Non-Invasive Luciferase Imaging of Type I Interferon Induction in a Transgenic Mouse Model of Biomaterial Associated Bacterial Infections: Microbial Specificity and Inter-Bacterial Species Interactions
Abstract
1. Introduction
2. Materials and Methods
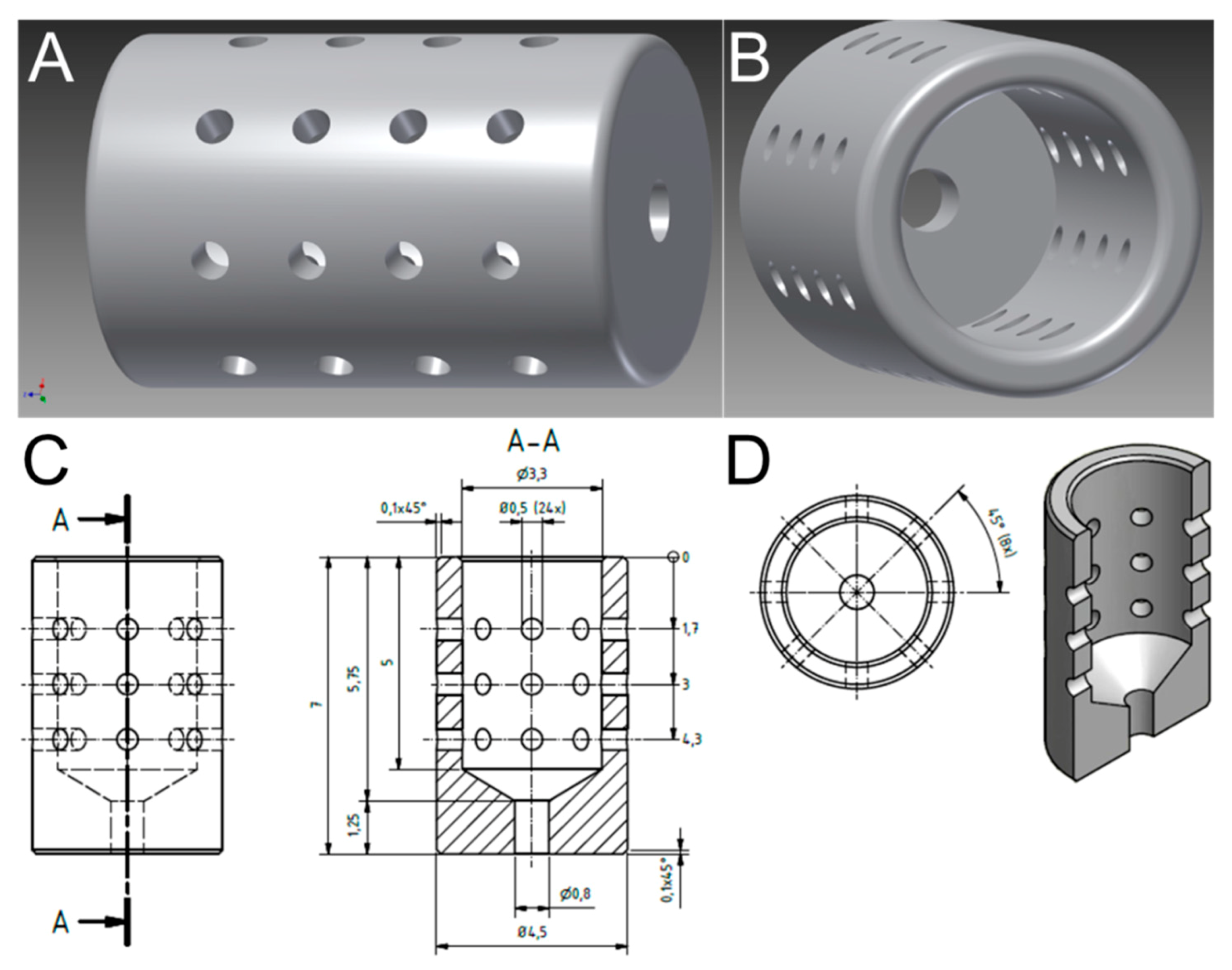
2.1. Preparation of Implants
2.2. Culture of Bacteria
2.3. In Vivo Implantation and Infection Procedures
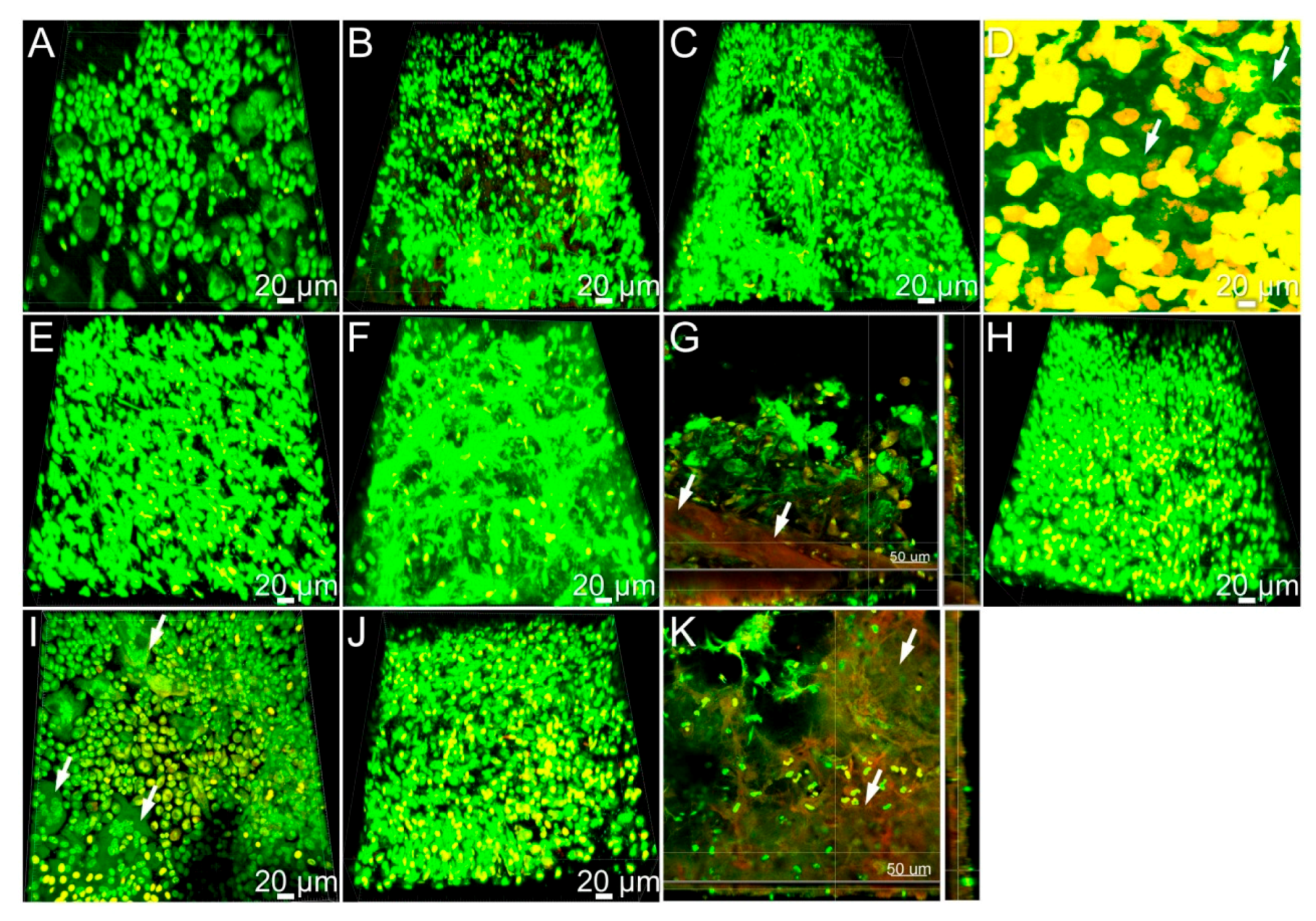
2.4. Histology and Biofilm Staining
2.5. Statistical Analysis
3. Results
3.1. Optimized Morphological Modifications Dedicated in Implant Design to Promote Prolonged and Reproducible Implant-Associated Infections
3.2. Biomaterial-Associated Infections Induce IFN-β Expression
3.3. Dual Species Bacterial Infections Decrease IFN-β Induction
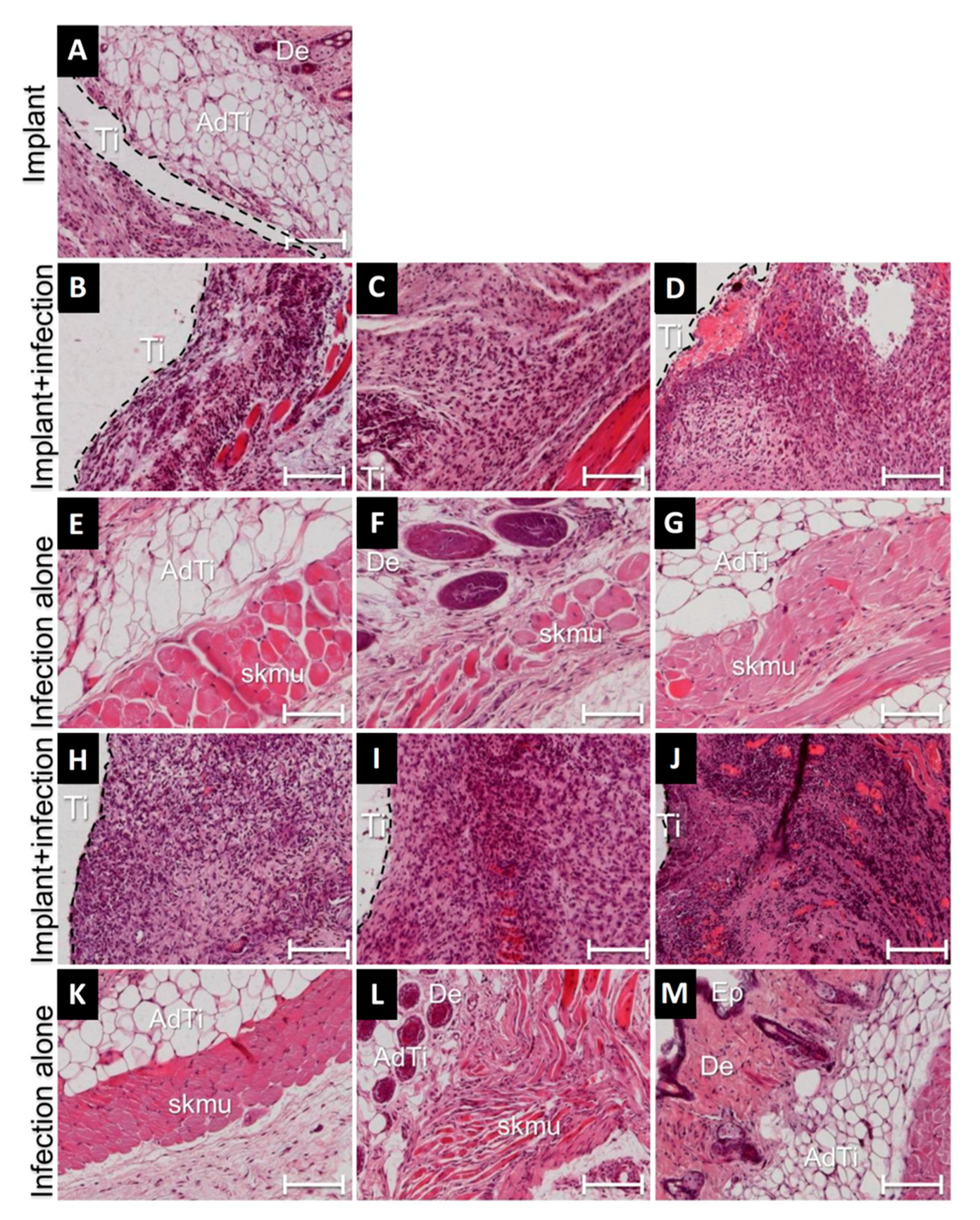
3.4. Cellular Responses during Implant-Related Infections
3.5. Tissue Implant Interfaces of Infected Implants Were Indicative of High Recruitment of Immune Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jinnouchi, H.; Torii, S.; Sakamoto, A.; Kolodgie, F.D.; Virmani, R.; Finn, A.V. Fully bioresorbable vascular scaffolds: Lessons learned and future directions. Nat. Rev. Cardiol. 2019, 16, 286–304. [Google Scholar] [CrossRef]
- Lee, P.C.; Dixon, J. Medical devices for the treatment of obesity. Nat. Rev. Gastroenterol. Hepatol. Amp. 2017, 14, 553. [Google Scholar] [CrossRef] [PubMed]
- Arciola, C.R.; Campoccia, D.; Montanaro, L. Implant infections: Adhesion, biofilm formation and immune evasion. Nat. Rev. Microbiol. 2018, 16, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Piattelli, A.; Cosci, F.; Scarano, A.; Trisi, P. Localized chronic suppurative bone infection as a sequel of peri-implantitis in a hydroxyapatite-coated dental implant. Biomaterials 1995, 16, 917–920. [Google Scholar] [CrossRef]
