Probiotics Treatment Improves Hippocampal Dependent Cognition in a Rodent Model of Parkinson’s Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
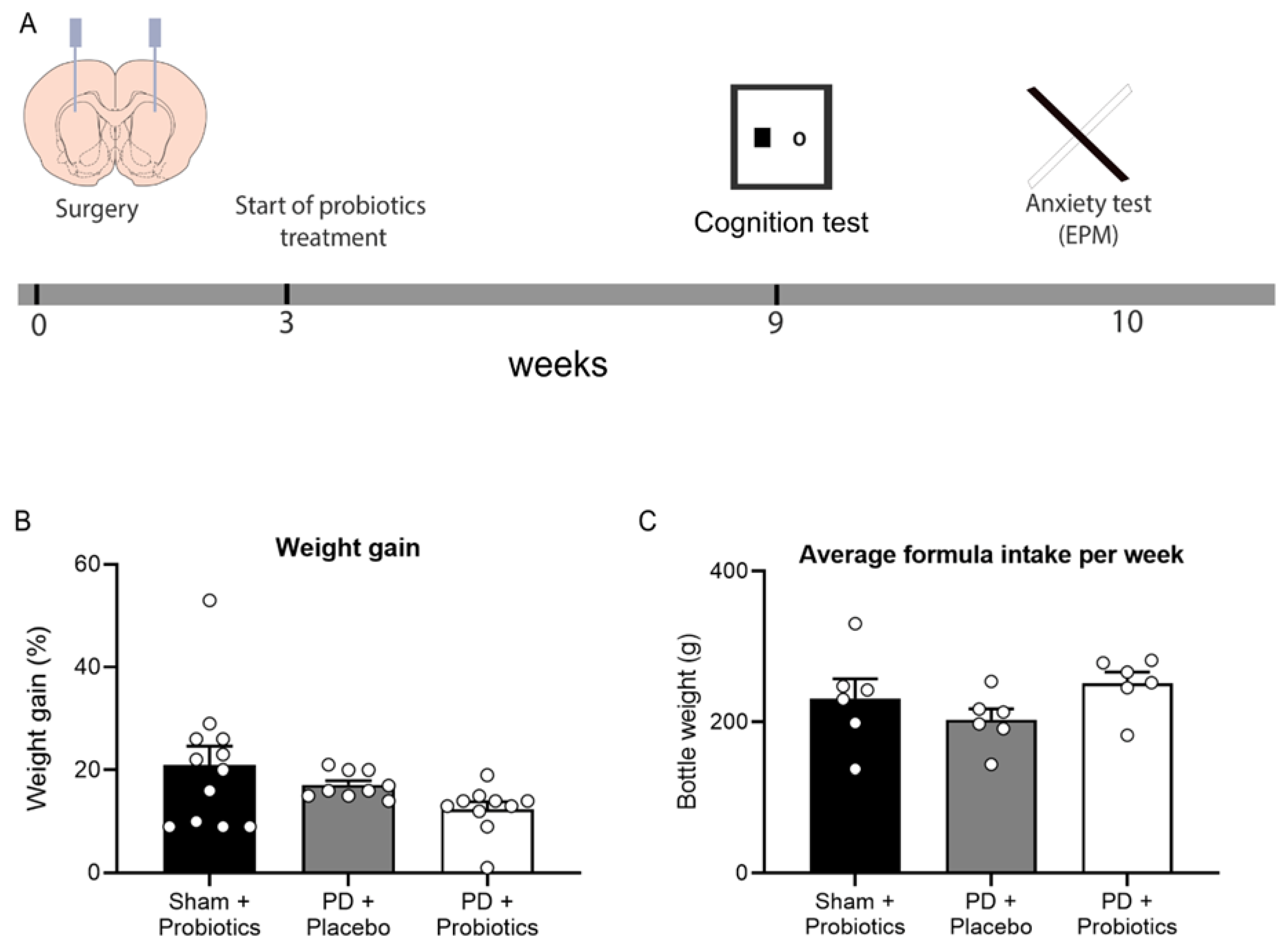
2.2. Surgery
2.3. Probiotic Treatment
2.4. Behavioural Procedures
2.5. Novel Object Recognition (NOR) Task
2.6. Novel Place Recognition (NPR) Task
2.7. Novelty Preference Ratio
2.8. Elevated Plus-Maze (EPM)
2.9. Immunohistochemistry
2.10. Statistical Analysis
3. Results
3.1. Weight and Formula Intake
3.2. Hippocampal Independent Cognition Remains Intact in 6-OHDA Lesioned Rats
3.3. Hippocampal Dependent Cognitive Deficits in 6-OHDA Lesioned Rats Are Reversed by Probiotics
3.4. Probiotics Treatment Does Not Impact Anxiety Behaviour in 6-OHDA-Lesioned Rats
3.5. Selective Dopamine Depletion in the Substantia Nigra
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Carlsson, A. Basic concepts underlying recent developments in the field of Parkinson’s disease. Contemp. Neurol. Ser. 1971, 8, 1–31. [Google Scholar]
- Chaudhuri, K.R.; Schapira, A.H.V. Non-motor symptoms of Parkinson’s disease: Dopaminergic pathophysiology and treatment. Lancet Neurol. 2009, 8, 464–474. [Google Scholar] [CrossRef]
- Emre, M.; Aarsland, D.; Brown, R.; Burn, D.J.; Duyckaerts, C.; Mizuno, Y.; Broe, G.A.; Cummings, J.; Dickson, D.W.; Gauthier, S.; et al. Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Mov. Disord. 2007, 22, 1689–1707. [Google Scholar] [CrossRef]
- Chaudhuri, K.R.; Healy, D.G.; Schapira, A.H.V. Non-motor symptoms of Parkinson’s disease: Diagnosis and management. Lancet Neurol. 2006, 5, 235–245. [Google Scholar] [CrossRef]
- Halliday, G.M.; Leverenz, J.B.; Schneider, J.S.; Adler, C.H. The neurobiological basis of cognitive impairment in Parkinson’s disease. Mov. Disord. 2014, 29, 634–650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adler, C.H.; Caviness, J.N.; Sabbagh, M.N.; Shill, H.A.; Connor, D.J.; Sue, L.; Evidente, V.G.; Driver-Dunckley, E.; Beach, T.G. Heterogeneous neuropathological findings in Parkinson’s disease with mild cognitive impairment. Acta Neuropathol. 2010, 120, 827–828. [Google Scholar] [CrossRef] [Green Version]
- Marras, C.; Chaudhuri, K.R. Nonmotor features of Parkinson’s disease subtypes. Mov. Disord. 2016, 31, 1095–1102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schapira, A.H.V.; Chaudhuri, K.R.; Jenner, P. Non-motor features of Parkinson disease. Nat. Rev. Neurosci. 2017, 18, 435. [Google Scholar] [CrossRef] [PubMed]
- Mueller, C.; Rajkumar, A.P.; Wan, Y.M.; Velayudhan, L.; Ffytche, D.; Chaudhuri, K.R.; Aarsland, D. Assessment and Management of Neuropsychiatric Symptoms in Parkinson’s Disease. CNS Drugs 2018, 32, 621–635. [Google Scholar] [CrossRef] [Green Version]
- Abbott, R.D.; Ross, G.W.; White, L.R.; Tanner, C.M.; Masaki, K.H.; Nelson, J.S.; Curb, J.D.; Petrovitch, H. Excessive daytime sleepiness and subsequent development of Parkinson disease. Neurology 2005, 65, 1442–1446. [Google Scholar] [CrossRef]
