–1218. [Google Scholar] [CrossRef] [PubMed]
- Bensing, B.A.; Khedri, Z.; Deng, L.; Yu, H.; Prakobphol, A.; Fisher, S.J.; Chen, X.; Iverson, T.M.; Varki, A.; Sullam, P.M. Novel aspects of sialoglycan recognition by the Siglec-like domains of streptococcal SRR glycoproteins. Glycobiology 2016, 26, 1222–1234. [Google Scholar] [CrossRef] [PubMed]
- Takamatsu, D.; Bensing, B.A.; Prakobphol, A.; Fisher, S.J.; Sullam, P.M. Binding of the streptococcal surface glycoproteins GspB and Hsa to human salivary proteins. Infect. Immun. 2006, 74, 1933–1940. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Fives-Taylor, P.M. Identification of dipeptide repeats and a cell wall sorting signal in the fimbriae-associated adhesin, Fap1, of Streptococcus parasanguis. Mol. Microbiol. 1999, 34, 1070–1081. [Google Scholar] [CrossRef]
- Bensing, B.A.; Lopez, J.A.; Sullam, P.M. The Streptococcus gordonii surface proteins GspB and Hsa mediate binding to sialylated carbohydrate epitopes on the platelet membrane glycoprotein Ibalpha. Infect. Immun. 2004, 72, 6528–6537. [Google Scholar] [CrossRef]
- Takamatsu, D.; Bensing, B.A.; Sullam, P.M. Two additional components of the accessory sec system mediating export of the Streptococcus gordonii platelet-binding protein GspB. J. Bacteriol. 2005, 187, 3878–3883. [Google Scholar] [CrossRef]
- Urano-Tashiro, Y.; Takahashi, Y.; Oguchi, R.; Konishi, K. Two arginine residues of Streptococcus gordonii sialic acid-binding adhesin Hsa are rssential for interaction to host cell receptors. PLoS ONE 2016, 11, e0154098. [Google Scholar] [CrossRef]
- Christie, J.; McNab, R.; Jenkinson, H.F. Expression of fibronectin-binding protein FbpA modulates adhesion in Streptococcus gordonii. Microbiology (Reading) 2002, 148, 1615–1625. [Google Scholar] [CrossRef]
- McNab, R.; Tannock, G.W.; Jenkinson, H.F. Characterization of CshA, a high molecular mass adhesin of Streptococcus gordonii. Dev. Biol. Stand. 1995, 85, 371–375. [Google Scholar] [PubMed]
- Moschioni, M.; Pansegrau, W.; Barocchi, M.A. Adhesion determinants of the Streptococcus species. Microb. Biotechnol. 2010, 3, 370–388. [Google Scholar] [CrossRef] [PubMed]
- Yajima, A.; Urano-Tashiro, Y.; Shimazu, K.; Takashima, E.; Takahashi, Y.; Konishi, K. Hsa, an adhesin of Streptococcus gordonii DL1, binds to alpha2-3-linked sialic acid on glycophorin A of the erythrocyte membrane. Microbiol. Immunol. 2008, 52, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.R.; Ahn, K.B.; Kim, H.Y.; Seo, H.S.; Yun, C.H.; Han, S.H. Serine-rich repeat adhesin gordonii surface protein B is important for Streptococcus gordonii biofilm formation. J. Endod. 2016, 42, 1767–1772. [Google Scholar] [CrossRef] [PubMed]
- Oguchi, R.; Takahashi, Y.; Shimazu, K.; Urano-Tashiro, Y.; Kawarai, T.; Konishi, K.; Karibe, H. Contribution of Streptococcus gordonii Hsa adhesin to biofilm formation. Jpn. J. Infect. Dis. 2017, 70, 399–404. [Google Scholar] [CrossRef]
- Ruhl, S.; Cisar, J.O.; Sandberg, A.L. Identification of polymorphonuclear leukocyte and HL-60 cell receptors for adhesins of Streptococcus gordonii and Actinomyces naeslundii. Infect. Immun. 2000, 68, 6346–6354. [Google Scholar] [CrossRef]
- Urano-Tashiro, Y.; Yajima, A.; Takahashi, Y.; Konishi, K. Streptococcus gordonii promotes rapid differentiation of monocytes into dendritic cells through interaction with the sialic acid-binding adhesin. Odontology 2012, 100, 144–148. [Google Scholar] [CrossRef]
- Urano-Tashiro, Y.; Yajima, A.; Takashima, E.; Takahashi, Y.; Konishi, K. Binding of the Streptococcus gordonii DL1 surface protein Hsa to the host cell membrane glycoproteins CD11b, CD43, and CD50. Infect. Immun. 2008, 76, 4686–4691. [Google Scholar] [CrossRef]
- Froeliger, E.H.; Fives-Taylor, P. Streptococcus parasanguis fimbria-associated adhesin fap1 is required for biofilm formation. Infect. Immun. 2001, 69, 2512–2519. [Google Scholar] [CrossRef]
