Convalescent Plasma against COVID-19: A Broad-Spectrum Therapeutic Approach for Emerging Infectious Diseases
Abstract
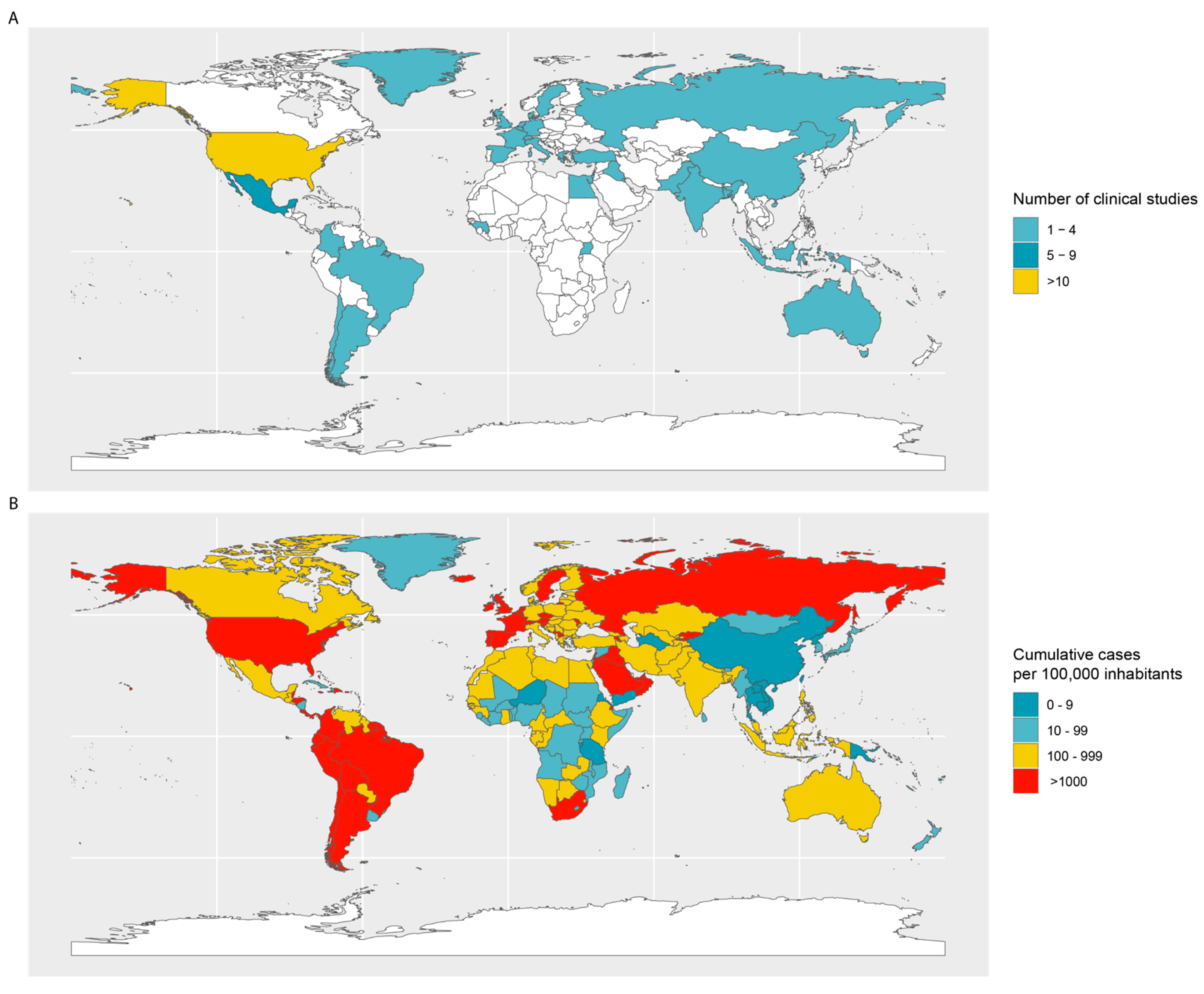
1. Introduction
2. Current Challenges for CP Therapy
2.1. Timing of CP Therapy Implementation
2.2. Source of Convalescent Plasma
2.3. Precautionary Requirements for Plasma Donors
2.4. Plasma Donation and Postdonation Challenges
2.5. Timing of CP Transfusion
2.6. Dosage of CP Therapy
2.7. Post-CP Transfusion Follow-Up
2.8. Risks
2.9. CP Technology: Hyperimmunoglobulin and Monoclonal Antibodies
3. Implementation of CP Therapy in Pandemic Preparedness
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Takian, A.; Raoofi, A.; Kazempour-Ardebili, S. COVID-19 battle during the toughest sanctions against Iran. Lancet 2020, 395, 1035–1036. [Google Scholar] [CrossRef]
- Zhou, P.; Yang, X.-L.; Wang, X.-G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.-R.; Zhu, Y.; Li, B.; Huang, C.-L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Zhang, D.; Wang, W. China Novel Coronavirus Investigating and Research Team. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO made the assessment that COVID-19 can be characterized as a pandemic. In WHO-Timeline COVID-19; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Gasparyan, A.Y.; Misra, D.P.; Yessirkepov, M.; Zimba, O. Perspectives of immune therapy in coronavirus disease 2019. J. Korean Med. Sci. 2020, 35, e176. [Google Scholar] [CrossRef] [PubMed]
- Matthay, M.A.; Aldrich, J.M.; Gotts, J.E. Treatment for severe acute respiratory distress syndrome from COVID-19. Lancet Respir. Med. 2020, 8, 433–434. [Google Scholar] [CrossRef]
- Grein, J.; Ohmagari, N.; Shin, D. Original: Compassionate Use of Remdesivir for Patients with Severe Covid-19. N. Engl. J. Med. 2020, 382, 2327–2336. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, D.; Du, R. Original: Remdesivir in Adults with Severe Covid-19: A Randomised, Double-Blind, Placebo; Campus Toledo, Universidade Federal do Paraná: Toledo, Brazil, 2020. [Google Scholar]
- Pan, H.; Peto, R.; Karim, Q.A.; Alejandria, M.; Restrepo, A.M.H.; Garcia, C.H.; Kieny, M.P.; Malekzadeh, R.; Murthy, S.; Preziosi, M.-P. Repurposed antiviral drugs for COVID-19; interim WHO Solidarity trial results. medRxiv 2020. [Google Scholar] [CrossRef]
- Von Behring, E.; Kitasato, S. The mechanism of diphtheria immunity and tetanus immunity in animals. 1890. Mol. Immunol. 1991, 28, 1317–1319. [Google Scholar] [PubMed]
- Klöppel, U. Enacting Cultural Boundaries in French and German Diphtheria Serum Research. Sci. Context 2008, 21, 161–180. [Google Scholar] [CrossRef] [PubMed]
- Casadevall, A.; Scharff, M.D. Return to the past: The case for antibody-based therapies in infectious diseases. Clin. Infect. Dis. 1995, 21, 150–161. [Google Scholar] [CrossRef]
- Rozowski, T. Emil Behring: Discoverer of antitoxins and father of serotherapy. Polski Tygodnik Lekarski 1955, 10, 1690. [Google Scholar] [PubMed]
- Keller, M.A.; Stiehm, E.R. Passive immunity in prevention and treatment of infectious diseases. Clin. Microbiol. Rev. 2000, 13, 602–614. [Google Scholar] [CrossRef]
- McGuire, L.; Redden, W. The use of convalescent human serum in influenza pneumonia—A preliminary report. Am. J. Public Health 1918, 8, 741–744. [Google Scholar] [CrossRef]
- Bloch, E.M.; Shoham, S.; Casadevall, A.; Sachais, B.S.; Shaz, B.; Winters, J.L.; van Buskirk, C.; Grossman, B.J.; Joyner, M.; Henderson, J.P. Deployment of convalescent plasma for the prevention and treatment of COVID-19. J. Clin. Investig. 2020, 130, 2757–2765. [Google Scholar] [CrossRef]
- Lai, S. Treatment of severe acute respiratory syndrome. Eur. J. Clin. Microbiol. Infect. Dis. 2005, 24, 583–591. [Google Scholar] [CrossRef]
- Hung, I.F.; To, K.K.; Lee, C.-K.; Lee, K.-L.; Chan, K.; Yan, W.-W.; Liu, R.; Watt, C.-L.; Chan, W.-M.; Lai, K.-Y. Convalescent plasma treatment reduced mortality in patients with severe pandemic influenza A (H1N1) 2009 virus infection. Clin. Infect. Dis. 2011, 52, 447–456. [Google Scholar] [CrossRef]
- Arabi, Y.M.; Hajeer, A.H.; Luke, T.; Raviprakash, K.; Balkhy, H.; Johani, S.; Al-Dawood, A.; Al-Qahtani, S.; Al-Omari, A.; Al-Hameed, F. Feasibility of using convalescent plasma immunotherapy for MERS-CoV infection, Saudi Arabia. Emerg. Infect. Dis. 2016, 22, 1554–1661. [Google Scholar] [CrossRef] [PubMed]