- Lewis, S.J.; Dove, A.; Robbins, T.W.; Barker, R.A.; Owen, A.M. Cognitive impairments in early Parkinson’s disease are accompanied by reductions in activity in frontostriatal neural circuitry. J. Neurosci. 2003, 23, 6351–6356. [Google Scholar] [CrossRef] [Green Version]
- Hely, M.A.; Reid, W.G.J.; Adena, M.A.; Halliday, G.M.; Morris, J.G.L. The Sydney multicenter study of Parkinson’s disease: The inevitability of dementia at 20 years. Mov. Disord. 2008, 23, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Schrag, A.; Jahanshahi, M.; Quinn, N. How does Parkinson’s disease affect quality of life? A comparison with quality of life in the general population. Mov. Disord. 2000, 15, 1112–1118. [Google Scholar] [CrossRef]
- Martínez-Rivera, F.J.; Rodriguez-Romaguera, J.; Lloret-Torres, M.E.; Do Monte, F.H.; Quirk, G.J.; Barreto-Estrada, J.L. Bidirectional Modulation of Extinction of Drug Seeking by Deep Brain Stimulation of the Ventral Striatum. Biol. Psychiatry 2016, 80, 682–690. [Google Scholar] [CrossRef] [Green Version]
- Klingelhoefer, L.; Reichmann, H. Pathogenesis of Parkinson disease--the gut-brain axis and environmental factors. Nat. Rev. Neurol. 2015, 11, 625–636. [Google Scholar] [CrossRef]
- Damier, P.; Hirsch, E.C.; Agid, Y.; Graybiel, A.M. The substantia nigra of the human brain: II. Patterns of loss of dopamine-containing neurons in Parkinson’s disease. Brain 1999, 122, 1437–1448. [Google Scholar] [CrossRef] [PubMed]
- Kordower, J.H.; Olanow, C.W.; Dodiya, H.B.; Chu, Y.; Beach, T.G.; Adler, C.H.; Halliday, G.M.; Bartus, R.T. Disease duration and the integrity of the nigrostriatal system in Parkinson’s disease. Brain 2013, 136, 2419–2431. [Google Scholar] [CrossRef] [Green Version]
- Glinka, Y.; Gassen, M.; Youdim, M.B. Mechanism of 6-hydroxydopamine neurotoxicity. J. Neural Transm. Suppl. 1997, 50, 55–66. [Google Scholar]
- Tieu, K. A guide to neurotoxic animal models of Parkinson’s disease. Cold Spring Harb. Perspect. Med. 2011, 1, a009316. [Google Scholar] [CrossRef] [PubMed]
- Cenci, M.A.; Lundblad, M. Ratings of L-DOPA-Induced Dyskinesia in the Unilateral 6-OHDA Lesion Model of Parkinson’s Disease in Rats and Mice. Curr. Protoc. Neurosci. 2007, 41, 9.25.1–9.25.23. [Google Scholar] [CrossRef]
- Sakai, K.; Gash, D.M. Effect of bilateral 6-OHDA lesions of the substantia nigra on locomotor activity in the rat. Brain Res. 1994, 633, 144–150. [Google Scholar] [CrossRef]
- Ferro, M.M.; Bellissimo, M.I.; Anselmo-Franci, J.A.; Angellucci, M.E.; Canteras, N.S.; Da Cunha, C. Comparison of bilaterally 6-OHDA- and MPTP-lesioned rats as models of the early phase of Parkinson’s disease: Histological, neurochemical, motor and memory alterations. J. Neurosci. Methods 2005, 148, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Branchi, I.; D’Andrea, I.; Armida, M.; Cassano, T.; Pèzzola, A.; Potenza, R.L.; Morgese, M.G.; Popoli, P.; Alleva, E. Nonmotor symptoms in Parkinson’s disease: Investigating early-phase onset of behavioral dysfunction in the 6-hydroxydopamine-lesioned rat model. J. Neurosci. Res. 2008, 86, 2050–2061. [Google Scholar] [CrossRef]