- Handley, P.S.; Correia, F.F.; Russell, K.; Rosan, B.; DiRienzo, J.M. Association of a novel high molecular weight, serine-rich protein (SrpA) with fibril-mediated adhesion of the oral biofilm bacterium Streptococcus cristatus. Oral Microbiol. Immunol. 2005, 20, 131–140. [Google Scholar] [CrossRef]
- Sanchez, C.J.; Shivshankar, P.; Stol, K.; Trakhtenbroit, S.; Sullam, P.M.; Sauer, K.; Hermans, P.W.; Orihuela, C.J. The pneumococcal serine-rich repeat protein is an intra-species bacterial adhesin that promotes bacterial aggregation in vivo and in biofilms. PLoS Pathog. 2010, 6, e1001044. [Google Scholar] [CrossRef] [PubMed]
- Love, R.M.; McMillan, M.D.; Jenkinson, H.F. Invasion of dentinal tubules by oral streptococci is associated with collagen recognition mediated by the antigen I/II family of polypeptides. Infect. Immun. 1997, 65, 5157–5164. [Google Scholar] [CrossRef] [PubMed]
- Lamont, R.J.; El-Sabaeny, A.; Park, Y.; Cook, G.S.; Costerton, J.W.; Demuth, D.R. Role of the Streptococcus gordonii SspB protein in the development of Porphyromonas gingivalis biofilms on streptococcal substrates. Microbiology (Reading) 2002, 148, 1627–1636. [Google Scholar] [CrossRef] [PubMed]
- McNab, R.; Forbes, H.; Handley, P.S.; Loach, D.M.; Tannock, G.W.; Jenkinson, H.F. Cell wall-anchored CshA polypeptide (259 kilodaltons) in Streptococcus gordonii forms surface fibrils that confer hydrophobic and adhesive properties. J. Bacteriol. 1999, 181, 3087–3095. [Google Scholar] [CrossRef] [PubMed]
- McNab, R.; Holmes, A.R.; Clarke, J.M.; Tannock, G.W.; Jenkinson, H.F. Cell surface polypeptide CshA mediates binding of Streptococcus gordonii to other oral bacteria and to immobilized fibronectin. Infect. Immun. 1996, 64, 4204–4210. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.R.; Svensater, G.; Herzberg, M.C. Identification of novel LPXTG-linked surface proteins from Streptococcus gordonii. Microbiology (Reading) 2009, 155, 1977–1988. [Google Scholar] [CrossRef]
- Moses, P.J.; Power, D.A.; Jesionowski, A.M.; Jenkinson, H.F.; Pantera, E.A., Jr.; Vickerman, M.M. Streptococcus gordonii collagen-binding domain protein CbdA may enhance bacterial survival in instrumented root canals ex vivo. J. Endod. 2013, 39, 39–43. [Google Scholar] [CrossRef]
- Haworth, J.A.; Jenkinson, H.F.; Petersen, H.J.; Back, C.R.; Brittan, J.L.; Kerrigan, S.W.; Nobbs, A.H. Concerted functions of Streptococcus gordonii surface proteins PadA and Hsa mediate activation of human platelets and interactions with extracellular matrix. Cell Microbiol. 2017, 19. [Google Scholar] [CrossRef]
- Petersen, H.J.; Keane, C.; Jenkinson, H.F.; Vickerman, M.M.; Jesionowski, A.; Waterhouse, J.C.; Cox, D.; Kerrigan, S.W. Human platelets recognize a novel surface protein, PadA, on Streptococcus gordonii through a unique interaction involving fibrinogen receptor GPIIbIIIa. Infect. Immun. 2010, 78, 413–422. [Google Scholar] [CrossRef]
- Tanzer, J.M.; Grant, L.; Thompson, A.; Li, L.; Rogers, J.D.; Haase, E.M.; Scannapieco, F.A. Amylase-binding proteins A (AbpA) and B (AbpB) differentially affect colonization of rats’ teeth by Streptococcus gordonii. Microbiology (Reading) 2003, 149, 2653–2660. [Google Scholar] [CrossRef]
- Kang, J.Y.; Nan, X.; Jin, M.S.; Youn, S.J.; Ryu, Y.H.; Mah, S.; Han, S.H.; Lee, H.; Paik, S.G.; Lee, J.O. Recognition of lipopeptide patterns by Toll-like receptor 2-Toll-like receptor 6 heterodimer. Immunity 2009, 31, 873–884. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.; Arimoto, T.; Igarashi, T.; Yamamoto, M. Involvement of lipoprotein PpiA of Streptococcus gordonii in evasion of phagocytosis by macrophages. Mol. Oral Microbiol. 2013, 28, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Hutchings, M.I.; Palmer, T.; Harrington, D.J.; Sutcliffe, I.C. Lipoprotein biogenesis in Gram-positive bacteria: Knowing when to hold ‘em, knowing when to fold ‘em. Trends Microbiol. 2009, 17, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, H.; Kurokawa, K.; Lee, B.L. Lipoproteins in bacteria: Structures and biosynthetic pathways. FEBS J. 2012, 279, 4247–4268. [Google Scholar] [CrossRef]