- WHO. Use of Convalescent Whole Blood or Plasma Collected from Patients Recovered from Ebola Virus Disease for Transfusion, as an Empirical Treatment During Outbreaks: Interim Guidance for National Health Authorities and Blood Transfusion Services; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Sahr, F.; Ansumana, R.; Massaquoi, T.; Idriss, B.; Sesay, F.; Lamin, J.; Baker, S.; Nicol, S.; Conton, B.; Johnson, W. Evaluation of convalescent whole blood for treating Ebola Virus Disease in Freetown, Sierra Leone. J. Infect. 2017, 74, 302–309. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Y. Potential interventions for novel coronavirus in China: A systematic review. J. Med. Virol. 2020, 92, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Mair-Jenkins, J.; Saavedra-Campos, M.; Baillie, J.K.; Cleary, P.; Khaw, F.-M.; Lim, W.S.; Makki, S.; Rooney, K.D.; Group, C.P.S.; Nguyen-Van-Tam, J.S. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: A systematic review and exploratory meta-analysis. J. Infect. Dis. 2015, 211, 80–90. [Google Scholar] [CrossRef]
- Luke, T.C.; Kilbane, E.M.; Jackson, J.L.; Hoffman, S.L. Meta-analysis: Convalescent blood products for Spanish influenza pneumonia: A future H5N1 treatment? Ann. Intern. Med. 2006, 145, 599–609. [Google Scholar] [CrossRef]
- Rajendran, K.; Narayanasamy, K.; Rangarajan, J.; Rathinam, J.; Natarajan, M.; Ramachandran, A. Convalescent plasma transfusion for the treatment of COVID-19: Systematic review. J. Med. Virol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Wong, R.; Soo, Y.; Wong, W.; Lee, C.; Ng, M.; Chan, P.; Wong, K.; Leung, C.; Cheng, G. Use of convalescent plasma therapy in SARS patients in Hong Kong. Eur. J. Clin. Microbiol. Infect. Dis. 2005, 24, 44–46. [Google Scholar] [CrossRef] [PubMed]
- Arabi, Y.; Balkhy, H.; Hajeer, A.H.; Bouchama, A.; Hayden, F.G.; Al-Omari, A.; Al-Hameed, F.M.; Taha, Y.; Shindo, N.; Whitehead, J. Feasibility, safety, clinical, and laboratory effects of convalescent plasma therapy for patients with Middle East respiratory syndrome coronavirus infection: A study protocol. SpringerPlus 2015, 4, 1–8. [Google Scholar] [CrossRef]
- Chen, L.; Xiong, J.; Bao, L.; Shi, Y. Convalescent plasma as a potential therapy for COVID-19. Lancet Infect. Dis. 2020, 20, 398–400. [Google Scholar] [CrossRef]
- Joyner, M.J.; Wright, R.S.; Fairweather, D.; Senefeld, J.W.; Bruno, K.A.; Klassen, S.A.; Carter, R.E.; Klompas, A.M.; Wiggins, C.C.; Shepherd, J.R.; et al. Early safety indicators of COVID-19 convalescent plasma in 5000 patients. J. Clin. Investig. 2020, 130, 4791–4797. [Google Scholar] [CrossRef]
- Li, L.; Zhang, W.; Hu, Y.; Tong, X.; Zheng, S.; Yang, J.; Kong, Y.; Ren, L.; Wei, Q.; Mei, H. Effect of Convalescent Plasma Therapy on Time to Clinical Improvement in Patients with Severe and Life-threatening COVID-19: A Randomized Clinical Trial. JAMA 2020, 324, 460–470. [Google Scholar] [CrossRef]
- Salazar, E.; Perez, K.K.; Ashraf, M.; Chen, J.; Castillo, B.; Christensen, P.A.; Eubank, T.; Bernard, D.W.; Eagar, T.N.; Long, S.W. Treatment of COVID-19 Patients with Convalescent Plasma. Am. J. Pathol. 2020. [Google Scholar] [CrossRef]
- Cortegiani, A.; Ingoglia, G.; Ippolito, M.; Giarratano, A.; Einav, S. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. J. Crit. Care 2020. [Google Scholar] [CrossRef]
- Shen, C.; Wang, Z.; Zhao, F.; Yang, Y.; Li, J.; Yuan, J.; Wang, F.; Li, D.; Yang, M.; Xing, L. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA 2020, 323, 1582–1589. [Google Scholar] [CrossRef]
- Duan, K.; Liu, B.; Li, C.; Zhang, H.; Yu, T.; Qu, J.; Zhou, M.; Chen, L.; Meng, S.; Hu, Y. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc. Natl. Acad. Sci. USA 2020, 117, 9490–9496. [Google Scholar] [CrossRef]
- Zhang, W.; Du, R.-H.; Li, B.; Zheng, X.-S.; Yang, X.-L.; Hu, B.; Wang, Y.-Y.; Xiao, G.-F.; Yan, B.; Shi, Z.-L. Molecular and serological investigation of 2019-nCoV infected patients: Implication of multiple shedding routes. Emerg. Microbes Infect. 2020, 9, 386–389. [Google Scholar] [CrossRef]
- Tanne, J.H. Covid-19: FDA approves use of convalescent plasma to treat critically ill patients. BMJ 2020, 368, m1256. [Google Scholar] [CrossRef] [PubMed]
- FDA. Recommendations for Investigational COVID-19 Convalescent Plasma. Available online: https://www.fda.gov/vaccines-blood-biologics/investigational-new-drug-ind-or-device-exemption-ide-process-cber/recommendations-investigational-covid-19-convalescent-plasma (accessed on 1 May 2020).