- Tadaiesky, M.T.; Dombrowski, P.A.; Figueiredo, C.P.; Cargnin-Ferreira, E.; Da Cunha, C.; Takahashi, R.N. Emotional, cognitive and neurochemical alterations in a premotor stage model of Parkinson’s disease. Neuroscience 2008, 156, 830–840. [Google Scholar] [CrossRef]
- Santiago, R.M.; Barbieiro, J.; Lima, M.M.; Dombrowski, P.A.; Andreatini, R.; Vital, M.A. Depressive-like behaviors alterations induced by intranigral MPTP, 6-OHDA, LPS and rotenone models of Parkinson’s disease are predominantly associated with serotonin and dopamine. Prog. Neuropsychopharmacol. Biol. Psychiatry 2010, 34, 1104–1114. [Google Scholar] [CrossRef] [PubMed]
- McDowell, K.; Chesselet, M.F. Animal models of the non-motor features of Parkinson’s disease. Neurobiol. Dis. 2012, 46, 597–606. [Google Scholar] [CrossRef] [Green Version]
- Campos, F.; Carvalho, M.; Cristovão, A.; Je, G.; Baltazar, G.; Salgado, A.; Kim, Y.S.; Sousa, N. Rodent models of Parkinson’s disease: Beyond the motor symptomatology. Front. Behav. Neurosci. 2013, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magnard, R.; Vachez, Y.; Carcenac, C.; Krack, P.; David, O.; Savasta, M.; Boulet, S.; Carnicella, S. What can rodent models tell us about apathy and associated neuropsychiatric symptoms in Parkinson’s disease? Transl. Psychiatry 2016, 6, e753. [Google Scholar] [CrossRef]
- Carnicella, S.; Drui, G.; Boulet, S.; Carcenac, C.; Favier, M.; Duran, T.; Savasta, M. Implication of dopamine D3 receptor activation in the reversion of Parkinson’s disease-related motivational deficits. Transl. Psychiatry 2014, 4, e401. [Google Scholar] [CrossRef]
- Drui, G.; Carnicella, S.; Carcenac, C.; Favier, M.; Bertrand, A.; Boulet, S.; Savasta, M. Loss of dopaminergic nigrostriatal neurons accounts for the motivational and affective deficits in Parkinson’s disease. Mol. Psychiatry 2014, 19, 358–367. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Deltheil, T.; Turle-Lorenzo, N.; Liberge, M.; Rosier, C.; Watabe, I.; Sreng, L.; Amalric, M.; Mourre, C. SK channel blockade reverses cognitive and motor deficits induced by nigrostriatal dopamine lesions in rats. Int. J. Neuropsychopharmacol. 2014, 17, 1295–1306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deumens, R.; Blokland, A.; Prickaerts, J. Modeling Parkinson’s Disease in Rats: An Evaluation of 6-OHDA Lesions of the Nigrostriatal Pathway. Exp. Neurol. 2002, 175, 303–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fahn, S.; Poewe, W. Levodopa: 50 years of a revolutionary drug for Parkinson disease. Mov. Disord. 2015, 30, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Muzerengi, S.; Contrafatto, D.; Chaudhuri, K.R. Non-motor symptoms: Identification and management. Parkinsonism Relat. Disord. 2007, 13, S450–S456. [Google Scholar] [CrossRef]
