Proteomic Adaptation of Streptococcus pneumoniae to the Antimicrobial Peptide Human Beta Defensin 3 (hBD3) in Comparison to Other Cell Surface Stresses
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kang, X.; Elson, C.; Penfield, J.; Kirui, A.; Chen, A.; Zhang, L.; Wang, T. Integrated solid-state NMR and molecular dynamics modeling determines membrane insertion of human beta-defensin analog. Commun. Biol. 2019, 2. [Google Scholar] [CrossRef] [PubMed]
- Hiemstra, P.S.; Amatngalim, G.D.; van der Does, A.M.; Taube, C. Antimicrobial peptides and innate lung defenses: Role in infectious and noninfectious lung diseases and therapeutic applications. Chest 2016, 149, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Hurdle, J.G.; O’Neill, A.J.; Chopra, I.; Lee, R.E. Targeting bacterial membrane function: An underexploited mechanism for treating persistent infections. Nat. Rev. Microbiol. 2011, 9, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Perron, G.G.; Zasloff, M.; Bell, G. Experimental evolution of resistance to an antimicrobial peptide. Proc. Biol. Sci. 2006, 273, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Shelburne, C.E.; Coulter, W.A.; Olguin, D.; Lantz, M.S.; Lopatin, D.E. Induction of beta-defensin resistance in the oral anaerobe Porphyromonas gingivalis. Antimicrob. Agents Chemother. 2005, 49, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Mücke, P.-A.; Maaß, S.; Kohler, T.P.; Hammerschmidt, S.; Becher, D. Proteomic adaptation of Streptococcus pneumoniae to the human antimicrobial peptide LL-37. Microorganisms 2020, 8, 413. [Google Scholar] [CrossRef] [PubMed]
- Schibli, D.J.; Hunter, H.N.; Aseyev, V.; Starner, T.D.; Wiencek, J.M.; McCray, P.B., Jr.; Tack, B.F.; Vogel, H.J. The solution structures of the human beta-defensins lead to a better understanding of the potent bactericidal activity of HBD3 against Staphylococcus aureus. J. Biol. Chem. 2002, 277, 8279–8289. [Google Scholar] [CrossRef]
- Wang, G. Structures of human host defense cathelicidin LL-37 and its smallest antimicrobial peptide KR-12 in lipid micelles. J. Biol. Chem. 2008, 283, 32637–32643. [Google Scholar] [CrossRef]