- Perotti, C.; Del Fante, C.; Baldanti, F.; Franchini, M.; Percivalle, E.; Vecchio Nepita, E.; Seminari, E.; De Silvestri, A.; Bruno, R.; Klersy, C. Plasma from donors recovered from the new Coronavirus 2019 as therapy for critical patients with COVID-19 (COVID-19 plasma study): A multicentre study protocol. Intern. Emerg Med. 2020, 19, 819–824. [Google Scholar] [CrossRef] [PubMed]
- Haematology, T.L. The resurgence of convalescent plasma therapy. Lancet. Haematol. 2020, 7, e353. [Google Scholar] [CrossRef]
- Langhi, D.M.; Junior, G.C.D.S.; Bordin, J.O. COVID-19 convalescent plasma transfusion. Hematol. Transfus. Cell Ther. 2020, 42, 113. [Google Scholar] [CrossRef] [PubMed]
- Tiberghien, P.; de Lamballerie, X.; Morel, P.; Gallian, P.; Lacombe, K.; Yazdanpanah, Y. Collecting and evaluating convalescent plasma for COVID-19 treatment: Why and how? Vox Sang. 2020, 115, 488–494. [Google Scholar] [CrossRef]
- Syal, K. COVID-19: Herd immunity and convalescent plasma transfer therapy. J. Med. Virol. 2020. [Google Scholar] [CrossRef]
- Franchini, M.; Marano, G.; Velati, C.; Pati, I.; Pupella, S.; Liumbruno, G.M. Operational protocol for donation of anti-COVID-19 convalescent plasma in Italy. Vox Sang. 2020. [Google Scholar] [CrossRef]
- Epstein, J.; Burnouf, T. Points to consider in the preparation and transfusion of COVID-19 convalescent plasma. Vox Sang. 2020. [Google Scholar] [CrossRef]
- Xie, L.; Liu, Y.; Fan, B.; Xiao, Y.; Tian, Q.; Chen, L.; Zhao, H.; Chen, W. Dynamic changes of serum SARS-coronavirus IgG, pulmonary function and radiography in patients recovering from SARS after hospital discharge. Respir. Res. 2005, 6, 5. [Google Scholar] [CrossRef]
- Liu, W.; Fontanet, A.; Zhang, P.-H.; Zhan, L.; Xin, Z.-T.; Baril, L.; Tang, F.; Lv, H.; Cao, W.-C. Two-year prospective study of the humoral immune response of patients with severe acute respiratory syndrome. J. Infect. Dis. 2006, 193, 792–795. [Google Scholar] [CrossRef]
- Haveri, A.; Smura, T.; Kuivanen, S.; Österlund, P.; Hepojoki, J.; Ikonen, N.; Pitkäpaasi, M.; Blomqvist, S.; Rönkkö, E.; Kantele, A. Serological and molecular findings during SARS-CoV-2 infection: The first case study in Finland, January to February 2020. Eurosurveillance 2020, 25, 2000266. [Google Scholar] [CrossRef] [PubMed]
- Muruato, A.E.; Fontes-Garfias, C.R.; Ren, P.; Garcia-Blanco, M.A.; Menachery, V.D.; Xie, X.; Shi, P.-Y. A high-throughput neutralizing antibody assay for COVID-19 diagnosis and vaccine evaluation. bioRxiv 2020. [Google Scholar] [CrossRef]
- Wu, F.; Wang, A.; Liu, M.; Wang, Q.; Chen, J.; Xia, S.; Ling, Y.; Zhang, Y.; Xun, J.; Lu, L. Neutralizing antibody responses to SARS-CoV-2 in a COVID-19 recovered patient cohort and their implications. medRxiv 2020. [Google Scholar] [CrossRef]
- Brown, J.F.; Dye, J.M.; Tozay, S.; Jeh-Mulbah, G.; Wohl, D.A.; Fischer, W.A., 2nd; Cunningham, C.K.; Rowe, K.; Zacharias, P.; van Hasselt, J. Anti–Ebola Virus Antibody Levels in Convalescent Plasma and Viral Load After Plasma Infusion in Patients With Ebola Virus Disease. J. Infect. Dis. 2018, 218, 555–562. [Google Scholar] [CrossRef]
- Amanat, F.; Stadlbauer, D.; Strohmeier, S.; Nguyen, T.H.; Chromikova, V.; McMahon, M.; Jiang, K.; Arunkumar, G.A.; Jurczyszak, D.; Polanco, J. A serological assay to detect SARS-CoV-2 seroconversion in humans. Nat. Med. 2020, 26, 1–4. [Google Scholar] [CrossRef]
- Wong, H.K.; Lee, C.K.; Hung, I.F.; Leung, J.N.; Hong, J.; Yuen, K.Y.; Lin, C.K. Practical limitations of convalescent plasma collection: A case scenario in pandemic preparation for influenza A (H1N1) infection. Transfusion 2010, 50, 1967–1971. [Google Scholar] [CrossRef]
- Sullivan, H.C.; Roback, J.D. Convalescent plasma: Therapeutic hope or hopeless strategy in the SARS-CoV-2 pandemic. Transfus. Med. Rev. 2020. [Google Scholar] [CrossRef]
- Long, Q.-X.; Tang, X.-J.; Shi, Q.-L.; Li, Q.; Deng, H.-J.; Yuan, J.; Hu, J.-L.; Xu, W.; Zhang, Y.; Lv, F.-J. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat. Med. 2020, 26, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Sekine, T.; Perez-Potti, A.; Rivera-Ballesteros, O.; Straling, K.; Gorin, J.-B.; Olsson, A.; Llewellyn-Lacey, S.; Kamal, H.; Bogdanovic, G.; Muschiol, S. Robust T cell immunity in convalescent individuals with asymptomatic or mild COVID-19. BioRXiv 2020. [Google Scholar] [CrossRef]
- Li, L.; Tong, X.; Chen, H.; He, R.; Lv, Q.; Yang, R.; Zhao, L.; Wang, J.; Xu, H.; Liu, C. Characteristics and serological patterns of COVID-19 convalescent plasma donors: Optimal donors and timing of donation. Transfusion 2020, 8, 1765–1772. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.-K.; Lee, C.-K. Pivotal role of convalescent plasma in managing emerging infectious diseases. Vox Sang. 2020. [Google Scholar] [CrossRef]
