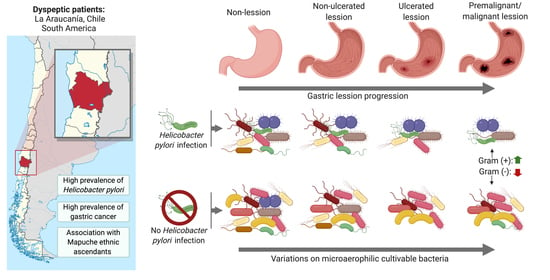
MALDI-TOF MS and 16S RNA Identification of Culturable Gastric Microbiota: Variability Associated with the Presence of Helicobacter pylori
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Clinical Specimens
2.2. Categorization of State Gastric Epithelial
2.3. Isolation of Culturable Microaerophilic Microbiota
2.4. Identification of Non-Pylori Species (Culturable Microaerophilic Microbiota)
2.5. Helicobacter pylori Confirmation
2.6. Statistical Analyses
3. Results
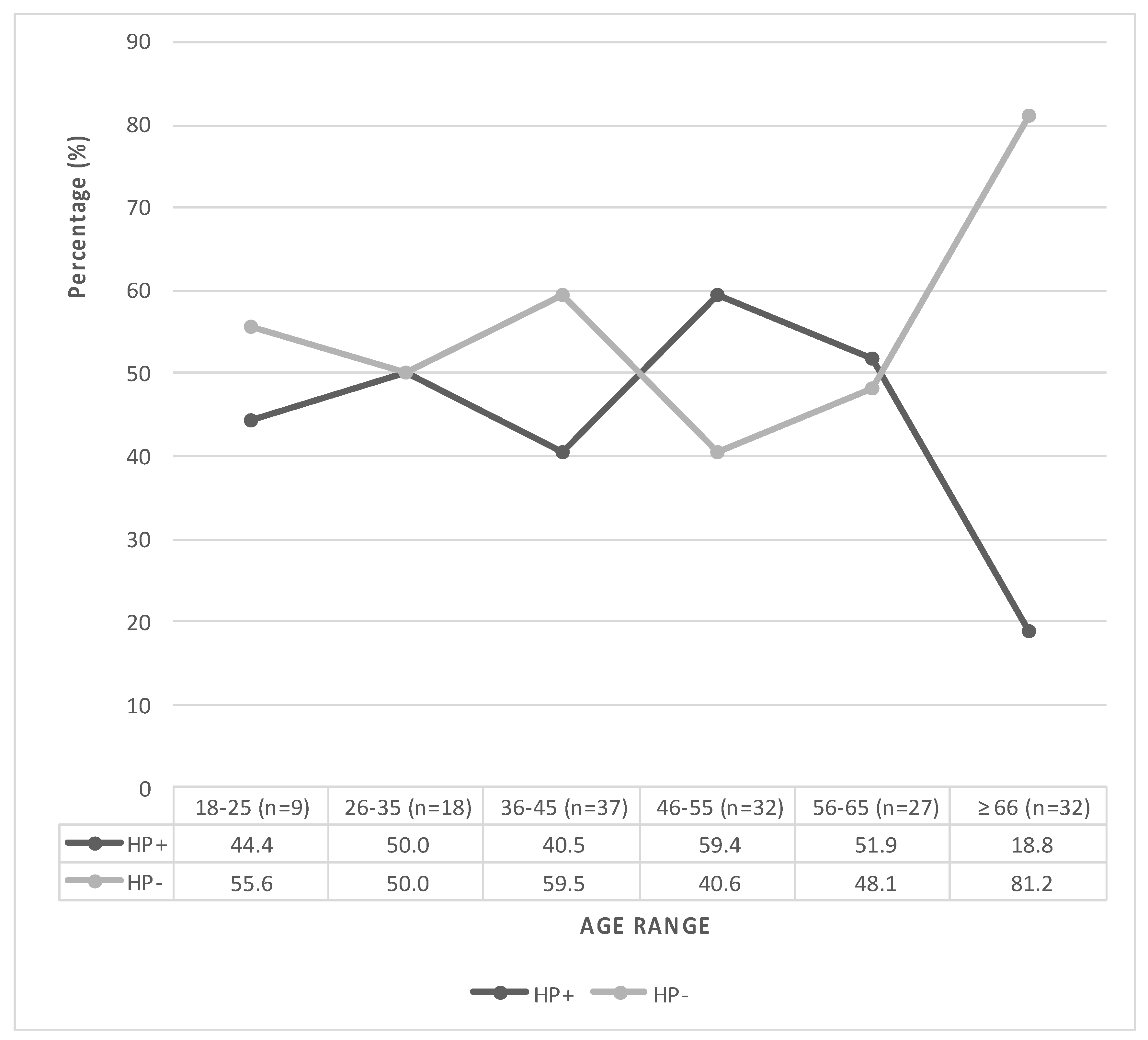
3.1. Characterization and Clinical Data of the Participants
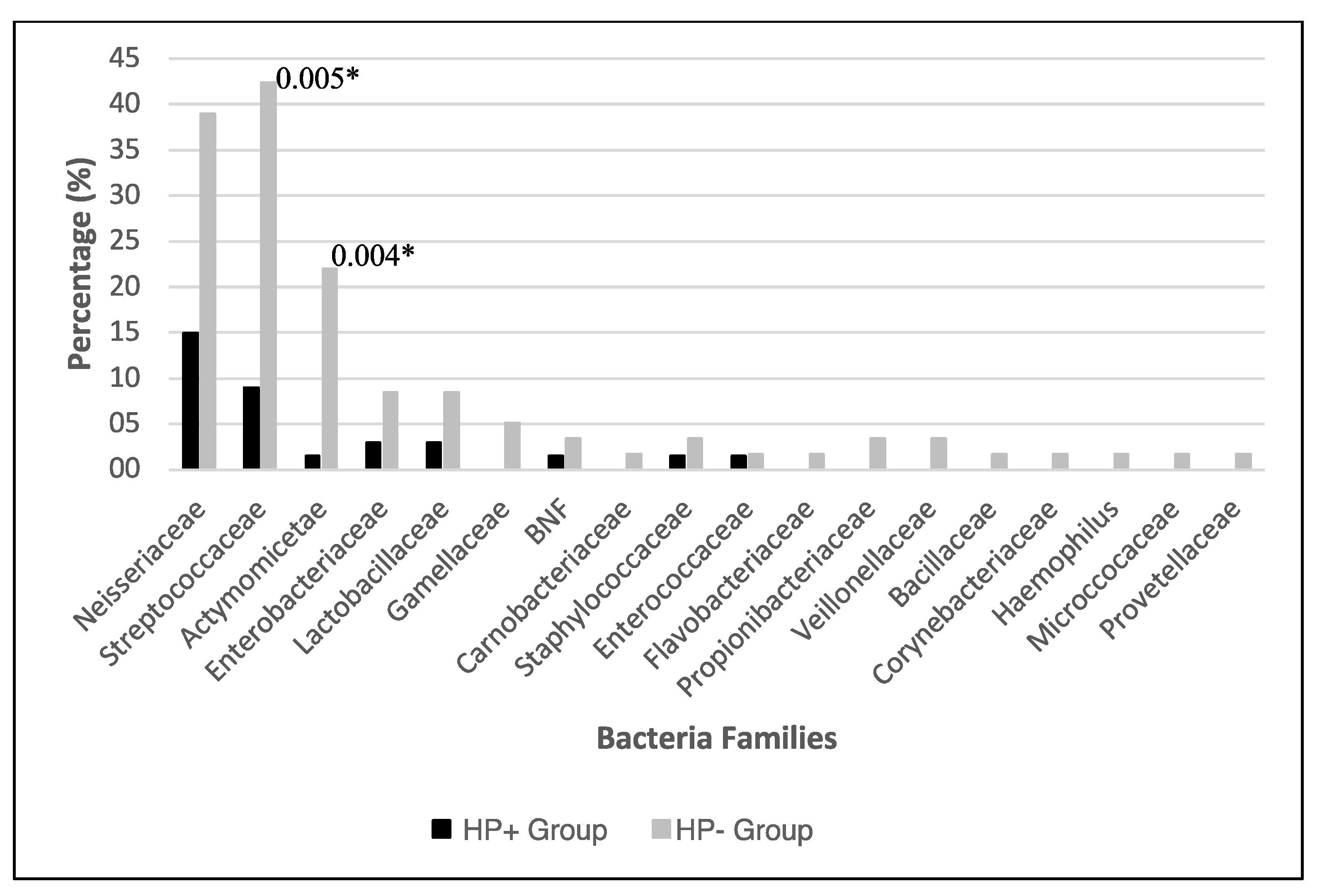
3.2. Bacterial Identification
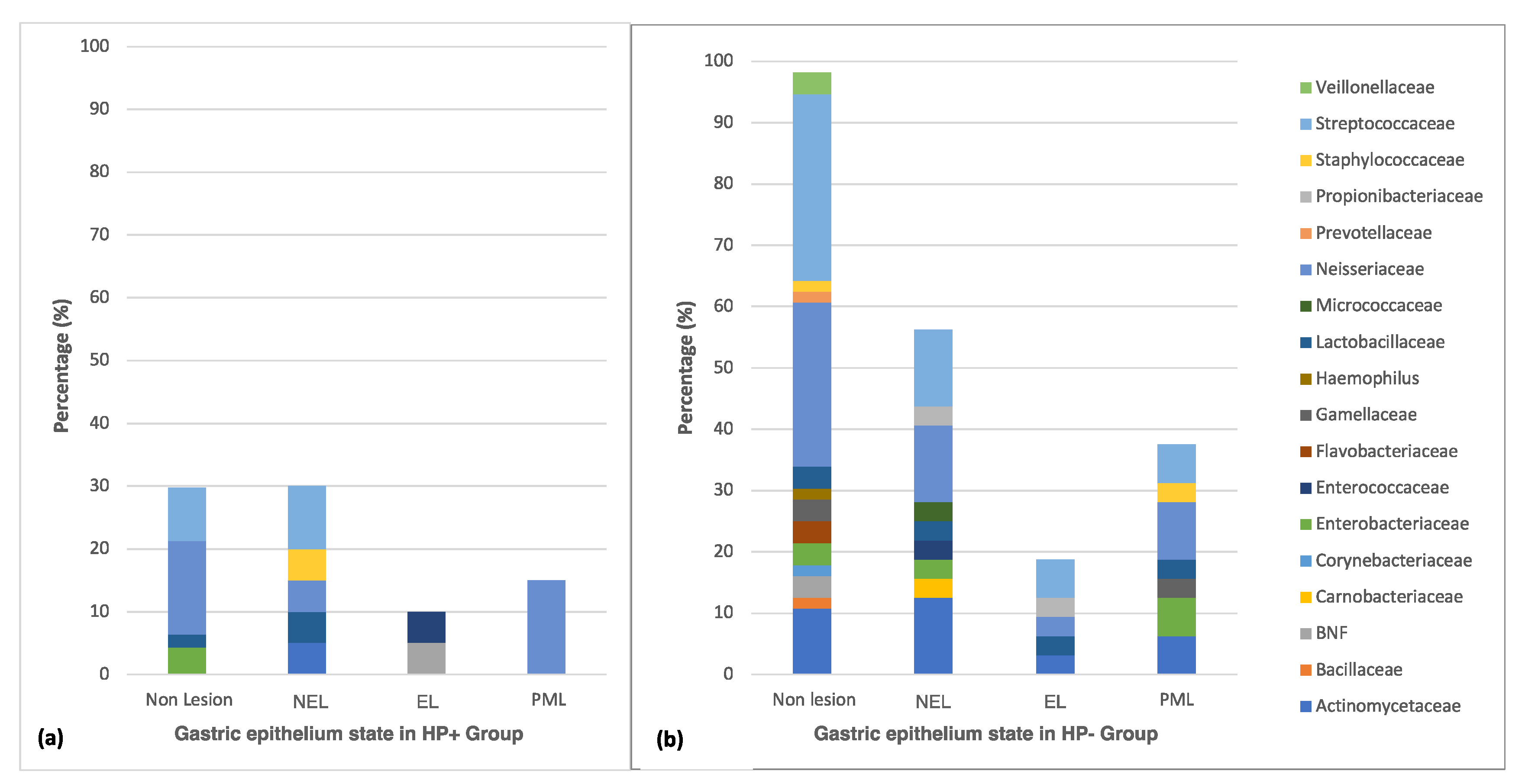
3.3. Distribution of Culturable Bacteria According to the State of the Gastric Epithelium
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Marshall, B.; Warren, J.R. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet 1984, 323, 1311–1314. [Google Scholar]
- Dicksved, J.; Lindberg, M.; Rosenquist, M.; Enroth, H.; Jansson, J.K.; Engstrand, L. Molecular characterization of the stomach microbiota in patients with gastric cancer and in controls. J. Med. Microbiol. 2009, 58, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Csendes, A.; Figueroa, M. Situación del cáncer gástrico en el mundo y en Chile. Rev. Chil. Cirugía 2016, 69, 502–507. [Google Scholar] [CrossRef]
- Castaño-rodríguez, N.; Goh, K.; Fock, K.M.; Mitchell, H.M.; Kaakoush, N.O. Dysbiosis of the microbiome in gastric carcinogenesis. Sci. Rep. 2017, 1–9. [Google Scholar] [CrossRef]
- Bik, E.M.; Eckburg, P.B.; Gill, S.R.; Nelson, K.E.; Purdom, E.A.; Francois, F.; Perez-Perez, G.; Blaser, M.J.; Relman, D.A. Molecular analysis of the bacterial microbiota in the human stomach. Proc. Natl. Acad. Sci. USA 2006, 103, 732–737. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhou, J.; Xin, Y.; Geng, C.; Tian, Z.; Yu, X.; Dong, Q. Bacterial overgrowth and diversification of microbiota in gastric cancer. Eur. J. Gastroenterol. Hepatol. 2016, 28, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Ouyang, Y.; Lu, N.; Li, N. The NF-κB Signaling Pathway, the Microbiota, and Gastrointestinal Tumorigenesis: Recent Advances. Front. Immunol. 2020, 11, 1–13. [Google Scholar] [CrossRef]
- Miftahussurur, M.; Waskito, L.A.; El-Serag, H.B.; Ajami, N.J.; Nusi, I.A.; Syam, A.F.; Matsumoto, T.; Rezkitha, Y.A.A.; Doohan, D.; Fauzia, K.A.; et al. Gastric microbiota and Helicobacter pylori in Indonesian population. Helicobacter 2020, 25, 1–10. [Google Scholar] [CrossRef]