- Busscher, H.J.; Rinastiti, M.; Siswomihardjo, W.; Van Der Mei, H.C. Biofilm Formation on Dental Restorative and Implant Materials. J. Dent. Res. 2010, 89, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Elter, C.; Heuer, W.; Demling, A.P.; Hannig, M.; Heidenblut, T.; Stiesch, M. Comparative analysis of biofilm formation on dental implant abutments with respect to supra- and subgingival areas: Polytetrafluoroethylene versus titanium. Int. J. Prosthodont. 2011, 24, 373–375. [Google Scholar] [PubMed]
- Szafrański, S.P.; Kilian, M.; Yang, I.; Bei Der Wieden, G.; Winkel, A.; Hegermann, J.; Stiesch, M. Diversity patterns of bacteriophages infecting Aggregatibacter and Haemophilus species across clades and niches. ISME J. 2019, 13, 2500–2522. [Google Scholar] [CrossRef]
- Moutsopoulos, N.M.; Konkel, J.E. Tissue-Specific Immunity at the Oral Mucosal Barrier. Trends Immunol. 2018, 39, 276–287. [Google Scholar] [CrossRef]
- Lamont, R.J.; Koo, H.; Hajishengallis, G. The oral microbiota: Dynamic communities and host interactions. Nat. Rev. Microbiol. 2018, 16, 745–759. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Lamont, R.J. Dancing with the Stars: How Choreographed Bacterial Interactions Dictate Nososymbiocity and Give Rise to Keystone Pathogens, Accessory Pathogens, and Pathobionts. Trends Microbiol. 2016, 24, 477–489. [Google Scholar] [CrossRef]
- Hajishengallis, G. Periodontitis: From microbial immune subversion to systemic inflammation. Nat. Rev. Immunol. 2014, 15, 30. [Google Scholar] [CrossRef] [PubMed]
- Bowen, W.H.; Burne, R.A.; Wu, H.; Koo, H. Oral Biofilms: Pathogens, Matrix, and Polymicrobial Interactions in Microenvironments. Trends Microbiol. 2018, 26, 229–242. [Google Scholar] [CrossRef] [PubMed]
- Olsen, I.; Lambris, J.D.; Hajishengallis, G. Porphyromonas gingivalis disturbs host–commensal homeostasis by changing complement function. J. Oral Microbiol. 2017, 9, 1340085. [Google Scholar] [CrossRef]
- Abranches, J.; Zeng, L.; Kajfasz, J.K.; Palmer, S.R.; Chakraborty, B.; Wen, Z.T.; Richards, V.P.; Brady, L.J.; Lemos, J.A. Biology of Oral Streptococci. Microbiol. Spectr. 2018, 6. [Google Scholar] [CrossRef] [PubMed]