- Zhao, J.; Yuan, Q.; Wang, H.; Liu, W.; Liao, X.; Su, Y.; Wang, X.; Yuan, J.; Li, T.; Li, J. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019. Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Okba, N.M.; Müller, M.A.; Li, W.; Wang, C.; GeurtsvanKessel, C.H.; Corman, V.M.; Lamers, M.M.; Sikkema, R.S.; de Bruin, E.; Chandler, F.D. Severe acute respiratory syndrome coronavirus 2-specific antibody responses in coronavirus disease 2019 patients. Emerg. Infect. Dis. 2020, 26. [Google Scholar] [CrossRef]
- Guo, L.; Ren, L.; Yang, S.; Xiao, M.; Chang, D.; Yang, F.; Dela Cruz, C.S.; Wang, Y.; Wu, C.; Xiao, Y. Profiling early humoral response to diagnose novel coronavirus disease (COVID-19). Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Bullard, J.; Dust, K.; Funk, D.; Strong, J.E.; Alexander, D.; Garnett, L.; Boodman, C.; Bello, A.; Hedley, A.; Schiffman, Z. Predicting infectious SARS-CoV-2 from diagnostic samples. Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wu, Q.; Xu, W.; Qiao, B.; Wang, J.; Zheng, H.; Jiang, S.; Mei, J.; Wu, Z.; Deng, Y. Clinical diagnosis of 8274 samples with 2019-novel coronavirus in Wuhan. medRxiv 2020. [Google Scholar] [CrossRef]
- Diao, B.; Wen, K.; Zhang, J.; Chen, J.; Han, C.; Chen, Y.; Wang, S.; Deng, G.; Zhou, H.; Wu, Y. Accuracy of a nucleocapsid protein antigen rapid test in the diagnosis of SARS-CoV-2 infection. Clin. Microbiol. Infect. 2020. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Fu, D.; Ren, Y.; Wang, F.; Wang, D.; Zhang, F.; Xia, X.; Lv, T. Treatment with convalescent plasma for COVID-19 patients in Wuhan, China. J. Med. Virol. 2020. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, M.; Shen, C.; Wang, F.; Yuan, J.; Li, J.; Zhang, M.; Wang, Z.; Xing, L.; Wei, J. Laboratory diagnosis and monitoring the viral shedding of 2019-nCoV infections. MedRxiv 2020. [Google Scholar] [CrossRef]
- Chan, J.F.-W.; Yuan, S.; Kok, K.-H.; To, K.K.-W.; Chu, H.; Yang, J.; Xing, F.; Liu, J.; Yip, C.C.-Y.; Poon, R.W.-S. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: A study of a family cluster. Lancet 2020, 395, 514–523. [Google Scholar] [CrossRef]
- Lamers, M.M.; Beumer, J.; van der Vaart, J.; Knoops, K.; Puschhof, J.; Breugem, T.I.; Ravelli, R.B.; van Schayck, J.P.; Mykytyn, A.Z.; Duimel, H.Q. SARS-CoV-2 productively infects human gut enterocytes. Science 2020. [Google Scholar] [CrossRef]
- Jeong, H.W.; Kim, S.-M.; Kim, H.-S.; Kim, Y.-I.; Kim, J.H.; Cho, J.Y.; Kim, S.-h.; Kang, H.; Kim, S.-G.; Park, S.-J. Viable SARS-CoV-2 in various specimens from COVID-19 patients. Clin. Microbiol. Infect. 2020. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.T.; Lin, H.-M.; Baine, I.; Wajnberg, A.; Gumprecht, J.P.; Rahman, F.; Rodriguez, D.; Tandon, P.; Bassily-Marcus, A.; Bander, J. Convalescent plasma treatment of severe COVID-19: A matched control study. medRxiv 2020. [Google Scholar] [CrossRef]
- Burnouf, T.; Dye, J.M.; Abayomi, A. Convalescent plasma and the dose of Ebola virus antibodies. N. Engl. J. Med. 2017, 376, 1296–1297. [Google Scholar]
- Meyer, B.; Reimerink, J.; Torriani, G.; Brouwer, F.; Godeke, G.-J.; Yerly, S.; Hoogerwerf, M.; Vuilleumier, N.; Kaiser, L.; Eckerle, I. Validation and clinical evaluation of a SARS-CoV-2 Surrogate Virus Neutralisation Test (sVNT). Emerg. Microbes Infect. 2020, 9, 2394–2403. [Google Scholar] [CrossRef]
- Zhang, L.; Pang, R.; Xue, X.; Bao, J.; Ye, S.; Dai, Y.; Zheng, Y.; Fu, Q.; Hu, Z.; Yi, Y. Anti-SARS-CoV-2 virus antibody levels in convalescent plasma of six donors who have recovered from COVID-19. Aging 2020, 12, 6536–6542. [Google Scholar] [CrossRef]
- Ye, G.; Pan, Z.; Pan, Y.; Deng, Q.; Chen, L.; Li, J.; Li, Y.; Wang, X. Clinical characteristics of severe acute respiratory syndrome coronavirus 2 reactivation. J. Infect. 2020, 80, e14–e17. [Google Scholar] [CrossRef]
- Mallapaty, S. Will antibody tests for the coronavirus really change everything? Nature 2020, 571–572. [Google Scholar] [CrossRef]
- Okba, N.M.; Muller, M.A.; Li, W.; Wang, C.; GeurtsvanKessel, C.H.; Corman, V.M.; Lamers, M.M.; Sikkema, R.S.; de Bruin, E.; Chandler, F.D. SARS-CoV-2 specific antibody responses in COVID-19 patients. medRxiv 2020. [Google Scholar] [CrossRef]
- To, K.K.-W.; Hung, I.F.-N.; Ip, J.D.; Chu, A.W.-H.; Chan, W.-M.; Tam, A.R.; Fong, C.H.-Y.; Yuan, S.; Tsoi, H.-W.; Ng, A.C.-K. COVID-19 re-infection by a phylogenetically distinct SARS-coronavirus-2 strain confirmed by whole genome sequencing. Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Huang, A.T.; Garcia-Carreras, B.; Hitchings, M.D.; Yang, B.; Katzelnick, L.C.; Rattigan, S.M.; Borgert, B.A.; Moreno, C.A.; Solomon, B.D.; Rodriguez-Barraquer, I. A systematic review of antibody mediated immunity to coronaviruses: Antibody kinetics, correlates of protection, and association of antibody responses with severity of disease. medRxiv 2020. [Google Scholar] [CrossRef]
- Avendano-Sola, C.; Ramos-Martinez, A.; Munez-Rubio, E.; Ruiz-Antoran, B.; de Molina, R.M.; Torres, F.; Fernandez-Cruz, A.; Callejas-Diaz, A.; Calderon, J.; Payares-Herrera, C. Convalescent Plasma for COVID-19: A multicenter, randomized clinical trial. medRxiv 2020. [Google Scholar] [CrossRef]