- Sehnal, D.; Rose, A.S.; Koca, J.; Burley, S.K.; Velankar, S. Mol*: Towards a common library and tools for web molecular graphics. In MolVa: Workshop on Molecular Graphics and Visual Analysis of Molecular Data 2018; Eurographics: Brno, Czech Republic, 2018. [Google Scholar] [CrossRef]
- Schulz, C.; Gierok, P.; Petruschka, L.; Lalk, M.; Mader, U.; Hammerschmidt, S. Regulation of the arginine deiminase system by ArgR2 interferes with arginine metabolism and fitness of Streptococcus pneumoniae. mBio 2014, 5. [Google Scholar] [CrossRef]
- Cox, J.; Neuhauser, N.; Michalski, A.; Scheltema, R.A.; Olsen, J.V.; Mann, M. Andromeda: A peptide search engine integrated into the MaxQuant environment. J. Proteome Res. 2011, 10, 1794–1805. [Google Scholar] [CrossRef]
- Tyanova, S.; Temu, T.; Sinitcyn, P.; Carlson, A.; Hein, M.Y.; Geiger, T.; Mann, M.; Cox, J. The Perseus computational platform for comprehensive analysis of (prote)omics data. Nat. Methods 2016, 13, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Slager, J.; Aprianto, R.; Veening, J.W. Deep genome annotation of the opportunistic human pathogen Streptococcus pneumoniae D39. Nucleic Acids Res. 2018, 46, 9971–9989. [Google Scholar] [CrossRef]
- Peabody, M.A.; Laird, M.R.; Vlasschaert, C.; Lo, R.; Brinkman, F.S. PSORTdb: Expanding the bacteria and archaea protein subcellular localization database to better reflect diversity in cell envelope structures. Nucleic Acids Res. 2016, 44, D663–D668. [Google Scholar] [CrossRef]
- Perez-Riverol, Y.; Csordas, A.; Bai, J.; Bernal-Llinares, M.; Hewapathirana, S.; Kundu, D.J.; Inuganti, A.; Griss, J.; Mayer, G.; Eisenacher, M.; et al. The PRIDE database and related tools and resources in 2019: Improving support for quantification data. Nucleic Acids Res. 2019, 47, D442–D450. [Google Scholar] [CrossRef] [PubMed]
- Halfmann, A.; Kovacs, M.; Hakenbeck, R.; Bruckner, R. Identification of the genes directly controlled by the response regulator CiaR in Streptococcus pneumoniae: Five out of 15 promoters drive expression of small non-coding RNAs. Mol. Microbiol. 2007, 66, 110–126. [Google Scholar] [CrossRef]