- Scheperjans, F.; Aho, V.; Pereira, P.A.B.; Koskinen, K.; Paulin, L.; Pekkonen, E.; Haapaniemi, E.; Kaakkola, S.; Eerola-Rautio, J.; Pohja, M.; et al. Gut microbiota are related to Parkinson’s disease and clinical phenotype. Mov. Disord. 2015, 30, 350–358. [Google Scholar] [CrossRef]
- Gorecki, A.M.; Preskey, L.; Bakeberg, M.C.; Kenna, J.E.; Gildenhuys, C.; MacDougall, G.; Dunlop, S.A.; Mastaglia, F.L.; Akkari, P.A.; Koengten, F.; et al. Altered Gut Microbiome in Parkinson’s Disease and the Influence of Lipopolysaccharide in a Human α-Synuclein Over-Expressing Mouse Model. Front. Neurosci. 2019, 13, 839. [Google Scholar] [CrossRef] [Green Version]
- Pietrucci, D.; Cerroni, R.; Unida, V.; Farcomeni, A.; Pierantozzi, M.; Mercuri, N.B.; Biocca, S.; Stefani, A.; Desideri, A. Dysbiosis of gut microbiota in a selected population of Parkinson’s patients. Parkinsonism Relat. Disord. 2019, 65, 124–130. [Google Scholar] [CrossRef]
- Unger, M.M.; Spiegel, J.; Dillmann, K.U.; Grundmann, D.; Philippeit, H.; Bürmann, J.; Faßbender, K.; Schwiertz, A.; Schäfer, K.H. Short chain fatty acids and gut microbiota differ between patients with Parkinson’s disease and age-matched controls. Parkinsonism Relat. Disord. 2016, 32, 66–72. [Google Scholar] [CrossRef]
- Houser, M.C.; Chang, J.; Factor, S.A.; Molho, E.S.; Zabetian, C.P.; Hill-Burns, E.M.; Payami, H.; Hertzberg, V.S.; Tansey, M.G. Stool Immune Profiles Evince Gastrointestinal Inflammation in Parkinson’s Disease. Mov. Disord. 2018, 33, 793–804. [Google Scholar] [CrossRef]
- Forsyth, C.B.; Shannon, K.M.; Kordower, J.H.; Voigt, R.M.; Shaikh, M.; Jaglin, J.A.; Estes, J.D.; Dodiya, H.B.; Keshavarzian, A. Increased intestinal permeability correlates with sigmoid mucosa alpha-synuclein staining and endotoxin exposure markers in early Parkinson’s disease. PLoS ONE 2011, 6, e28032. [Google Scholar] [CrossRef] [Green Version]
- Aho, V.T.E.; Pereira, P.A.B.; Voutilainen, S.; Paulin, L.; Pekkonen, E.; Auvinen, P.; Scheperjans, F. Gut microbiota in Parkinson’s disease: Temporal stability and relations to disease progression. EBioMedicine 2019, 44, 691–707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castelli, V.; d’Angelo, M.; Lombardi, F.; Alfonsetti, M.; Antonosante, A.; Catanesi, M.; Benedetti, E.; Palumbo, P.; Cifone, M.G.; Giordano, A.; et al. Effects of the probiotic formulation SLAB51 in in vitro and in vivo Parkinson’s disease models. Aging 2020, 12, 4641–4659. [Google Scholar] [CrossRef]
- Heinzel, S.; Aho, V.T.E.; Suenkel, U.; von Thaler, A.K.; Schulte, C.; Deuschle, C.; Paulin, L.; Hantunen, S.; Brockmann, K.; Eschweiler, G.W.; et al. Gut Microbiome Signatures of Risk and Prodromal Markers of Parkinson Disease. Ann. Neurol. 2020, 88, 320–331. [Google Scholar] [CrossRef]