- Agarwal, A.; Mukherjee, A.; Kumar, G.; Chatterjee, P.; Bhatnagar, T.; Malhotra, P.; Latha, B.; Bundas, S.; Kumar, V.; Dosi, R. Convalescent plasma in the management of moderate COVID-19 in India: An open-label parallel-arm phase II multicentre randomized controlled trial (PLACID Trial). MedRxiv 2020. [Google Scholar] [CrossRef]
- Gharbharan, A.; Jordans, C.C.; GeurtsvanKessel, C.; den Hollander, J.G.; Karim, F.; Mollema, F.P.; Stalenhoef, J.E.; Dofferhoff, A.; Ludwig, I.; Koster, A. Convalescent Plasma for COVID-19. A randomized clinical trial. MedRxiv 2020. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, S.; Tan, T.; Huang, W.; Dong, Y.; Chen, L.; Chen, Q.; Zhang, L.; Zhong, Q.; Zhang, X. Treatment with convalescent plasma for critically ill patients with SARS-CoV-2 infection. Chest 2020. [Google Scholar] [CrossRef]
- Pei, S.; Yuan, X.; Zhang, Z.Z.; Yao, R.R.; Xie, Y.; Shen, M.M.; Li, B.B.; Chen, X.; Yin, M. Convalescent plasma to treat covid-19: Chinese strategy and experiences. medRxiv 2020. [Google Scholar] [CrossRef]
- Salazar, E.; Christensen, P.A.; Graviss, E.A.; Nguyen, D.T.; Castillo, B.; Chen, J.; Lopez, B.V.; Eagar, T.N.; Yi, X.; Zhao, P. Treatment of coronavirus disease 2019 patients with convalescent plasma reveals a signal of significantly decreased mortality. Am. J. Pathol. 2020, 190, 2290–2303. [Google Scholar] [CrossRef]
- Ahn, J.Y.; Sohn, Y.; Lee, S.H.; Cho, Y.; Hyun, J.H.; Baek, Y.J.; Jeong, S.J.; Kim, J.H.; Ku, N.S.; Yeom, J.-S. Use of convalescent plasma therapy in two COVID-19 patients with acute respiratory distress syndrome in Korea. J. Korean Med. Sci. 2020, 35, e149. [Google Scholar] [CrossRef]
- Zeng, Q.-L.; Yu, Z.-J.; Gou, J.-J.; Li, G.-M.; Ma, S.-H.; Zhang, G.-F.; Xu, J.-H.; Lin, W.-B.; Cui, G.-L.; Zhang, M.-M. Effect of convalescent plasma therapy on viral shedding and survival in patients with coronavirus disease 2019. J. Infect. Dis. 2020, 222, 38–43. [Google Scholar] [CrossRef]
- Joyner, M.J.; Senefeld, J.W.; Klassen, S.A.; Mills, J.R.; Johnson, P.W.; Theel, E.S.; Wiggins, C.C.; Bruno, K.A.; Klompas, A.M.; Lesser, E.R. Effect of convalescent plasma on mortality among hospitalized patients with COVID-19: Initial three-month experience. medrxiv 2020. [Google Scholar] [CrossRef]
- Casadevall, A.; Joyner, M.J.; Pirofski, L.-A. A Randomized Trial of Convalescent Plasma for COVID-19—Potentially Hopeful Signals. JAMA 2020, 324, 455. [Google Scholar] [CrossRef]
- Morabito, C.J.; Gangadharan, B. Active Therapy with Passive Immunotherapy May Be Effective in the Fight against Covid-19. Clin. Transl. Sci. 2020, 13, 835–837. [Google Scholar]
- Rubin, R. Testing an old therapy against a new disease: Convalescent plasma for COVID-19. JAMA 2020, 323, 2114. [Google Scholar] [CrossRef]
- Yeh, K.-M.; Chiueh, T.-S.; Siu, L.; Lin, J.-C.; Chan, P.K.; Peng, M.-Y.; Wan, H.-L.; Chen, J.-H.; Hu, B.-S.; Perng, C.-L. Experience of using convalescent plasma for severe acute respiratory syndrome among healthcare workers in a Taiwan hospital. J. Antimicrob. Chemother. 2005, 56, 919–922. [Google Scholar] [CrossRef]
- Zhou, B.; Zhong, N.; Guan, Y. Treatment with convalescent plasma for influenza A (H5N1) infection. N. Engl. J. Med. 2007, 357, 1450–1451. [Google Scholar] [CrossRef] [PubMed]
- Burnouf, T.; Radosevich, M. Treatment of severe acute respiratory syndrome with convalescent plasma. Hong Kong Med. J. 2003, 9, 309. [Google Scholar] [PubMed]
- Garcia, P.D.W.; Fumeaux, T.; Guerci, P.; Heuberger, D.M.; Montomoli, J.; Roche-Campo, F.; Schuepbach, R.A.; Hilty, M.P.; RISC-19-ICU Investigators. Prognostic factors associated with mortality risk and disease progression in 639 critically ill patients with COVID-19 in Europe: Initial report of the international RISC-19-ICU prospective observational cohort. EClinicalMedicine 2020, 25, 100449. [Google Scholar] [CrossRef]
- Richardson, S.; Hirsch, J.S.; Narasimhan, M.; Crawford, J.M.; McGinn, T.; Davidson, K.W.; Barnaby, D.P.; Becker, L.B.; Chelico, J.D.; Cohen, S.L. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA 2020. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, Z.; Yu, M.; Tao, Y.; Xie, M. 15-day mortality and associated risk factors for hospitalized patients with COVID-19 in Wuhan, China: An ambispective observational cohort study. Intensive Care Med. 2020, 46, 1–3. [Google Scholar] [CrossRef]
- Nurtop, E.; Villarroel, P.M.S.; Pastorino, B.; Ninove, L.; Drexler, J.F.; Roca, Y.; Gake, B.; Dubot-Peres, A.; Grard, G.; Peyrefitte, C. Combination of ELISA screening and seroneutralisation tests to expedite Zika virus seroprevalence studies. Virol. J. 2018, 15, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Eickmann, M.; Gravemann, U.; Handke, W.; Tolksdorf, F.; Reichenberg, S.; Müller, T.H.; Seltsam, A. Inactivation of Ebola virus and Middle East respiratory syndrome coronavirus in platelet concentrates and plasma by ultraviolet C light and methylene blue plus visible light, respectively. Transfusion 2018, 58, 2202–2207. [Google Scholar] [CrossRef]