- Vogtmann, E.; Goedert, J.J. Epidemiologic studies of the human microbiome and cancer. Br. J. Cancer 2016, 114, 237–242. [Google Scholar] [CrossRef]
- Khosravi, Y.; Dieye, Y.; Poh, B.H.; Ng, C.G.; Loke, M.F.; Goh, K.L.; Vadivelu, J. Culturable bacterial microbiota of the stomach of helicobacter pylori positive and negative gastric disease patients. Sci. World J. 2014, 2014. [Google Scholar] [CrossRef]
- Tang, C.-L. Helicobacter pylori tumor necrosis factor-α inducing protein promotes cytokine expression via nuclear factor-κB. World J. Gastroenterol. 2013, 19, 399. [Google Scholar] [CrossRef] [PubMed]
- Dias-Jácome, E.; Libânio, D.; Borges-Canha, M.; Galaghar, A.; Pimentel-Nunes, P. Gastric microbiota and carcinogenesis: The role of non-Helicobacter pylori bacteria—A systematic review. Rev. Esp. Enfermedades Dig. 2016, 108, 530–540. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Pereira, V.; Saxena, S.; Ghosh, T.S.; Anbumani, D. Gastric microbiome of Indian patients with Helicobacter pylori infection, and their interaction networks. Sci. Rep. 2017, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Cleveland, K.; Schnoll-sussman, F.; Mcclure, B.; Bigg, M.; Thakkar, P.; Schultz, N.; Shah, M.A.; Betel, D. Identification of low abundance microbiome in clinical samples using whole genome sequencing. Genome Biol. 2015, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Pero, R.; Brancaccio, M.; Laneri, S.; De Biasi, M.G.; Lombardo, B.; Scudiero, O. A novel view of human Helicobacter pylori infections: Interplay between microbiota and beta-defensins. Biomolecules 2019, 9, 237. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; He, L.H.; Xiao, D.; Liu, G.D.; Gu, Y.X.; Tao, X.X.; Zhang, J.Z. Bacterial flora concurrent with helicobacter pylori in the stomach of patients with upper gastrointestinal diseases. World J. Gastroenterol. 2012, 18, 1257–1261. [Google Scholar] [CrossRef]
- Da Costa, D.; Guidotti, F.; Cabello, N.; Trigo, F.; Contreras, C.; Vergara, F.; Miranda, J.P.; Montenegro, C.; Muñoz, P.; Berger, Z. Disminución en la frecuencia de infección por Helicobacter pylori en endoscopías digestivas altas. Rev. Med. Chil. 2018, 146, 555–561. [Google Scholar] [CrossRef]
- González, I.; Romero, J.; Rodríuez, B.; Llanos, J.; Morales, E.; Figueroa, H.; Perez-Castro, R.; Valdés, E.; Cofre, C.; Rojas, A. High prevalence of virulence-associated genotypes in Helicobacter pylori clinical isolates in the Region del Maule, Chile. Scand. J. Infect. Dis. 2011, 43, 652–655. [Google Scholar] [CrossRef]