- Yang, H.B.; Hou, W.T.; Cheng, M.T.; Jiang, Y.L.; Chen, Y.; Zhou, C.Z. Structure of a MacAB-like efflux pump from Streptococcus pneumoniae. Nat. Commun. 2018, 9, 196. [Google Scholar] [CrossRef] [PubMed]
- Damjanovic, M.; Kharat, A.S.; Eberhardt, A.; Tomasz, A.; Vollmer, W. The essential tacF gene is responsible for the choline-dependent growth phenotype of Streptococcus pneumoniae. J. Bacteriol. 2007, 189, 7105–7111. [Google Scholar] [CrossRef]
- Lysenko, E.S.; Gould, J.; Bals, R.; Wilson, J.M.; Weiser, J.N. Bacterial phosphorylcholine decreases susceptibility to the antimicrobial peptide LL-37/hCAP18 expressed in the upper respiratory tract. Infect. Immun. 2000, 68, 1664–1671. [Google Scholar] [CrossRef]
- Waldow, F.; Kohler, T.P.; Hess, N.; Schwudke, D.; Hammerschmidt, S.; Gisch, N. Attachment of phosphorylcholine residues to pneumococcal teichoic acids and modification of substitution patterns by the phosphorylcholine esterase. J. Biol. Chem. 2018, 293, 10620–10629. [Google Scholar] [CrossRef]
- Kovacs, M.; Halfmann, A.; Fedtke, I.; Heintz, M.; Peschel, A.; Vollmer, W.; Hakenbeck, R.; Bruckner, R. A functional dlt operon, encoding proteins required for incorporation of D-alanine in teichoic acids in Gram-positive bacteria, confers resistance to cationic antimicrobial peptides in Streptococcus pneumoniae. J. Bacteriol. 2006, 188, 5797–5805. [Google Scholar] [CrossRef]
- May, J.J.; Finking, R.; Wiegeshoff, F.; Weber, T.T.; Bandur, N.; Koert, U.; Marahiel, M.A. Inhibition of the D-alanine:D-alanyl carrier protein ligase from Bacillus subtilis increases the bacterium’s susceptibility to antibiotics that target the cell wall. FEBS J. 2005, 272, 2993–3003. [Google Scholar] [CrossRef] [PubMed]
- Cooper, V.S.; Honsa, E.; Rowe, H.; Deitrick, C.; Iverson, A.R.; Whittall, J.J.; Neville, S.L.; McDevitt, C.A.; Kietzman, C.; Rosch, J.W. Experimental evolution in vivo to identify selective pressures during pneumococcal colonization. mSystems 2020, 5. [Google Scholar] [CrossRef] [PubMed]