- Kovacs-Simon, A.; Titball, R.W.; Michell, S.L. Lipoproteins of bacterial pathogens. Infect. Immun. 2011, 79, 548–561. [Google Scholar] [CrossRef]
- Chimalapati, S.; Cohen, J.M.; Camberlein, E.; MacDonald, N.; Durmort, C.; Vernet, T.; Hermans, P.W.; Mitchell, T.; Brown, J.S. Effects of deletion of the Streptococcus pneumoniae lipoprotein diacylglyceryl transferase gene lgt on ABC transporter function and on growth in vivo. PLoS ONE 2012, 7, e41393. [Google Scholar] [CrossRef]
- Nguyen, M.T.; Gotz, F. Lipoproteins of Gram-positive bacteria: Key players in the immune response and virulence. Microbiol. Mol. Biol. Rev. 2016, 80, 891–903. [Google Scholar] [CrossRef]
- Kim, H.Y.; Kim, A.R.; Seo, H.S.; Baik, J.E.; Ahn, K.B.; Yun, C.H.; Han, S.H. Lipoproteins in Streptococcus gordonii are critical in the infection and inflammatory responses. Mol. Immunol. 2018, 101, 574–584. [Google Scholar] [CrossRef]
- Mayer, M.L.; Phillips, C.M.; Townsend, R.A.; Halperin, S.A.; Lee, S.F. Differential activation of dendritic cells by Toll-like receptor agonists isolated from the Gram-positive vaccine vector Streptococcus gordonii. Scand. J. Immunol. 2009, 69, 351–356. [Google Scholar] [CrossRef]
- Saeki, A.; Segawa, T.; Abe, T.; Sugiyama, M.; Arimoto, T.; Hara, H.; Hasebe, A.; Ohtani, M.; Tanizume, N.; Ohuchi, M.; et al. Toll-like receptor 2-mediated modulation of growth and functions of regulatory T cells by oral streptococci. Mol. Oral Microbiol. 2013, 28, 267–280. [Google Scholar] [CrossRef]
- Kawasaki, T.; Kawai, T. Toll-like receptor signaling pathways. Front. Immunol. 2014, 5, 461. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Yang, J.; Park, O.J.; Kang, S.S.; Kim, W.S.; Kurokawa, K.; Yun, C.H.; Kim, H.H.; Lee, B.L.; Han, S.H. Lipoproteins are an important bacterial component responsible for bone destruction through the induction of osteoclast differentiation and activation. J. Bone Miner. Res. 2013, 28, 2381–2391. [Google Scholar] [CrossRef] [PubMed]
- Farges, J.C.; Carrouel, F.; Keller, J.F.; Baudouin, C.; Msika, P.; Bleicher, F.; Staquet, M.J. Cytokine production by human odontoblast-like cells upon Toll-like receptor-2 engagement. Immunobiology 2011, 216, 513–517. [Google Scholar] [CrossRef]
- Becker, K.; Sander, P. Mycobacterium tuberculosis lipoproteins in virulence and immunity - fighting with a double-edged sword. FEBS Lett. 2016, 590, 3800–3819. [Google Scholar] [CrossRef] [PubMed]
- Delcour, J.; Ferain, T.; Deghorain, M.; Palumbo, E.; Hols, P. The biosynthesis and functionality of the cell-wall of lactic acid bacteria. Antonie Van Leeuwenhoek 1999, 76, 159–184. [Google Scholar] [CrossRef]
- Brown, S.; Santa Maria, J.P., Jr.; Walker, S. Wall teichoic acids of Gram-positive bacteria. Annu. Rev. Microbiol. 2013, 67, 313–336. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.S.; Sim, J.R.; Yun, C.H.; Han, S.H. Lipoteichoic acids as a major virulence factor causing inflammatory responses via Toll-like receptor 2. Arch. Pharm Res. 2016, 39, 1519–1529. [Google Scholar] [CrossRef]
- Lima, B.P.; Kho, K.; Nairn, B.L.; Davies, J.R.; Svensater, G.; Chen, R.; Steffes, A.; Vreeman, G.W.; Meredith, T.C.; Herzberg, M.C. Streptococcus gordonii type I lipoteichoic acid contributes to surface protein biogenesis. mSphere 2019, 4. [Google Scholar] [CrossRef]
- Kang, S.S.; Ryu, Y.H.; Baik, J.E.; Yun, C.H.; Lee, K.; Chung, D.K.; Han, S.H. Lipoteichoic acid from Lactobacillus plantarum induces nitric oxide production in the presence of interferon-gamma in murine macrophages. Mol. Immunol. 2011, 48, 2170–2177. [Google Scholar] [CrossRef]
- Baik, J.E.; Ryu, Y.H.; Han, J.Y.; Im, J.; Kum, K.Y.; Yun, C.H.; Lee, K.; Han, S.H. Lipoteichoic acid partially contributes to the inflammatory responses to Enterococcus faecalis. J. Endod. 2008, 34, 975–982. [Google Scholar] [CrossRef]