- Kolenbrander, P.E.; Palmer, R.J.; Periasamy, S.; Jakubovics, N.S. Oral multispecies biofilm development and the key role of cell–cell distance. Nat. Rev. Microbiol. 2010, 8, 471–480. [Google Scholar] [CrossRef]
- Pye, A.; Lockhart, D.; Dawson, M.; Murray, C.; Smith, A. A review of dental implants and infection. J. Hosp. Infect. 2009, 72, 104–110. [Google Scholar] [CrossRef]
- Giaouris, E.; Eheir, E.; Edesvaux, M.; Ehébraud, M.; Emøretrø, T.; Elangsrud, S.; Edoulgeraki, A.; Enychas, G.-J.; eKačániová, M.; Eczaczyk, K.; et al. Intra- and inter-species interactions within biofilms of important foodborne bacterial pathogens. Front. Microbiol. 2015, 6, 841. [Google Scholar] [CrossRef]
- Silva, N.; Abusleme, L.; Bravo, D.; Dutzan, N.; Garcia-Sesnich, J.; Vernal, R.; Hernández, M.; Gamonal, J. Host response mechanisms in periodontal diseases. J. Appl. Oral Sci. 2015, 23, 329–355. [Google Scholar] [CrossRef]
- Lamont, R.J.; Hajishengallis, G. Polymicrobial synergy and dysbiosis in inflammatory disease. Trends Mol. Med. 2015, 21, 172–183. [Google Scholar] [CrossRef]
- Mandakhalikar, K.D.; Rahmat, J.N.; Chiong, E.; Neoh, K.G.; Shen, L.; Tambyah, P.A. Extraction and quantification of biofilm bacteria: Method optimized for urinary catheters. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef]
- Mezzanotte, L.; Root, M.V.T.; Karatas, H.; Goun, E.A.; Löwik, C.W. In Vivo Molecular Bioluminescence Imaging: New Tools and Applications. Trends Biotechnol. 2017, 35, 640–652. [Google Scholar] [CrossRef] [PubMed]
- Rahim, M.I.; Babbar, A.; Lienenklaus, S.; Pils, M.C.; Rohde, M. Degradable magnesium implant-associated infections by bacterial biofilms induce robust localized and systemic inflammatory reactions in a mouse model. Biomed. Mater. 2017, 12, 055006. [Google Scholar] [CrossRef] [PubMed]
- Rahim, M.I.; Rohde, M.; Rais, B.; Seitz, J.-M.; Mueller, P.P. Susceptibility of metallic magnesium implants to bacterial biofilm infections. J. Biomed. Mater. Res. Part A 2016, 104, 1489–1499. [Google Scholar] [CrossRef] [PubMed]