- Minsal Plan Nacional d.e. Cancer 2018–2028; Documento de consulta pública; Ministerio de Salud: Chile, 2018; pp. 1–27. Available online: https//www.gob.cl/plannacionaldecancer/ (accessed on 16 August 2019).
- Ministerio de Desarrollo Social Informe de Desarrollo Social 2017 (informe en proceso de edición). 2017. Available online: http://www.desarrollosocialyfamilia.gob.cl/pdf/upload/IDS2017.pdf (accessed on 16 January 2020).
- Liu, J.; Xue, Y.; Zhou, L. Detection of gastritis-associated pathogens by culturing of gastric juice and mucosa. Int. J. Clin. Exp. Pathol. 2018, 11, 2214–2220. [Google Scholar]
- Brawner, K.M.; Kumar, R.; Serrano, C.A.; Ptacek, T.; Lefkowitz, E.; Morrow, C.D.; Zhi, D.; Kyanam-Kabir-Baig, K.R.; Smythies, L.E.; Harris, P.R.; et al. Helicobacter pylori infection is associated with an altered gastric microbiota in children. Mucosal Immunol. 2017, 10, 1169–1177. [Google Scholar] [CrossRef]
- Sotelo, S.; Manterola, C. Morfología y Repercusiones Diagnóstico-Terapéuticas de las Lesiones Preneoplásicas Gástricas. Int. J. Morphol. 2019, 37, 917–927. [Google Scholar] [CrossRef]
- Correa, P.; Piazuelo, M.B. The gastric precancerous cascade. J. Dig. Dis. 2012, 13, 2–9. [Google Scholar] [CrossRef] [PubMed]
- García-Rodríguez, J.; Quiles-Melero, I.; Gómez-López, A.; Mingorance, J. Evaluation of matrix-assisted laser desorption/ionisation time-of-flight (MALDI-TOF) mass spectrometry for identification of Candida parapsilosis, C. orthopsilosis and C. metapsilosis. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 67–71. [Google Scholar] [CrossRef]
- Srinivasan, R.; Karaoz, U.; Volegova, M.; MacKichan, J.; Kato-Maeda, M.; Miller, S.; Nadarajan, R.; Brodie, E.L.; Lynch, S.V. Use of 16S rRNA gene for identification of a broad range of clinically relevant bacterial pathogens. PLoS ONE 2015, 10, e0117617. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Wormwood, T.; Parra, A.; Bresky, G.; Madariaga, J.A.; Sergio, H.; Jacqueline, F.; Bernal, G. Prevalencia de cepas cagA-positivo en la región de Coquimbo, determinada mediante nested-qPCR en muestras fecales. Rev. Med. Chil. 2018, 146, 596–602. [Google Scholar] [CrossRef]
- Zeng, B.; Sun, L.; Chen, Y.; Qian, Y.; Cao, Q.; Zhang, Z.; Li, Z. Neisseria flavescens: A Urease-Expressing Potential Pathogen Isolated from Gastritis Patients. Curr. Microbiol. 2018, 75, 186–193. [Google Scholar] [CrossRef]
- Gupta, V.K.; Paul, S.; Dutta, C. Geography, ethnicity or subsistence-specific variations in human microbiome composition and diversity. Front. Microbiol. 2017, 8. [Google Scholar] [CrossRef]