- Perez-Pardo, P.; Kliest, T.; Dodiya, H.B.; Broersen, L.M.; Garssen, J.; Keshavarzian, A.; Kraneveld, A.D. The gut-brain axis in Parkinson’s disease: Possibilities for food-based therapies. Eur. J. Pharmacol. 2017, 817, 86–95. [Google Scholar] [CrossRef]
- Cani, P.D. Human gut microbiome: Hopes, threats and promises. Gut 2018, 67, 1716–1725. [Google Scholar] [CrossRef] [PubMed]
- Bercik, P.; Park, A.J.; Sinclair, D.; Khoshdel, A.; Lu, J.; Huang, X.; Deng, Y.; Blennerhassett, P.A.; Fahnestock, M.; Moine, D.; et al. The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut–brain communication. Neurogastroenterol. Motil. 2011, 23, 1132–1139. [Google Scholar] [CrossRef] [Green Version]
- Ait-Belgnaoui, A.; Colom, A.; Braniste, V.; Ramalho, L.; Marrot, A.; Cartier, C.; Houdeau, E.; Theodorou, V.; Tompkins, T. Probiotic gut effect prevents the chronic psychological stress-induced brain activity abnormality in mice. Neurogastroenterol. Motil. 2014, 26, 510–520. [Google Scholar] [CrossRef] [PubMed]
- Davari, S.; Talaei, S.A.; Alaei, H.; Salami, M. Probiotics treatment improves diabetes-induced impairment of synaptic activity and cognitive function: Behavioral and electrophysiological proofs for microbiome–gut–brain axis. Neuroscience 2013, 240, 287–296. [Google Scholar] [CrossRef]
- Rezaei Asl, Z.; Sepehri, G.; Salami, M. Probiotic treatment improves the impaired spatial cognitive performance and restores synaptic plasticity in an animal model of Alzheimer’s disease. Behav. Brain Res. 2019, 376, 112183. [Google Scholar] [CrossRef]
- Akbari, E.; Asemi, Z.; Daneshvar Kakhaki, R.; Bahmani, F.; Kouchaki, E.; Tamtaji, O.R.; Hamidi, G.A.; Salami, M. Effect of Probiotic Supplementation on Cognitive Function and Metabolic Status in Alzheimer’s Disease: A Randomized, Double-Blind and Controlled Trial. Front. Aging Neurosci. 2016, 8, 256. [Google Scholar] [CrossRef] [Green Version]
- Bonfili, L.; Cecarini, V.; Berardi, S.; Scarpona, S.; Suchodolski, J.S.; Nasuti, C.; Fiorini, D.; Boarelli, M.C.; Rossi, G.; Eleuteri, A.M. Microbiota modulation counteracts Alzheimer’s disease progression influencing neuronal proteolysis and gut hormones plasma levels. Sci. Rep. 2017, 7, 2426. [Google Scholar] [CrossRef]
- Cowan, C.S.; Callaghan, B.L.; Richardson, R. The effects of a probiotic formulation (Lactobacillus rhamnosus and L. helveticus) on developmental trajectories of emotional learning in stressed infant rats. Transl. Psychiatry 2016, 6, e823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walf, A.A.; Frye, C.A. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat. Protoc. 2007, 2, 322–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beilharz, J.E.; Maniam, J.; Morris, M.J. Short exposure to a diet rich in both fat and sugar or sugar alone impairs place, but not object recognition memory in rats. Brain Behav. Immun. 2014, 37, 134–141. [Google Scholar] [CrossRef]