- Rahim, M.I.; Szafrański, S.P.; Ingendoh-Tsakmakidis, A.; Stiesch, M.; Mueller, P.P. Evidence for inoculum size and gas interfaces as critical factors in bacterial biofilm formation on magnesium implants in an animal model. Colloids Surf. B Biointerfaces 2020, 186, 110684. [Google Scholar] [CrossRef]
- Hsieh, C.-L.; Xie, Z.; Liu, Z.-Y.; E Green, J.; Martin, W.D.; Datta, M.W.; Yeung, F.; Pan, D.; Chung, L.W.K. A luciferase transgenic mouse model: Visualization of prostate development and its androgen responsiveness in live animals. J. Mol. Endocrinol. 2005, 35, 293–304. [Google Scholar] [CrossRef]
- Sansonetti, P.J.; Di Santo, J.P. Debugging how Bacteria Manipulate the Immune Response. Immunity 2007, 26, 149–161. [Google Scholar] [CrossRef]
- González-Navajas, J.M.; Lee, J.; David, M.; Raz, E. Immunomodulatory functions of type I interferons. Nat. Rev. Immunol. 2012, 12, 125–135. [Google Scholar] [CrossRef]
- Mizraji, G.; Nassar, M.; Segev, H.; Sharawi, H.; Eli-Berchoer, L.; Capucha, T.; Nir, T.; Tabib, Y.; Maimon, A.; Dishon, S.; et al. Porphyromonas gingivalis Promotes Unrestrained Type I Interferon Production by Dysregulating TAM Signaling via MYD88 Degradation. Cell Rep. 2017, 18, 419–431. [Google Scholar] [CrossRef]
- Wright, H.J.; Matthews, J.B.; Chapple, I.L.; Ling-Mountford, N.; Cooper, P.R. Periodontitis associates with a type 1 IFN signature in peripheral blood neutrophils. J. Immunol. 2008, 181, 5775–5784. [Google Scholar] [CrossRef]
- Henderson, B.; Ward, J.M.; Ready, D. Aggregatibacter (Actinobacillus) actinomycetemcomitans: A triple A* periodontopathogen? Periodontology 2000 2010, 54, 78–105. [Google Scholar] [CrossRef]
- Huang, N.; Gibson, F.C. Immuno-Pathogenesis of Periodontal Disease: Current and Emerging Paradigms. Curr. Oral Health Rep. 2014, 1, 124–132. [Google Scholar] [CrossRef] [PubMed]
- You, M.; Chan, Y.; Lacap-Bugler, D.; Huo, Y.-B.; Gao, W.; Leung, W.K.; Watt, R.M. Oral Treponeme Major Surface Protein: Sequence Diversity and Distributions within Periodontal Niches. Mol. Oral Microbiol. 2017, 32, 455–474. [Google Scholar] [CrossRef] [PubMed]