- Faddy, H.M.; Fryk, J.J.; Hall, R.A.; Young, P.R.; Reichenberg, S.; Tolksdorf, F.; Sumian, C.; Gravemann, U.; Seltsam, A.; Marks, D.C. Inactivation of yellow fever virus in plasma after treatment with methylene blue and visible light and in platelet concentrates following treatment with ultraviolet C light. Transfusion 2019, 59, 2223–2227. [Google Scholar] [CrossRef]
- Ragan, I.; Hartson, L.; Pidcoke, H.; Bowen, R.; Goodrich, R. Pathogen reduction of SARS-CoV-2 virus in plasma and whole blood using riboflavin and UV light. PLoS ONE 2020, 15, e0233947. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Yan, Y.; Wang, L. Coronavirus disease 2019: Coronaviruses and blood safety. Transfus. Med. Rev. 2020, 34, 75–80. [Google Scholar] [CrossRef]
- Rodriguez-Morales, A.J.; Cardona-Ospina, J.A.; Gutiérrez-Ocampo, E.; Villamizar-Peña, R.; Holguin-Rivera, Y.; Escalera-Antezana, J.P.; Alvarado-Arnez, L.E.; Bonilla-Aldana, D.K.; Franco-Paredes, C.; Henao-Martinez, A.F. Clinical, laboratory and imaging features of COVID-19: A systematic review and meta-analysis. Travel Med. Infect. Dis. 2020, 34, 101623. [Google Scholar] [CrossRef]
- Benson, A.B.; Moss, M.; Silliman, C.C. Transfusion-related acute lung injury (TRALI): A clinical review with emphasis on the critically ill. Br. J. Haematol. 2009, 147, 431–443. [Google Scholar] [CrossRef]
- Ooley, P. AABB Standards for Blood Banks and Transfusion Services; AABB Press: Bethesda, MD, USA, 2017. [Google Scholar]
- Mora-Rillo, M.; Arsuaga, M.; Ramírez-Olivencia, G.; de la Calle, F.; Borobia, A.M.; Sánchez-Seco, P.; Lago, M.; Figueira, J.C.; Fernández-Puntero, B.; Viejo, A. Acute respiratory distress syndrome after convalescent plasma use: Treatment of a patient with Ebola virus disease contracted in Madrid, Spain. Lancet Respir. Med. 2015, 3, 554–562. [Google Scholar] [CrossRef]
- Llitjos, J.F.; Leclerc, M.; Chochois, C.; Monsallier, J.M.; Ramakers, M.; Auvray, M.; Merouani, K. High incidence of venous thromboembolic events in anticoagulated severe COVID-19 patients. J. Thromb. Haemost. 2020, 18, 1743–1746. [Google Scholar] [CrossRef]
- Flipse, J.; Diosa-Toro, M.A.; Hoornweg, T.E.; Van De Pol, D.P.; Urcuqui-Inchima, S.; Smit, J.M. Antibody-dependent enhancement of dengue virus infection in primary human macrophages; balancing higher fusion against antiviral responses. Sci. Rep. 2016, 6, 29201. [Google Scholar] [CrossRef]
- Liu, L.; Wei, Q.; Lin, Q.; Fang, J.; Wang, H.; Kwok, H.; Tang, H.; Nishiura, K.; Peng, J.; Tan, Z. Anti–spike IgG causes severe acute lung injury by skewing macrophage responses during acute SARS-CoV infection. JCI Insight 2019, 4, 4. [Google Scholar] [CrossRef]
- Tirado, S.M.C.; Yoon, K.-J. Antibody-dependent enhancement of virus infection and disease. Viral Immunol. 2003, 16, 69–86. [Google Scholar] [CrossRef]
- Wan, Y.; Shang, J.; Sun, S.; Tai, W.; Chen, J.; Geng, Q.; He, L.; Chen, Y.; Wu, J.; Shi, Z. Molecular mechanism for antibody-dependent enhancement of coronavirus entry. J. Virol. 2020, 94, e02015-19. [Google Scholar] [CrossRef] [PubMed]
- Katzelnick, L.C.; Gresh, L.; Halloran, M.E.; Mercado, J.C.; Kuan, G.; Gordon, A.; Balmaseda, A.; Harris, E. Antibody-dependent enhancement of severe dengue disease in humans. Science 2017, 358, 929–932. [Google Scholar] [CrossRef]
- Robinson Jr, W.E.; Montefiori, D.C.; Gillespie, D.H.; Mitchell, W.M. Complement-mediated, antibody-dependent enhancement of HIV-1 infection in vitro is characterized by increased protein and RNA syntheses and infectious virus release. J. Acquir. Immune Defic. Syndr. 1989, 2, 33–42. [Google Scholar]
- Uhr, J.W.; Baumann, J.B. Antibody formation: I. The suppression of antibody formation by passively administered antibody. J. Exp. Med. 1961, 113, 935–957. [Google Scholar] [CrossRef]
- Hung, I.F.; To, K.K.; Lee, C.-K.; Lee, K.-L.; Yan, W.-W.; Chan, K.; Chan, W.-M.; Ngai, C.-W.; Law, K.-I.; Chow, F.-L. Hyperimmune IV immunoglobulin treatment: A multicenter double-blind randomized controlled trial for patients with severe 2009 influenza A (H1N1) infection. Chest 2013, 144, 464–473. [Google Scholar] [CrossRef]
- Traggiai, E.; Becker, S.; Subbarao, K.; Kolesnikova, L.; Uematsu, Y.; Gismondo, M.R.; Murphy, B.R.; Rappuoli, R.; Lanzavecchia, A. An efficient method to make human monoclonal antibodies from memory B cells: Potent neutralization of SARS coronavirus. Nat. Med. 2004, 10, 871–875. [Google Scholar] [CrossRef]
- Corti, D.; Zhao, J.; Pedotti, M.; Simonelli, L.; Agnihothram, S.; Fett, C.; Fernandez-Rodriguez, B.; Foglierini, M.; Agatic, G.; Vanzetta, F. Prophylactic and postexposure efficacy of a potent human monoclonal antibody against MERS coronavirus. Proc. Natl. Acad. Sci. USA 2015, 112, 10473–10478. [Google Scholar] [CrossRef]
- Corti, D.; Misasi, J.; Mulangu, S.; Stanley, D.A.; Kanekiyo, M.; Wollen, S.; Ploquin, A.; Doria-Rose, N.A.; Staupe, R.P.; Bailey, M. Protective monotherapy against lethal Ebola virus infection by a potently neutralizing antibody. Science 2016, 351, 1339–1342. [Google Scholar] [CrossRef]