- Yang, I.; Woltemate, S.; Piazuelo, M.B.; Bravo, L.E.; Yepez, M.C.; Romero-Gallo, J.; Delgado, A.G.; Wilson, K.T.; Peek, R.M.; Correa, P.; et al. Different gastric microbiota compositions in two human populations with high and low gastric cancer risk in Colombia. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef]
- Wang, D.; Li, Y.; Zhong, H.; Ding, Q.; Lin, Y.; Tang, S.; Zong, Y.; Wang, Q.; Zhang, X.; Yang, H.; et al. Alterations in the human gut microbiome associated with Helicobacter pylori infection. FEBS Open Bio 2019, 9, 1552–1560. [Google Scholar] [CrossRef]
- Ianiro, G.; Molina-infante, J.; Gasbarrini, A. Gastric Microbiota. Helicobacter 2015, 20, 68–71. [Google Scholar] [CrossRef] [PubMed]
- Li, X.X.; Wong, G.L.H.; To, K.F.; Wong, V.W.S.; Lai, L.H.; Chow, D.K.L.; Lau, J.Y.W.; Sung, J.J.Y.; Ding, C. Bacterial microbiota profiling in gastritis without Helicobacter pylori infection or non-steroidal anti-inflammatory drug use. PLoS ONE 2009, 4, e7985. [Google Scholar] [CrossRef] [PubMed]
- Andersson, A.F.; Lindberg, M.; Jakobsson, H.; Ba, F. Comparative Analysis of Human Gut Microbiota by Barcoded Pyrosequencing. PLoS ONE 2008, 3, e2836. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhang, Y.; Gerhard, M.; Gao, J.J.; Mejias-Luque, R.; Zhang, L.; Vieth, M.; Ma, J.L.; Bajbouj, M.; Suchanek, S.; et al. Effect of Helicobacter pylori on gastrointestinal microbiota: A population-based study in Linqu, a high-risk area of gastric cancer. Gut 2019, 69, 1598–1607. [Google Scholar] [CrossRef]
- Ailloud, F.; Didelot, X.; Woltemate, S.; Pfaffinger, G.; Overmann, J.; Bader, R.C.; Schulz, C.; Malfertheiner, P.; Suerbaum, S. Within-host evolution of Helicobacter pylori shaped by niche-specific adaptation, intragastric migrations and selective sweeps. Nat. Commun. 2019. [Google Scholar] [CrossRef]
- Lan, H.C.; Chen, T.S.; Li, A.F.Y.; Chang, F.Y.; Lin, H.C. Additional corpus biopsy enhances the detection of Helicobacter pylori infection in a background of gastritis with atrophy. BMC Gastroenterol. 2012. [Google Scholar] [CrossRef]
- Serrano, C.A.; Pierre, R.; Van Der Pol, W.J.; Morrow, C.D.; Smith, P.D.; Harris, P.R. Eradication of Helicobacter pylori in Children Restores the Structure of the Gastric Bacterial Community to That of Noninfected Children. Gastroenterology 2019, 157, 1673–1675. [Google Scholar] [CrossRef]
- Muto, M.; Hitomi, Y.; Ohtsu, A.; Shimada, H.; Kashiwase, Y.; Sasaki, H.; Yoshida, S.; Esumi, H. Acetaldehyde production by non-pathogenic Neisseria in human oral microflora: Implications for carcinogenesis in upper aerodigestive tract. Int. J. Cancer 2000, 88, 342–350. [Google Scholar] [CrossRef]