- Ehow, K.Y.; Esong, K.P.; Echan, K.G. Porphyromonas gingivalis: An Overview of Periodontopathic Pathogen below the Gum Line. Front. Microbiol. 2016, 7, 53. [Google Scholar] [CrossRef]
- Heuer, W.; Kettenring, A.; Stumpp, S.N.; Eberhard, J.; Gellermann, E.; Winkel, A.; Stiesch, M. Metagenomic analysis of the peri-implant and periodontal microflora in patients with clinical signs of gingivitis or mucositis. Clin. Oral Investig. 2012, 16, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Williams, D. Implants in dental and maxillofacial surgery. Biomaterials 1981, 2, 133–146. [Google Scholar] [CrossRef]
- Kommerein, N.; Stumpp, S.N.; Musken, M.; Ehlert, N.; Winkel, A.; Häussler, S.; Behrens, P.; Buettner, F.F.R.; Stiesch, M. An oral multispecies biofilm model for high content screening applications. PLoS ONE 2017, 12, e0173973. [Google Scholar] [CrossRef]
- Chan, E.C.S.; Siboo, R.; Keng, T.; Psarra, N.; Hurley, R.; Cheng, S.-L.; Iugovaz, I. Treponema denticola (ex Brumpt 1925) sp. nov., nom. rev., and Identification of New Spirochete Isolates from Periodontal Pockets. Int. J. Syst. Evol. Microbiol. 1993, 43, 196–203. [Google Scholar] [CrossRef]
- Zheng, J.; Xu, L.; Zhou, H.; Zhang, W.; Chen, Z. Quantitative analysis of cell tracing by in vivo imaging system. J. Huazhong Univ. Sci. Tech. Med. Sci. 2010, 30, 541–545. [Google Scholar] [CrossRef]
- Kommerein, N.; Doll, K.; Stumpp, N.S.; Stiesch, M. Development and characterization of an oral multispecies biofilm implant flow chamber model. PLoS ONE 2018, 13, e0196967. [Google Scholar] [CrossRef]
- Diekmann, J.; Bauer, S.; Weizbauer, A.; Willbold, E.; Windhagen, H.; Helmecke, P.; Lucas, A.; Reifenrath, J.; Nolte, I.; Ezechieli, M. Examination of a biodegradable magnesium screw for the reconstruction of the anterior cruciate ligament: A pilot in vivo study in rabbits. Mater. Sci. Eng. C 2016, 59, 1100–1109. [Google Scholar] [CrossRef]