- Hong, S.W.; Baik, J.E.; Kang, S.S.; Yun, C.H.; Seo, D.G.; Han, S.H. Lipoteichoic acid of Streptococcus mutans interacts with Toll-like receptor 2 through the lipid moiety for induction of inflammatory mediators in murine macrophages. Mol. Immunol. 2014, 57, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.G.; Kim, N.R.; Gim, M.G.; Lee, J.M.; Lee, S.Y.; Ko, M.Y.; Kim, J.Y.; Han, S.H.; Chung, D.K. Lipoteichoic acid isolated from Lactobacillus plantarum inhibits lipopolysaccharide-induced TNF-alpha production in THP-1 cells and endotoxin shock in mice. J. Immunol. 2008, 180, 2553–2561. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.W.; Kang, S.S.; Woo, S.J.; Park, O.J.; Ahn, K.B.; Song, K.D.; Lee, H.K.; Yun, C.H.; Han, S.H. Lipoteichoic acid of probiotic Lactobacillus plantarum attenuates Poly I:C-induced IL-8 production in porcine intestinal epithelial cells. Front. Microbiol. 2017, 8, 1827. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.S.; Kim, S.K.; Baik, J.E.; Ko, E.B.; Ahn, K.B.; Yun, C.H.; Han, S.H. Staphylococcal LTA antagonizes the B cell-mitogenic potential of LPS. Sci. Rep. 2018, 8, 1496. [Google Scholar] [CrossRef] [PubMed]
- Biswas, R.; Martinez, R.E.; Gohring, N.; Schlag, M.; Josten, M.; Xia, G.; Hegler, F.; Gekeler, C.; Gleske, A.K.; Gotz, F.; et al. Proton-binding capacity of Staphylococcus aureus wall teichoic acid and its role in controlling autolysin activity. PLoS ONE 2012, 7, e41415. [Google Scholar] [CrossRef]
- Hong, S.J.; Kim, S.K.; Ko, E.B.; Yun, C.H.; Han, S.H. Wall teichoic acid is an essential component of Staphylococcus aureus for the induction of human dendritic cell maturation. Mol. Immunol. 2017, 81, 135–142. [Google Scholar] [CrossRef]
- Swaminathan, C.P.; Brown, P.H.; Roychowdhury, A.; Wang, Q.; Guan, R.; Silverman, N.; Goldman, W.E.; Boons, G.J.; Mariuzza, R.A. Dual strategies for peptidoglycan discrimination by peptidoglycan recognition proteins (PGRPs). Proc. Natl. Acad. Sci. USA 2006, 103, 684–689. [Google Scholar] [CrossRef]
- Alderwick, L.J.; Harrison, J.; Lloyd, G.S.; Birch, H.L. The mycobacterial cell wall--peptidoglycan and arabinogalactan. Cold Spring Harb. Perspect. Med. 2015, 5, a021113. [Google Scholar] [CrossRef]
- Vollmer, W. Structural variation in the glycan strands of bacterial peptidoglycan. FEMS Microbiol. Rev. 2008, 32, 287–306. [Google Scholar] [CrossRef]
- Vollmer, W.; Blanot, D.; de Pedro, M.A. Peptidoglycan structure and architecture. FEMS Microbiol. Rev. 2008, 32, 149–167. [Google Scholar] [CrossRef]
- Girardin, S.E.; Boneca, I.G.; Carneiro, L.A.; Antignac, A.; Jehanno, M.; Viala, J.; Tedin, K.; Taha, M.K.; Labigne, A.; Zahringer, U.; et al. Nod1 detects a unique muropeptide from gram-negative bacterial peptidoglycan. Science 2003, 300, 1584–1587. [Google Scholar] [CrossRef] [PubMed]
- Girardin, S.E.; Travassos, L.H.; Herve, M.; Blanot, D.; Boneca, I.G.; Philpott, D.J.; Sansonetti, P.J.; Mengin-Lecreulx, D. Peptidoglycan molecular requirements allowing detection by Nod1 and Nod2. J. Biol. Chem. 2003, 278, 41702–41708. [Google Scholar] [CrossRef] [PubMed]
- Kufer, T.A.; Banks, D.J.; Philpott, D.J. Innate immune sensing of microbes by Nod proteins. Ann. N. Y. Acad. Sci. 2006, 1072, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Jeon, D.I.; Park, S.R.; Ahn, M.Y.; Ahn, S.G.; Park, J.H.; Yoon, J.H. NOD1 and NOD2 stimulation triggers innate immune responses of human periodontal ligament cells. Int. J. Mol. Med. 2012, 29, 699–703. [Google Scholar] [CrossRef] [PubMed]
- Bidault, P.; Chandad, F.; Grenier, D. Risk of bacterial resistance associated with systemic antibiotic therapy in periodontology. J. Can. Dent. Assoc. 2007, 73, 721–725. [Google Scholar]
- Ivanova, E.N. [The comparative efficacy of local anticaries agents]. Stomatologiia (Mosk) 1990, 69, 60–61. [Google Scholar] [PubMed]
- Maltz, M.; Jardim, J.J.; Alves, L.S. Health promotion and dental caries. Braz. Oral Res. 2010, 24 (Suppl 1.), 18–25. [Google Scholar] [CrossRef]
- Van Strydonck, D.A.; Timmerman, M.F.; van der Velden, U.; van der Weijden, G.A. Plaque inhibition of two commercially available chlorhexidine mouthrinses. J. Clin. Periodontol. 2005, 32, 305–309. [Google Scholar] [CrossRef]