- Levine, M.M. Monoclonal Antibody Therapy for Ebola Virus Disease; Mass Medical Society: Waltham, MA, USA, 2019. [Google Scholar]
- Regeneron. Regeneron’s Regn-Cov2 Antibody Cocktail Reduced Viral Levels and Improved Symptoms in Non-Hospitalized Covid-19 Patients. Available online: https://investor.regeneron.com/news-releases/news-release-details/regenerons-regn-cov2-antibody-cocktail-reduced-viral-levels-and/ (accessed on 1 November 2020).
- Cohen, J. The Race Is on for Antibodies That Stop the New Coronavirus. Available online: https://www.sciencemag.org/news/2020/05/race-antibodies-stop-new-coronavirus (accessed on 1 November 2020).
- Company ELA. Lilly Statement on the NIAID Decision to Pause Enrollment in ACTIV-3 Clinical Trial; Company ELA: Butler, PA, USA, 2020. [Google Scholar]
- Cao, Y.; Su, B.; Guo, X.; Sun, W.; Deng, Y.; Bao, L.; Zhu, Q.; Zhang, X.; Zheng, Y.; Geng, C. Potent neutralizing antibodies against SARS-CoV-2 identified by high-throughput single-cell sequencing of convalescent patients’ B cells. Cell 2020, 182, 73–84.e16. [Google Scholar] [CrossRef] [PubMed]
- Shanmugaraj, B.; Siriwattananon, K.; Wangkanont, K.; Phoolcharoen, W. Perspectives on monoclonal antibody therapy as potential therapeutic intervention for Coronavirus disease-19 (COVID-19). Asian Pac. J. Allergy Immunol. 2020, 38, 10–18. [Google Scholar]
- Tian, X.; Li, C.; Huang, A.; Xia, S.; Lu, S.; Shi, Z.; Lu, L.; Jiang, S.; Yang, Z.; Wu, Y. Potent binding of 2019 novel coronavirus spike protein by a SARS coronavirus-specific human monoclonal antibody. Emerg. Microbes Infect. 2020, 9, 382–385. [Google Scholar] [CrossRef]
- Burki, T.K. Completion of clinical trials in light of COVID-19. Lancet. Respir. Med. 2020. [Google Scholar] [CrossRef]
- Wise, J.; Coombes, R. Covid-19: The inside story of the RECOVERY trial. BMJ 2020, 370, m2670. [Google Scholar] [CrossRef]
- Park, W.B.; Perera, R.A.; Choe, P.G.; Lau, E.H.; Choi, S.J.; Chun, J.Y.; Oh, H.S.; Song, K.-H.; Bang, J.H.; Kim, E.S. Kinetics of serologic responses to MERS coronavirus infection in humans, South Korea. Emerg. Infect. Dis. 2015, 21, 2186–2189. [Google Scholar] [CrossRef]
- Roback, J.D.; Guarner, J. Convalescent plasma to treat COVID-19: Possibilities and challenges. JAMA 2020, 323, 1561–1562. [Google Scholar] [CrossRef]
- Pourkarim, M.; Van Espen, L.; Thijssen, M.; Van Ranst, M.; Pourkarim, M. How adequate social media management supports the viral Hepatitis elimination program. Hepat. Mon. 2018, 18, e69791. [Google Scholar] [CrossRef]
- Pourkarim, M.R.; Razavi, H.; Lemey, P.; Van Ranst, M. Iran’s hepatitis elimination programme is under threat. Lancet 2018, 392, 1009. [Google Scholar] [CrossRef]
- Pourkarim, M.R.; Thijssen, M.; Alavian, S.M.; Van Ranst, M. Natural disasters pose a challenge for hepatitis elimination in Iran. Lancet. Gastroenterol. Hepatol. 2019, 4, 581–582. [Google Scholar] [CrossRef]
- Kissler, S.M.; Tedijanto, C.; Goldstein, E.; Grad, Y.H.; Lipsitch, M. Projecting the transmission dynamics of SARS-CoV-2 through the postpandemic period. Science 2020, 368, 860–868. [Google Scholar] [CrossRef]
- Hall, M.A.; Studdert, D.M. Privileges and immunity certification during the COVID-19 pandemic. JAMA 2020, 323, 2243. [Google Scholar] [CrossRef]

| Reference | Study Design | Time of Transfusion (Days Postadmission) | *Neutralizing Ab Titer **Anti-SARS-CoV-2 Ab Titer (Spike-Antigen Antibody) | Transfused Volume (mL/units) | Clinical Outcome | Data Collection (Days after Infusion) | Conclusion |
|---|---|---|---|---|---|---|---|
| [33] | Case series, 5 critically ill patients | 10–22 | *>1:40 **>1:1000 | 400/2 | Normalizing body temperature Resolution of ARDS Decrease in SOFA Decrease/undetectable viral load Development of neutralizing Ab | 12 | Efficacy + no severe adverse events |
| [34] | Case series, 10 severely ill patients (ChiCTR2000030046) | 11–20 | *>1:640 | 200/1 | Decrease/undetectable viral load, decrease in CRP Increased oxygen saturation, increased lymphocyte count, absorption of lung lesions No ARDS | 3–7 | Efficacy + no severe adverse events |
| [82] | Case series, 4 severely ill patients | 12–19 | **IgG titer >1:320 **IgM, OD ratio 1.22 (weakly reactive) | 200–2400/1–2 | Undetectable viral load Weaning from mechanical ventilation Absorption of lung lesions | 11 | Efficacy + no severe adverse events |
| [83] | Case series, 3 patients | 12–27 | **IgG titer >1:160 | 200–500/ | Undetectable viral load Hospital discharge | 4–26 | Efficacy + anaphylactic shock in one case (plasma donor had a history of pregnancy) |
| [64] | Case series, 6 patients | 33–50 | Was not defined in the article | 200–600/1–3 | Development of neutralizing antibodies, resolution of consolidation | Efficacy + no severe adverse events | |