- Thorell, K.; Yahara, K.; Berthenet, E.; Lawson, D.J.; Mikhail, J.; Kato, I.; Mendez, A.; Rizzato, C.; Bravo, M.M.; Suzuki, R.; et al. Rapid evolution of distinct Helicobacter pylori subpopulations in the Americas. PLoS Genet. 2017, 13, e1006546. [Google Scholar] [CrossRef]
- Oporto, M.; Pavez, M.; Troncoso, C.; Cerda, A.; Hofmann, E.; Sierralta, A.; Rios, E.; Coppelli, L.; Barrientos, L. Prevalence of Infection and Antibiotic Susceptibility of Helicobacter pylori: An Evaluation in Public and Private Health Systems of Southern Chile. Pathogens 2019, 8, 226. [Google Scholar] [CrossRef]
- Ndegwa, N.; Ploner, A.; Andersson, A.F.; Zagai, U.; Andreasson, A.; Vieth, M.; Talley, N.J.; Agreus, L.; Ye, W. Gastric Microbiota in a Low-Helicobacter pylori Prevalence General Population and Their Associations With Gastric Lesions. Clin. Transl. Gastroenterol. 2020, 11, e00191. [Google Scholar] [CrossRef] [PubMed]
- Weng, M.T.; Chiu, Y.T.; Wei, P.Y.; Chiang, C.W.; Fang, H.L.; Wei, S.C. Microbiota and gastrointestinal cancer. J. Formos. Med. Assoc. 2019, 118, S32–S41. [Google Scholar] [CrossRef] [PubMed]
- Diehl, G.E.; Longman, R.S.; Zhang, J.X.; Breart, B.; Galan, C.; Cuesta, A.; Schwab, S.R.; Littman, D.R. Microbiota restricts trafficking of bacteria to mesenteric lymph nodes by CX 3 CR1 hi cells. Nature 2013, 494, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Eun, C.S.; Kim, B.K.; Han, D.S.; Kim, S.Y.; Kim, K.M.; Choi, B.Y.; Song, K.S.; Kim, Y.S.; Kim, J.F. Differences in gastric mucosal microbiota profiling in patients with chronic gastritis, intestinal metaplasia, and gastric cancer using pyrosequencing methods. Helicobacter 2014, 19, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Gantuya, B.; El Serag, H.B.; Matsumoto, T.; Ajami, N.J.; Uchida, T.; Oyuntsetseg, K.; Bolor, D.; Yamaoka, Y. Gastric mucosal microbiota in a Mongolian population with gastric cancer and precursor conditions. Aliment. Pharmacol. Ther. 2020, 51, 770–780. [Google Scholar] [CrossRef]
- Schulz, C.; Schütte, K.; Koch, N.; Vilchez-Vargas, R.; Wos-Oxley, M.L.; Oxley, A.P.A.; Vital, M.; Malfertheiner, P.; Pieper, D.H. The active bacterial assemblages of the upper Gi tract in individuals with and without Helicobacter infection. Gut 2016, 67. [Google Scholar] [CrossRef]
- Ge, Z.; Sheh, A.; Feng, Y.; Muthupalani, S.; Ge, L.; Wang, C.; Kurnick, S.; Mannion, A.; Whary, M.T.; Fox, J.G. Helicobacter pylori-infected C57BL/6 mice with different gastrointestinal microbiota have contrasting gastric pathology, microbial and host immune responses. Sci. Rep. 2018, 8, 1–15. [Google Scholar] [CrossRef]