- Rasko, D.A.; Sperandio, V. Anti-virulence strategies to combat bacteria-mediated disease. Nat. Rev. Drug Discov. 2010, 9, 117–128. [Google Scholar] [CrossRef]
- Algburi, A.; Comito, N.; Kashtanov, D.; Dicks, L.M.T.; Chikindas, M.L. Control of biofilm formation: Antibiotics and beyond. Appl. Environ. Microbiol. 2017, 83. [Google Scholar] [CrossRef]
- Kerrigan, S.W.; Cox, D. Platelet-bacterial interactions. Cell Mol. Life Sci. 2010, 67, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Moreillon, P.; Que, Y.A.; Bayer, A.S. Pathogenesis of streptococcal and staphylococcal endocarditis. Infect. Dis. Clin. North. Am. 2002, 16, 297–318. [Google Scholar] [CrossRef]
- Mancini, S.; Menzi, C.; Oechslin, F.; Moreillon, P.; Entenza, J.M. Antibodies targeting Hsa and PadA prevent platelet aggregation and protect rats against experimental endocarditis induced by Streptococcus gordonii. Infect. Immun. 2016, 84, 3557–3563. [Google Scholar] [CrossRef] [PubMed]
- Rosema, N.; Slot, D.E.; van Palenstein Helderman, W.H.; Wiggelinkhuizen, L.; Van der Weijden, G.A. The efficacy of powered toothbrushes following a brushing exercise: A systematic review. Int. J. Dent. Hyg. 2016, 14, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Slot, D.E.; Wiggelinkhuizen, L.; Rosema, N.A.; Van der Weijden, G.A. The efficacy of manual toothbrushes following a brushing exercise: A systematic review. Int J. Dent. Hyg 2012, 10, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Drisko, C.H. Nonsurgical periodontal therapy. Periodontol 2001, 25, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Khosravi, M.; Bahrami, Z.S.; Atabaki, M.S.; Shokrgozar, M.A.; Shokri, F. Comparative effectiveness of hand and ultrasonic instrumentations in root surface planing in vitro. J. Clin. Periodontol. 2004, 31, 160–165. [Google Scholar] [CrossRef]
- Du, T.; Shi, Q.; Shen, Y.; Cao, Y.; Ma, J.; Lu, X.; Xiong, Z.; Haapasalo, M. Effect of modified nonequilibrium plasma with chlorhexidine digluconate against endodontic biofilms in vitro. J. Endod. 2013, 39, 1438–1443. [Google Scholar] [CrossRef]
- Jiao, Y.; Tay, F.R.; Niu, L.N.; Chen, J.H. Advancing antimicrobial strategies for managing oral biofilm infections. Int. J. Oral Sci. 2019, 11, 28. [Google Scholar] [CrossRef]
- Parsons, G.J.; Patterson, S.S.; Miller, C.H.; Katz, S.; Kafrawy, A.H.; Newton, C.W. Uptake and release of chlorhexidine by bovine pulp and dentin specimens and their subsequent acquisition of antibacterial properties. Oral Surg. Oral Med. Oral Pathol. 1980, 49, 455–459. [Google Scholar] [CrossRef]
- Chen, X.; Stewart, P.S. Biofilm removal caused by chemical treatments. Water Res. 2000, 34, 4229–4233. [Google Scholar] [CrossRef]
- Jakubovics, N.S.; Yassin, S.A.; Rickard, A.H. Community interactions of oral streptococci. Adv. Appl. Microbiol. 2014, 87, 43–110. [Google Scholar] [CrossRef] [PubMed]
- Kreth, J.; Merritt, J.; Qi, F. Bacterial and host interactions of oral streptococci. DNA Cell Biol. 2009, 28, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Nobbs, A.H.; Jenkinson, H.F.; Jakubovics, N.S. Stick to your gums: Mechanisms of oral microbial adherence. J. Dent. Res. 2011, 90, 1271–1278. [Google Scholar] [CrossRef] [PubMed]
- Nobbs, A.H.; Shearer, B.H.; Drobni, M.; Jepson, M.A.; Jenkinson, H.F. Adherence and internalization of Streptococcus gordonii by epithelial cells involves beta1 integrin recognition by SspA and SspB (antigen I/II family) polypeptides. Cell Microbiol. 2007, 9, 65–83. [Google Scholar] [CrossRef]
- Rampioni, G.; Leoni, L.; Williams, P. The art of antibacterial warfare: Deception through interference with quorum sensing-mediated communication. Bioorg. Chem. 2014, 55, 60–68. [Google Scholar] [CrossRef]
- Scutera, S.; Zucca, M.; Savoia, D. Novel approaches for the design and discovery of quorum-sensing inhibitors. Expert Opin. Drug Discov. 2014, 9, 353–366. [Google Scholar] [CrossRef]