- Westbrook, S.R.; Brennan, L.E.; Stanton, M.E. Ontogeny of object versus location recognition in the rat: Acquisition and retention effects. Dev. Psychobiol. 2014, 56, 1492–1506. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.A.; McNally, G.P. Ventral Pallidum Output Pathways in Context-Induced Reinstatement of Alcohol Seeking. J. Neurosci. 2016, 36, 11716–11726. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates; Academic: Sydney, Australia, 2009. [Google Scholar]
- Stylianakis, A.A.; Harmon-Jones, S.K.; Richardson, R.; Baker, K.D. Differences in the persistence of spatial memory deficits induced by a chronic stressor in adolescents compared to juveniles. Dev. Psychobiol. 2018, 60, 805–813. [Google Scholar] [CrossRef]
- Pellow, S.; Chopin, P.; File, S.E.; Briley, M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J. Neurosci. Methods 1985, 14, 149–167. [Google Scholar] [CrossRef]
- Matheus, F.C.; Rial, D.; Real, J.I.; Lemos, C.; Takahashi, R.N.; Bertoglio, L.J.; Cunha, R.A.; Prediger, R.D. Temporal Dissociation of Striatum and Prefrontal Cortex Uncouples Anhedonia and Defense Behaviors Relevant to Depression in 6-OHDA-Lesioned Rats. Mol. Neurobiol. 2016, 53, 3891–3899. [Google Scholar] [CrossRef]
- Bubser, M.; Schmidt, W.J. 6-Hydroxydopamine lesion of the rat prefrontal cortex increases locomotor activity, impairs acquisition of delayed alternation tasks, but does not affect uninterrupted tasks in the radial maze. Behav. Brain Res. 1990, 37, 157–168. [Google Scholar] [CrossRef]
- Kulisevsky, J.; Pascual-Sedano, B.; Barbanoj, M.; Gironell, A.; Pagonabarraga, J.; García-Sánchez, C. Acute effects of immediate and controlled-release levodopa on mood in Parkinson’s disease: A double-blind study. Mov. Disord. 2007, 22, 62–67. [Google Scholar] [CrossRef]
- Carola, V.; D’Olimpio, F.; Brunamonti, E.; Mangia, F.; Renzi, P. Evaluation of the elevated plus-maze and open-field tests for the assessment of anxiety-related behaviour in inbred mice. Behav. Brain Res. 2002, 134, 49–57. [Google Scholar] [CrossRef]
- Holzmann, C.; Dräger, D.; Mix, E.; Hawlitschka, A.; Antipova, V.; Benecke, R.; Wree, A. Effects of intrastriatal botulinum neurotoxin A on the behavior of Wistar rats. Behav. Brain Res. 2012, 234, 107–116. [Google Scholar] [CrossRef]
- Choleris, E.; Thomas, A.W.; Kavaliers, M.; Prato, F.S. A detailed ethological analysis of the mouse open field test: Effects of diazepam, chlordiazepoxide and an extremely low frequency pulsed magnetic field. Neurosci. Biobehav. Rev. 2001, 25, 235–260. [Google Scholar] [CrossRef]
- Seibenhener, M.L.; Wooten, M.C. Use of the Open Field Maze to measure locomotor and anxiety-like behavior in mice. J. Vis. Exp. 2015, 96, e52434. [Google Scholar] [CrossRef] [Green Version]
- Au-Nunez, J. Morris Water Maze Experiment. J. Vis. Exp. 2008, 19, 897. [Google Scholar] [CrossRef] [Green Version]
- Barker, G.R.I.; Warburton, E.C. When Is the Hippocampus Involved in Recognition Memory? J. Neurosci. 2011, 31, 10721–10731. [Google Scholar] [CrossRef] [Green Version]