- Kienesberger, S.; Cox, L.M.; Livanos, A.; Zhang, X.S.; Chung, J.; Perez-Perez, G.I.; Gorkiewicz, G.; Zechner, E.L.; Blaser, M.J. Gastric Helicobacter pylori Infection Affects Local and Distant Microbial Populations and Host Responses. Cell Rep. 2016, 14, 1395–1407. [Google Scholar] [CrossRef]
- Xia, Y.; Meng, G.; Zhang, Q.; Liu, L.; Wu, H.; Shi, H.; Bao, X.; Su, Q. Dietary Patterns are Associated with Helicobacter Pylori Infection in Chinese Adults: A Cross-Sectional Study. Sci. Rep. 2016, 1–8. [Google Scholar] [CrossRef]
| Data | HP+ n = 67 n (%) | HP- n = 88 n (%) | Total Participants n = 155 n (%) | p-Value |
|---|---|---|---|---|
| Age (years ± SD) | 48.2 ± 13.3 a | 53.4 ± 17 a | 51.1 ± 15.7 a | 0.041 * |
| Gender | 0.865 | |||
| Female | 44 (42.7) | 59 (57.3) | 103 (66.4) | |
| Male | 23 (44.2) | 29 (55.8) | 52 (33.5) | |
| Residence | 0.833 | |||
| Rural | 11 (40.7) | 16 (59.3) | 27 (17.4) | |
| Urban | 55 (43.6) | 71 (56.4) | 126 (81.3) | |
| Ethnicity | 0.097 | |||
| Mapuche | 22 (55.0) | 18 (45.0) | 40 (25.8) | |
| Caucasian | 45 (39.1) | 70 (60.9) | 115 (74.2) | |
| Other rurality factors | ||||
| Household (≥5 members) | 23 (45.1) | 28 (54.9) | 51 (32.9) | 0.863 |
| Education level (≤12 years) | 37 (41.1) | 53 (58.9) | 90 (58.1) | 0.603 |
| Addictive habits | ||||
| Smoker | 13 (32.5) | 27 (67.5) | 40 (25.8) | 0.139 |
| Drinker (Alcohol) | 31 (43.0) | 41 (57.0) | 72 (46.4) | >0.999 |
| Metabolic diseases | ||||
| Diabetes | 12 (40.0) | 18 (60.0) | 30 (19.3) | 0.838 |
| Hypercholesterolemia | 13 (28.9) | 32 (71.1) | 45 (29.0) | 0.032 * |
| Cardiovascular diseases | ||||
| Arterial hypertension | 16 (37.2) | 27 (62.8) | 43 (27.7) | 0.371 |
| Family history of gastric cancer | 19 (42.2) | 26 (57.8) | 45 (29.0) | >0.999 |
| H. pylori eradication treatment | 11 (37.9) | 18 (62.1) | 29 (18.7) | 0.678 |
| Gastric Epithelium | HP+ n = 67 n (%) | HP- n = 88 n (%) | Total Participants n = 155 n (%) | p-Value |
|---|---|---|---|---|
| Non- Lesion | 47 (45.6) | 56 (54.4) | 103 (66.4) | 0.492 |
| Lesion | 20 (38.5) | 32 (61.5) | 52 (33.6) | 0.772 |
| 11 (35.5) | 20 (64.5) | 31 (59.6) | 0.299 |
| 6 (54.5) | 5 (45.5) | 11 (21.2) | 0.722 |
| 3 (30.0) | 7 (70.0) | 10 (19.2) | >0.999 |
| 2 (40.0) | 3 (60.0) | 5 (50.0) | |
| 1 (20.0) | 4 (80.0) | 5 (50.0) |
| Nº | Bacteria | Biopsies n = 155 n (%) | HP+ n = 67 n (%) | HP- n = 88 n (%) |
|---|---|---|---|---|
| 1 | Neisseriaceae | 33 (21.3) | 10 (30.3) | 23 (69.7) |
| 1.1 | N. flavescens | 13 (8.4) | 5 (38.5) | 8 (61.5) |
| 1.2 | N. subflava | 10 (6.5) | 2 (20.0) | 8 (80.0) |