- Jang, Y.J.; Sim, J.; Jun, H.K.; Choi, B.K. Differential effect of autoinducer 2 of Fusobacterium nucleatum on oral streptococci. Arch. Oral Biol. 2013, 58, 1594–1602. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Ling, J. Streptococcus gordonii LuxS/autoinducer-2 quorum-sensing system modulates the dual-species biofilm formation with Streptococcus mutans. J. Basic Microbiol. 2017, 57, 605–616. [Google Scholar] [CrossRef]
- Taylor, P.K.; Yeung, A.T.; Hancock, R.E. Antibiotic resistance in Pseudomonas aeruginosa biofilms: Towards the development of novel anti-biofilm therapies. J. Biotechnol. 2014, 191, 121–130. [Google Scholar] [CrossRef]
- Ahn, K.B.; Baik, J.E.; Yun, C.H.; Han, S.H. Lipoteichoic acid inhibits Staphylococcus aureus biofilm formation. Front. Microbiol. 2018, 9, 327. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Park, O.J.; Kim, A.R.; Ahn, K.B.; Lee, D.; Kum, K.Y.; Yun, C.H.; Han, S.H. Lipoteichoic acids of lactobacilli inhibit Enterococcus faecalis biofilm formation and disrupt the preformed biofilm. J. Microbiol. 2019, 57, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.R.; Kang, M.; Yoo, Y.J.; Yun, C.H.; Perinpanayagam, H.; Kum, K.Y.; Han, S.H. Lactobacillus plantarum lipoteichoic acid disrupts mature Enterococcus faecalis biofilm. J. Microbiol. 2020, 58, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Chang, S.W.; Perinpanayagam, H.; Lim, S.M.; Park, Y.J.; Han, S.H.; Baek, S.H.; Zhu, Q.; Bae, K.S.; Kum, K.Y. Antibacterial efficacy of a human beta-defensin-3 peptide on multispecies biofilms. J. Endod. 2013, 39, 1625–1629. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Park, Y.J.; Kum, K.Y.; Han, S.H.; Chang, S.W.; Kaufman, B.; Jiang, J.; Zhu, Q.; Safavi, K.; Spangberg, L. Antimicrobial efficacy of a human beta-defensin-3 peptide using an Enterococcus faecalis dentine infection model. Int. Endod. J. 2013, 46, 406–412. [Google Scholar] [CrossRef]
- Murthy, A.K.; Chaganty, B.K.; Troutman, T.; Guentzel, M.N.; Yu, J.J.; Ali, S.K.; Lauriano, C.M.; Chambers, J.P.; Klose, K.E.; Arulanandam, B.P. Mannose-containing oligosaccharides of non-specific human secretory immunoglobulin A mediate inhibition of Vibrio cholerae biofilm formation. PLoS ONE 2011, 6, e16847. [Google Scholar] [CrossRef]
- Barraud, N.; Schleheck, D.; Klebensberger, J.; Webb, J.S.; Hassett, D.J.; Rice, S.A.; Kjelleberg, S. Nitric oxide signaling in Pseudomonas aeruginosa biofilms mediates phosphodiesterase activity, decreased cyclic di-GMP levels, and enhanced dispersal. J. Bacteriol. 2009, 191, 7333–7342. [Google Scholar] [CrossRef]
- Kurokawa, K.; Kim, M.S.; Ichikawa, R.; Ryu, K.H.; Dohmae, N.; Nakayama, H.; Lee, B.L. Environment-mediated accumulation of diacyl lipoproteins over their triacyl counterparts in Staphylococcus aureus. J. Bacteriol. 2012, 194, 3299–3306. [Google Scholar] [CrossRef]
- Lagousi, T.; Basdeki, P.; Routsias, J.; Spoulou, V. Novel protein-based Pneumococcal vaccines: Assessing the use of distinct protein fragments instead of full-length proteins as vaccine antigens. Vaccines (Basel) 2019, 7. [Google Scholar] [CrossRef]
- Gor, D.O.; Ding, X.; Li, Q.; Sultana, D.; Mambula, S.S.; Bram, R.J.; Greenspan, N.S. Enhanced immunogenicity of pneumococcal surface adhesin A (PsaA) in mice via fusion to recombinant human B lymphocyte stimulator (BLyS). Biol. Direct 2011, 6, 9. [Google Scholar] [CrossRef]
- Tai, S.S. Streptococcus pneumoniae protein vaccine candidates: Properties, activities and animal studies. Crit. Rev. Microbiol. 2006, 32, 139–153. [Google Scholar] [CrossRef] [PubMed]
- Lemjabbar, H.; Basbaum, C. Platelet-activating factor receptor and ADAM10 mediate responses to Staphylococcus aureus in epithelial cells. Nat. Med. 2002, 8, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Han, S.H.; Kim, J.H.; Seo, H.S.; Martin, M.H.; Chung, G.H.; Michalek, S.M.; Nahm, M.H. Lipoteichoic acid-induced nitric oxide production depends on the activation of platelet-activating factor receptor and Jak2. J. Immunol. 2006, 176, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Park, O.J.; Han, J.Y.; Baik, J.E.; Jeon, J.H.; Kang, S.S.; Yun, C.H.; Oh, J.W.; Seo, H.S.; Han, S.H. Lipoteichoic acid of Enterococcus faecalis induces the expression of chemokines via TLR2 and PAFR signaling pathways. J. Leukoc. Biol. 2013, 94, 1275–1284. [Google Scholar] [CrossRef] [PubMed]