- Nawrocki, K.L.; Crispell, E.K.; McBride, S.M. Antimicrobial peptide resistance mechanisms of Gram-positive bacteria. Antibiotics 2014, 3, 461–492. [Google Scholar] [CrossRef]
- Cole, J.N.; Nizet, V. Bacterial evasion of host antimicrobial peptide defenses. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef] [PubMed]
- Melin, M.; Di Paolo, E.; Tikkanen, L.; Jarva, H.; Neyt, C.; Kayhty, H.; Meri, S.; Poolman, J.; Vakevainen, M. Interaction of pneumococcal histidine triad proteins with human complement. Infect. Immun. 2010, 78, 2089–2098. [Google Scholar] [CrossRef] [PubMed]
- Bell, G.; Gouyon, P.H. Arming the enemy: The evolution of resistance to self-proteins. Microbiology 2003, 149, 1367–1375. [Google Scholar] [CrossRef] [PubMed]
- Mishra, B.; Wang, G. Individual and combined effects of engineered peptides and antibiotics on Pseudomonas aeruginosa biofilms. Pharmaceuticals 2017, 10, 58. [Google Scholar] [CrossRef]
- Chen, C.H.; Lu, T.K. Development and challenges of antimicrobial peptides for therapeutic applications. Antibiotics 2020, 9, 24. [Google Scholar] [CrossRef]
- Peters, B.M.; Shirtliff, M.E.; Jabra-Rizk, M.A. Antimicrobial peptides: Primeval molecules or future drugs? PLoS Pathog. 2010, 6, e1001067. [Google Scholar] [CrossRef]
- Lazar, V.; Martins, A.; Spohn, R.; Daruka, L.; Grezal, G.; Fekete, G.; Szamel, M.; Jangir, P.K.; Kintses, B.; Csorgo, B.; et al. Antibiotic-resistant bacteria show widespread collateral sensitivity to antimicrobial peptides. Nat. Microbiol. 2018, 3, 718–731. [Google Scholar] [CrossRef]
- Hashemi, M.M.; Holden, B.S.; Coburn, J.; Taylor, M.F.; Weber, S.; Hilton, B.; Zaugg, A.L.; McEwan, C.; Carson, R.; Andersen, J.L.; et al. Proteomic analysis of resistance of Gram-negative bacteria to chlorhexidine and impacts on susceptibility to colistin, antimicrobial peptides, and ceragenins. Front. Microbiol. 2019, 10, 210. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.Y.; Lee, M.W.; Wong, G.C.L. Modulation of toll-like receptor signaling by antimicrobial peptides. Semin. Cell Dev. Biol. 2019, 88, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Hancock, R.E.W. Concerns regarding resistance to self-proteins. Microbiology 2003, 149, 3343–3344. [Google Scholar] [CrossRef] [PubMed]