| 1.3 | N. perflava | 8 (5.2) | 2 (25.0) | 6 (75.0) |
| 1.4 | N. mucosa | 1 (0.6) | Not detected | 1 (100) |
| 1.5 | Eikenella corrodens | 1 (0.6) | 1 (100) | Not detected |
| 2 | Streptococaceae | 31 (20.0) | 6 (19.4) | 25 (80.6) * |
| 2.1 | S. pneumoniae | 9 (5.8) | 2 (22.2) | 7 (77.8) |
| 2.2 | S. parasanguinis | 8 (5.2) | 2 (25.0) | 6 (75.0 |
| 2.3 | S. salivarius | 3 (1.9) | 1 (33.3) | 2 (66.7) |
| 2.4 | S. anguinosus | 1 (0.6) | Not detected | 1 (100) |
| 2.5 | S. mitis | 2 (1.3) | Not detected | 2 (100) |
| 2.6 | S. oralis | 2 (1.3) | Not detected | 2 (100) |
| 2.7 | S. sanguinis | 2 (1.3) | Not detected | 2 (100) |
| 2.8 | S. vestibularis | 1 (0.6) | Not detected | 1 (100) |
| 2.9 | S. australis | 1 (0.6) | Not detected | 1 (100) |
| 2.10 | S. gordonii | 1 (0.6) | Not detected | 1 (100) |
| 2.11 | S. peroris | 1 (0.6) | 1 (100) | Not detected |
| 3 | Actinomyceteae | 14 (9.0) | 1 (7.1) | 13 (92.8) * |
| 3.1 | R. dentocariosa | 6 (3.9) | Not detected | 6 (100) |
| 3.2 | R. mucilaginosa | 3 (1.9) | Not detected | 3 (100) |
| 3.3 | A. odontolyticus | 5 (3.2) | 1 (20.0) | 4 (80.0) |
| 4. | Enterobacteriaceae | 7 (4.5) | 2 (28.6) | 5 (71.4) |
| 4.1 | E. coli | 4 (2.6) | 1 (25.0) | 3 (75.0) |
| 4.2 | E. cloaceae | 1 (0.6) | Not detected | 1 (100) |
| 4.3 | K. variicola | 1 (0.6) | Not detected | 1 (100) |
| 4.4 | P. penneri | 1 (0.6) | 1 (100) | Not detected |
| 5 | Lactobacillaceae | 7 (4.5) | 2 (28.6) | 5 (71.4) |
| 5.1 | L. paracasei | 3 (1.9) | 1 (33.3) | 2 (66.7) |
| 5.2 | L. agilis | 1 (0.6) | Not detected | 1 (100) |
| 5.3 | L. mucosae | 1 (0.6) | Not detected | 1 (100) |
| 5.4 | L. rhamnosus | 1 (0.6) | Not detected | 1 (100) |
| 5.5 | L. salivarius | 1 (0.6) | 1 (100) | Not detected |
| 6. | Gemellaceae | 3 (1.9) | Not detected | 3 (100) |
| 6.1 | G. haemolisans | 1 (0.6) | Not detected | 1 (100) |
| 6.2 | G. mobillorum | 1 (0.6) | Not detected | 1 (100) |
| 6.3 | G. sanguinis | 1 (0.6) | Not detected | 1 (100) |
| 7. | B. Non-Fermenters | 3 (1.9) | 1 (33.3) | 2 (66.7) |
| 7.1 | S. maltophilia | 2 (1.3) | Not detected | 2 (100) |
| 7.2 | A. johnsonii | 1 (0.6) | 1 (100) | Not detected |
| 8 | Staphylococcaceae | 3 (1.9) | 1 (33.3) | 2 (66.7) |
| 8.1 | S. pasteuri | 2 (1.3) | 1 (50.0) | 1 (50.0) |
| 8.2 | S. aureus | 1 (0.6) | Not detected | 1 (100) |
| 9 | Enterococcaceae | 2 (1.3) | 1 (50.0) | 1 (50.0) |
| 9.1 | E. faecium | 2 (1.3) | 1 (50.0) | 1 (50.0) |
| 10 | Propionibacteriaceae | 2 (1.3) | Not detected | 2 (100) |
| 10.1 | P. acnes | 1 (0.6) | Not detected | 1 (100) |
| 10.2 | P. granulosum | 1 (0.6) | Not detected | 1 (100) |