- Ohsawa, H.; Baba, T.; Enami, J.; Hiramatsu, K. Protective activity of anti-lipoteichoic acid monoclonal antibody in single or combination therapies in methicillin-resistant Staphylococcus aureus-induced murine sepsis models. J. Infect. Chemother. 2020, 26, 520–522. [Google Scholar] [CrossRef] [PubMed]
- Jung, D.J.; An, J.H.; Kurokawa, K.; Jung, Y.C.; Kim, M.J.; Aoyagi, Y.; Matsushita, M.; Takahashi, S.; Lee, H.S.; Takahashi, K.; et al. Specific serum Ig recognizing staphylococcal wall teichoic acid induces complement-mediated opsonophagocytosis against Staphylococcus aureus. J. Immunol. 2012, 189, 4951–4959. [Google Scholar] [CrossRef] [PubMed]
- Vickery, C.R.; Wood, B.M.; Morris, H.G.; Losick, R.; Walker, S. Reconstitution of Staphylococcus aureus lipoteichoic acid synthase activity identifies Congo Red as a selective inhibitor. J. Am. Chem. Soc. 2018, 140, 876–879. [Google Scholar] [CrossRef]
- Hernandez-Zamora, M.; Martinez-Jeronimo, F.; Cristiani-Urbina, E.; Canizares-Villanueva, R.O. Congo Red dye affects survival and reproduction in the cladoceran Ceriodaphnia dubia. Effects of direct and dietary exposure. Ecotoxicology 2016, 25, 1832–1840. [Google Scholar] [CrossRef]
- Bush, K.; Bradford, P.A. Interplay between beta-lactamases and new beta-lactamase inhibitors. Nat. Rev. Microbiol. 2019, 17, 295–306. [Google Scholar] [CrossRef]
- Culp, E.J.; Waglechner, N.; Wang, W.; Fiebig-Comyn, A.A.; Hsu, Y.P.; Koteva, K.; Sychantha, D.; Coombes, B.K.; Van Nieuwenhze, M.S.; Brun, Y.V.; et al. Evolution-guided discovery of antibiotics that inhibit peptidoglycan remodelling. Nature 2020, 578, 582–587. [Google Scholar] [CrossRef]
- Ling, L.L.; Schneider, T.; Peoples, A.J.; Spoering, A.L.; Engels, I.; Conlon, B.P.; Mueller, A.; Schaberle, T.F.; Hughes, D.E.; Epstein, S.; et al. Erratum: A new antibiotic kills pathogens without detectable resistance. Nature 2015, 520, 388. [Google Scholar] [CrossRef] [PubMed]
- Kohanski, M.A.; Dwyer, D.J.; Collins, J.J. How antibiotics kill bacteria: From targets to networks. Nat. Rev. Microbiol. 2010, 8, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Fischetti, V.A. Bacteriophage endolysins: A novel anti-infective to control Gram-positive pathogens. Int. J. Med. Microbiol. 2010, 300, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Entenza, J.M.; Loeffler, J.M.; Grandgirard, D.; Fischetti, V.A.; Moreillon, P. Therapeutic effects of bacteriophage Cpl-1 lysin against Streptococcus pneumoniae endocarditis in rats. Antimicrob. Agents Chemother. 2005, 49, 4789–4792. [Google Scholar] [CrossRef] [PubMed]
| Target Surfaces | SRR Adhesins | Functions | References |
|---|---|---|---|
| Dentin slices | GspB | Facilitating binding of S. gordonii Contributing to biofilm formation and colonization on teeth | [64] |
| Plates coated with saliva | Hsa | Facilitating dental biofilm formation | [65] |
| Platelets | GspB Hsa | Mediating attachment to host cell membrane Providing opportunities of translocation to other organs from the oral cavity Contributing to the aggregating of platelets | [17,40,57] |
| Erythrocytes | Hsa | [59,63] | |
| Polymorphonuclear leukocytes | Hsa | Promoting adherence to host cell surfaces | [66] |
| Monocytes | Hsa | Facilitating adherence to cluster of differentiation (CD)11b, CD43, and CD50 on the host cell membrane Inducing differentiation of monocytes into dendritic cells | [67,68] |
| Dendritic cells | GspB Hsa | Facilitating binding of S. gordonii Promoting induction of tumor necrosis factor-α (TNF-α), interleukin (IL)-6, and IL-12 production Activating maturation of dendritic cells | [16] |
| Cell Wall Protein | Host Binding Site | Functions | References | |
|---|---|---|---|---|