- Thurm, F.; Schuck, N.W.; Fauser, M.; Doeller, C.F.; Stankevich, Y.; Evens, R.; Riedel, O.; Storch, A.; Lueken, U.; Li, S.C. Dopamine modulation of spatial navigation memory in Parkinson’s disease. Neurobiol. Aging 2016, 38, 93–103. [Google Scholar] [CrossRef]
- Takeuchi, T.; Duszkiewicz, A.J.; Sonneborn, A.; Spooner, P.A.; Yamasaki, M.; Watanabe, M.; Smith, C.C.; Fernández, G.; Deisseroth, K.; Greene, R.W.; et al. Locus coeruleus and dopaminergic consolidation of everyday memory. Nature 2016, 537, 357–362. [Google Scholar] [CrossRef]
- Bradley, V.A.; Welch, J.L.; Dick, D.J. Visuospatial working memory in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 1989, 52, 1228–1235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Postle, B.R.; Jonides, J.; Smith, E.E.; Corkin, S.; Growdon, J.H. Spatial, but not object, delayed response is impaired in early Parkinson’s disease. Neuropsychology 1997, 11, 171–179. [Google Scholar] [CrossRef]
- Stav, A.L.; Johansen, K.K.; Auning, E.; Kalheim, L.F.; Selnes, P.; Bjørnerud, A.; Hessen, E.; Aarsland, D.; Fladby, T. Hippocampal subfield atrophy in relation to cerebrospinal fluid biomarkers and cognition in early Parkinson’s disease: A cross-sectional study. NPJ Parkinson’s Dis. 2016, 2, 15030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osadchiy, V.; Martin, C.R.; Mayer, E.A. The Gut–Brain Axis and the Microbiome: Mechanisms and Clinical Implications. Clin. Gastroenterol. Hepatol. 2019, 17, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Plaza-Diaz, J.; Ruiz-Ojeda, F.J.; Gil-Campos, M.; Gil, A. Mechanisms of Action of Probiotics. Adv. Nutr. 2019, 10, S49–S66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pearson-Leary, J.; Zhao, C.; Bittinger, K.; Eacret, D.; Luz, S.; Vigderman, A.S.; Dayanim, G.; Bhatnagar, S. The gut microbiome regulates the increases in depressive-type behaviors and in inflammatory processes in the ventral hippocampus of stress vulnerable rats. Mol. Psychiatry 2020, 25, 1068–1079. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, C.; Prasad, A.A. Probiotics Treatment Improves Hippocampal Dependent Cognition in a Rodent Model of Parkinson’s Disease. Microorganisms 2020, 8, 1661. https://doi.org/10.3390/microorganisms8111661
Xie C, Prasad AA. Probiotics Treatment Improves Hippocampal Dependent Cognition in a Rodent Model of Parkinson’s Disease. Microorganisms. 2020; 8(11):1661. https://doi.org/10.3390/microorganisms8111661
Chicago/Turabian StyleXie, Caroline, and Asheeta A. Prasad. 2020. "Probiotics Treatment Improves Hippocampal Dependent Cognition in a Rodent Model of Parkinson’s Disease" Microorganisms 8, no. 11: 1661. https://doi.org/10.3390/microorganisms8111661
APA StyleXie, C., & Prasad, A. A. (2020). Probiotics Treatment Improves Hippocampal Dependent Cognition in a Rodent Model of Parkinson’s Disease. Microorganisms, 8(11), 1661. https://doi.org/10.3390/microorganisms8111661