| [73] | Case series, 1 critically ill patient | 17 | **IgG titer >1:320 | 200/1 | Increased oxygen saturation Increased lymphocyte count Weaning from mechanical ventilation | 11 | Efficacy + no severe adverse events |
| [86] | Case series, 6 and 15 critically ill patients and controls, respectively | 12.5 | IgG-positive and IgM-negative | 200–600/1 | No viral shedding in most of both groups Death of 5/6 patients in the group and 14/15 in the control group | 3 | No severe adverse effects, CP infusion is not effective for critically ill patients at the late stages of the disease. Infusion in the early phase is recommended |
| [85] | Case series, 2 critically ill patient | 6 and 10 | IgG-positive | 500/2 | CRP and IL-6 normalization Decrease in viral load Resolution of lung infiltration Weaning from mechanical ventilation | 24 and 26 | Efficacy + no severe adverse events |
| [70] | Matched control study of 39 sever and life-threatening | 4 | **titer ≥1:320 | 250/2 | Improvement of survival in the CP-treated group | Variable | No severe adverse effects Positive impact on survival rate |
| [30] | Open-label, multicenter, randomized trial, 45 severe and 58 patients with life-threatening disease (ChiCTR2000029757) | 27 | **<1:160 **1:160–1:1280 or >1280 | 4 to 13 mL/kg | No statistically significant clinical improvements 28 days post-treatment (improvements in 52% of CP recipients versus 43% of controls) | 7–28 | Interpretation is limited by the early termination of the trial |
| [31] | Case series, 25 critically ill patients | 2 | **1:0–1:1350 | 300 | Resolution of ARDS Weaning from mechanical ventilation Improved clinical parameters Discharge in 20/25 patients | 7–14 | No severe adverse events Positive impact on survival rate |
| [84] | Matched control study of 316 patients with severe and life-threatening disease (NCT04554992) | 3 | **>1:1350 or <1:1350 (>1:150–1:1350) | 300/1 or more | Weaning from mechanical ventilation Discharge from ICU to the ward Decreased ventilation time | 3–28 | No severe adverse events Convalescent plasma was effective in the first 72 h after admission. Here, a reduced mortality rate was observed |
| [81] | Open-label randomized trial with 86 patients (NCT04342182) | >4 days | *>1:80 | 300/1 or 2 | No difference in mortality, hospital stay or disease severity was observed after 15 days | 15 | Prematurely stopped. At the time of inclusion, 53 of 66 patients had anti-SARS-CoV-2 antibodies at baseline |
| [87] | Open-label, multicenter, study with 35322 patients with severe or life-threatening (NCT04338360) | Within 3 or ≥4 days | Signal-to-cut-off (S/Co) ratio | 150–250/1 or 2 | 7- and 30-day mortality rates were reduced in patients who received plasma with antibody titers of 1:338 or higher | 7–30 | Earlier time to transfusion and higher antibody levels provide signatures of efficacy. No severe adverse events |
| [79] | Multicenter, randomized clinical trial on 87 hospitalized patients (NCT04345523) | 1 | *>1:80 | 250–300/1 | 38/81 of CP recipients died or developed severe disease and required mechanical ventilation | 15–29 | The trial was stopped due to the drop in available patients following control of the pandemic |
| [80] | Open-label, phase II, multicenter, randomized controlled trial, with 464 hospitalized patients (CTRI/2020/04/024775) | Not specified | *1:20–1:1280 | 200/2 | Resolution of dyspnea and fatigue, early clearance of viral RNA, reduce FiO2 requirement, weaning from mechanical ventilation CP was not associated with reduced mortality or progression to severe disease | Days 0, 1, 3, 5, 7, 14 and 28 | Minimal and non-life-threatening adverse events Mortality was assessed as possibly related to CP transfusion in three patients CP therapy seemed ineffective |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thijssen, M.; Devos, T.; Ejtahed, H.-S.; Amini-Bavil-Olyaee, S.; Pourfathollah, A.A.; Pourkarim, M.R. Convalescent Plasma against COVID-19: A Broad-Spectrum Therapeutic Approach for Emerging Infectious Diseases. Microorganisms 2020, 8, 1733. https://doi.org/10.3390/microorganisms8111733
Thijssen M, Devos T, Ejtahed H-S, Amini-Bavil-Olyaee S, Pourfathollah AA, Pourkarim MR. Convalescent Plasma against COVID-19: A Broad-Spectrum Therapeutic Approach for Emerging Infectious Diseases. Microorganisms. 2020; 8(11):1733. https://doi.org/10.3390/microorganisms8111733
Chicago/Turabian StyleThijssen, Marijn, Timothy Devos, Hanieh-Sadat Ejtahed, Samad Amini-Bavil-Olyaee, Ali Akbar Pourfathollah, and Mahmoud Reza Pourkarim. 2020. "Convalescent Plasma against COVID-19: A Broad-Spectrum Therapeutic Approach for Emerging Infectious Diseases" Microorganisms 8, no. 11: 1733. https://doi.org/10.3390/microorganisms8111733
APA StyleThijssen, M., Devos, T., Ejtahed, H.-S., Amini-Bavil-Olyaee, S., Pourfathollah, A. A., & Pourkarim, M. R. (2020). Convalescent Plasma against COVID-19: A Broad-Spectrum Therapeutic Approach for Emerging Infectious Diseases. Microorganisms, 8(11), 1733. https://doi.org/10.3390/microorganisms8111733