| 11 | Veillonellaceae | 2 (1.3) | Not detected | 2 (100) |
| 11.1 | V. atypical | 1 (0.6) | Not detected | 1 (100) |
| 11.2 | V. dispar | 1 (0.6) | Not detected | 1 (100) |
| 12 | Bacillaceae | 1 (0.6) | Not detected | 1 (100) |
| 12.1 | B. cereus | 1 (0.6) | Not detected | 1 (100) |
| 13 | Carnobacteriaceae | 1 (0.6) | Not detected | 1 (100) |
| 13.1 | G. adiacens | 1 (0.6) | Not detected | 1 (100) |
| 14 | Corynebacteriaceae | 1 (0.6) | Not detected | 1 (100) |
| 14.1 | C. glucuronolyticum | 1 (0.6) | Not detected | 1 (100) |
| 15 | Flavobacteriaceae | 1 (0.6) | Not detected | 1 (100) |
| 15.1 | C. sputigena | 1 (0.6) | Not detected | 1 (100) |
| 16 | Haemophilus | 1 (0.6) | Not detected | 1 (100) |
| 16.1 | H. haemolyticus | 1 (0.6) | Not detected | 1 (100) |
| 17 | Micrococcaceae | 1 (0.6) | Not detected | 1 (100) |
| 17.1 | M. luteus | 1 (0.6) | Not detected | 1 (100) |
| 18 | Prevotellaceae | 1 (0.6) | Not detected | 1 (100) |
| 18.1 | P. pallens | 1 (0.6) | Not detected | 1 (100) |
| Total bacteria species | 47 (100) | 16 (34.0) | 42 (89.4) * | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Troncoso, C.; Pavez, M.; Cerda, A.; Oporto, M.; Villarroel, D.; Hofmann, E.; Rios, E.; Sierralta, A.; Copelli, L.; Barrientos, L. MALDI-TOF MS and 16S RNA Identification of Culturable Gastric Microbiota: Variability Associated with the Presence of Helicobacter pylori. Microorganisms 2020, 8, 1763. https://doi.org/10.3390/microorganisms8111763
Troncoso C, Pavez M, Cerda A, Oporto M, Villarroel D, Hofmann E, Rios E, Sierralta A, Copelli L, Barrientos L. MALDI-TOF MS and 16S RNA Identification of Culturable Gastric Microbiota: Variability Associated with the Presence of Helicobacter pylori. Microorganisms. 2020; 8(11):1763. https://doi.org/10.3390/microorganisms8111763
Chicago/Turabian StyleTroncoso, Claudia, Monica Pavez, Alvaro Cerda, Marcelo Oporto, Daniel Villarroel, Edmundo Hofmann, Eddy Rios, Armando Sierralta, Luis Copelli, and Leticia Barrientos. 2020. "MALDI-TOF MS and 16S RNA Identification of Culturable Gastric Microbiota: Variability Associated with the Presence of Helicobacter pylori" Microorganisms 8, no. 11: 1763. https://doi.org/10.3390/microorganisms8111763
APA StyleTroncoso, C., Pavez, M., Cerda, A., Oporto, M., Villarroel, D., Hofmann, E., Rios, E., Sierralta, A., Copelli, L., & Barrientos, L. (2020). MALDI-TOF MS and 16S RNA Identification of Culturable Gastric Microbiota: Variability Associated with the Presence of Helicobacter pylori. Microorganisms, 8(11), 1763. https://doi.org/10.3390/microorganisms8111763