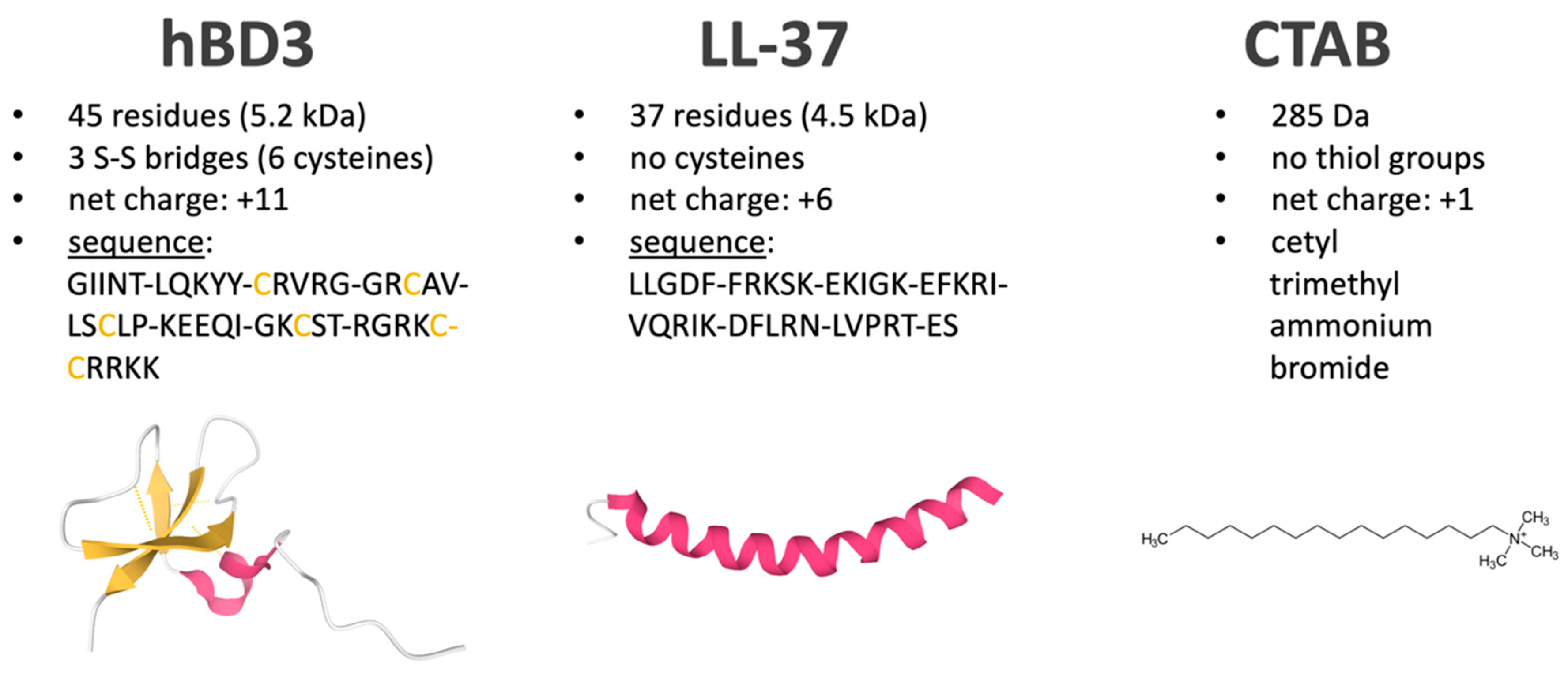

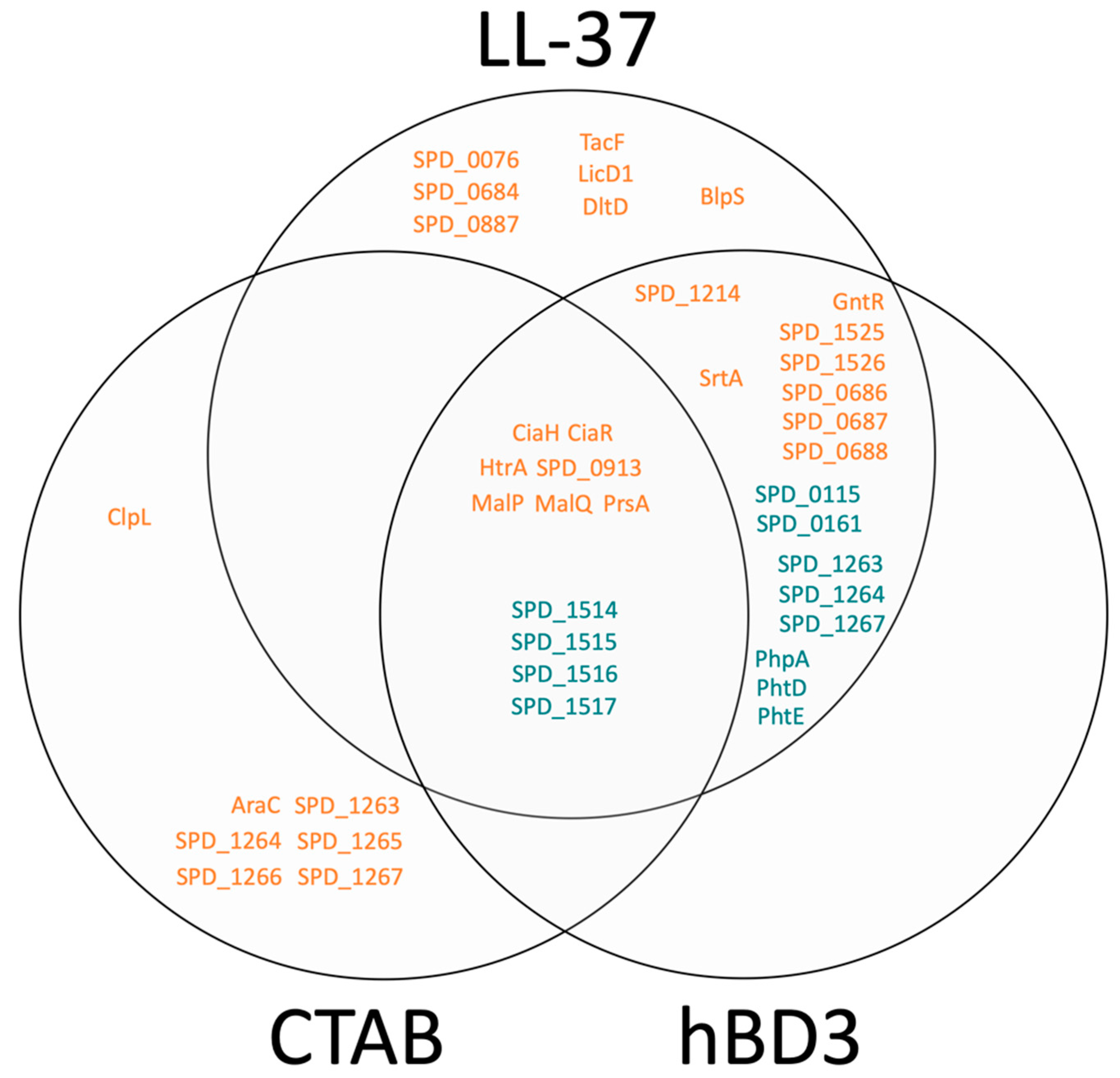
| Applied Compound | Identified Proteins (% of the Total Proteome) | Quantifiable Proteins (% of the Total Proteome) | Pearson Correlation between Samples of the Experiment | Number of Proteins with Significantly * Changed Abundance after 2 h of Stress Including on/off Proteins |
|---|---|---|---|---|
| hBD3 | 1241 (65%) | 1106 (58%) | 0.98–0.99 | 60 (18↑, 42↓) |
| LL-37 [6] | 1293 (68%) | 1118 (58%) | 0.96–0.99 | 80 (41↑, 39↓) |
| CTAB | 1275 (67%) | 1184 (62%) | 0.96–0.99 | 30 (15↑, 15↓) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mücke, P.-A.; Ostrzinski, A.; Hammerschmidt, S.; Maaß, S.; Becher, D. Proteomic Adaptation of Streptococcus pneumoniae to the Antimicrobial Peptide Human Beta Defensin 3 (hBD3) in Comparison to Other Cell Surface Stresses. Microorganisms 2020, 8, 1697. https://doi.org/10.3390/microorganisms8111697
Mücke P-A, Ostrzinski A, Hammerschmidt S, Maaß S, Becher D. Proteomic Adaptation of Streptococcus pneumoniae to the Antimicrobial Peptide Human Beta Defensin 3 (hBD3) in Comparison to Other Cell Surface Stresses. Microorganisms. 2020; 8(11):1697. https://doi.org/10.3390/microorganisms8111697
Chicago/Turabian StyleMücke, Pierre-Alexander, Anne Ostrzinski, Sven Hammerschmidt, Sandra Maaß, and Dörte Becher. 2020. "Proteomic Adaptation of Streptococcus pneumoniae to the Antimicrobial Peptide Human Beta Defensin 3 (hBD3) in Comparison to Other Cell Surface Stresses" Microorganisms 8, no. 11: 1697. https://doi.org/10.3390/microorganisms8111697
APA StyleMücke, P.-A., Ostrzinski, A., Hammerschmidt, S., Maaß, S., & Becher, D. (2020). Proteomic Adaptation of Streptococcus pneumoniae to the Antimicrobial Peptide Human Beta Defensin 3 (hBD3) in Comparison to Other Cell Surface Stresses. Microorganisms, 8(11), 1697. https://doi.org/10.3390/microorganisms8111697