| Antigen-related protein | SspA and SspB | Type I collagen or β1 integrin Salivary agglutinin glycoprotein (gp340) | Accelerating infection of S. gordonii into root dentinal tubules Mediating aggregation and adherence of cells Facilitating biofilm formation by interacting with other bacteria | [24,72,73] |
| CshA and CshB | Fibronectin | Facilitating invasion into endothelial cells | [74,75] | |
| Collagen-binding protein | CbdA | Type I collagen | Promoting S. gordonii to persist its survival in instrumented root canals | [76,77] |
| Fibronectin-binding protein | FbpA | Fibronectin | Controlling the attachment of S. gordonii to fibronectin through the regulation of CshA expression | [60] |
| Platelet adherence protein | PadA | Platelet receptor GPIIb/IIIa | Activating platelets by cooperating with Hsa Promoting biofilm formation by cooperating with Hsa to bind to salivary glycoproteins affecting Hsa active presentation on the cell wall | [78,79] |
| α-amylase-binding protein | AbpA and AbpB | α-amylase | Contributing to biofilm formation and colonization on teeth Facilitating the binding of S. gordonii to the salivary pellicle Providing nutritional benefit by capturing host enzymatic activity to compete with other oral microbial species | [80] |
| Host Cell | Stimuli | Receptor or Mechanisms | Reactions | References |
|---|---|---|---|---|
| Murine macrophage | S. gordonii lipoprotein extract | Toll-like receptor (TLR) 2-mediated nuclear factor-kappaB (NF-κB) pathway | Increased production of nitric oxide Reduced production of interferon-β (IFN-β) | [43] |
| Human and mouse macrophage | S. gordonii Δlgt | TLR2-mediated NF-κB pathway | Failed to induce tumor necrosis factor-α (TNF-α), interleukin (IL)-8, IL-1β Reduced mortality | [88] |
| S. gordonii lipoprotein extract | Increased expression of TNF-α, IL-8, and IL-1β | |||
| Murine dendritic cell | S. gordonii lipoprotein extract | TLR2-mediated MyD88 pathway | Increased TNF, IL-6, IL-12p70, and IL-10 production Increased cluster of differentiation 80 expression | [89] |
| Natural regulatory T cells in mouse splenocytes | S. gordonii wild type and Δlgt | TLR2-mediated NF-κB pathway | Only wild type reduced the frequency of natural regulatory T cells | [90] |
| Human embryonic kidney 293 cell | Heat-killed S. gordonii Δlgt | Failed to induce NF-κB activation | ||
| Human vascular endothelial cells | S. gordonii Δlgt | TLR2-mediated NF-κB pathway | Weak adherence to the human umbilical vein endothelial cells | [41] |
| Mouse tissues | Rapid clearance from the blood flow, liver, and spleen Reduced amounts of TNF-α production | |||
| Human periodontal ligament cell | S. gordonii lipoprotein extract | TLR2-mediated mitogen-activated protein kinase pathway | Increased production of IL-8 | [25] |
| Human dental pulp cell | S. gordonii lipoprotein extract | TLR2-mediated NF-κB pathway | Increased mRNA expression level of IL-8 and monocyte chemoattractant protein-1 | [26] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, O.-J.; Kwon, Y.; Park, C.; So, Y.J.; Park, T.H.; Jeong, S.; Im, J.; Yun, C.-H.; Han, S.H. Streptococcus gordonii: Pathogenesis and Host Response to Its Cell Wall Components. Microorganisms 2020, 8, 1852. https://doi.org/10.3390/microorganisms8121852
Park O-J, Kwon Y, Park C, So YJ, Park TH, Jeong S, Im J, Yun C-H, Han SH. Streptococcus gordonii: Pathogenesis and Host Response to Its Cell Wall Components. Microorganisms. 2020; 8(12):1852. https://doi.org/10.3390/microorganisms8121852
Chicago/Turabian StylePark, Ok-Jin, Yeongkag Kwon, Chaeyeon Park, Yoon Ju So, Tae Hwan Park, Sungho Jeong, Jintaek Im, Cheol-Heui Yun, and Seung Hyun Han. 2020. "Streptococcus gordonii: Pathogenesis and Host Response to Its Cell Wall Components" Microorganisms 8, no. 12: 1852. https://doi.org/10.3390/microorganisms8121852
APA StylePark, O.-J., Kwon, Y., Park, C., So, Y. J., Park, T. H., Jeong, S., Im, J., Yun, C.-H., & Han, S. H. (2020). Streptococcus gordonii: Pathogenesis and Host Response to Its Cell Wall Components. Microorganisms, 8(12), 1852. https://doi.org/10.3390/microorganisms8121852