- Svensson, S.; Trobos, M.; Hoffman, M.; Norlindh, B.; Petronis, S.; Lausmaa, J.; Suska, F.; Thomsen, P. A novel soft tissue model for biomaterial-associated infection and inflammation—Bacteriological, morphological and molecular observations. Biomaterials 2015, 41, 106–121. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T.; Jemt, T.; Mölne, J.; Tengvall, P.; Wennerberg, A. On inflammation-immunological balance theory-A critical apprehension of disease concepts around implants: Mucositis and marginal bone loss may represent normal conditions and not necessarily a state of disease. Clin. Implant. Dent. Relat. Res. 2019, 21, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhou, Y.; Jiang, T.; Liang, Y.-D.; Zhang, Z.; Wang, Y.-N. Evaluation of the osseointegration of dental implants coated with calcium carbonate: An animal study. Int. J. Oral Sci. 2017, 9, 133–138. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Croes, M.; Bakhshandeh, S.; Van Hengel, I.; Lietaert, K.; Van Kessel, K.; Pouran, B.; Van Der Wal, B.; Vogely, H.; Van Hecke, W.; Fluit, A.; et al. Antibacterial and immunogenic behavior of silver coatings on additively manufactured porous titanium. Acta Biomater. 2018, 81, 315–327. [Google Scholar] [CrossRef]
- DeMuth, D.R.; Irvine, D.C.; Costerton, J.W.; Cook, G.S.; Lamont, R.J. Discrete Protein Determinant Directs the Species-Specific Adherence of Porphyromonas gingivalis to Oral Streptococci. Infect. Immun. 2001, 69, 5736–5741. [Google Scholar] [CrossRef][Green Version]
- Maeda, K.; Nagata, H.; Ojima, M.; Amano, A. Proteomic and Transcriptional Analysis of Interaction between Oral Microbiota Porphyromonas gingivalis and Streptococcus oralis. J. Proteome Res. 2015, 14, 82–94. [Google Scholar] [CrossRef]
- Paju, S.; Pussinen, P.J.; Suominen-Taipale, L.; Hyvönen, M.; Knuuttila, M.; Könönen, E. Detection of Multiple Pathogenic Species in Saliva Is Associated with Periodontal Infection in Adults. J. Clin. Microbiol. 2009, 47, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Daghighi, S.; Sjollema, J.; Jaspers, V.; De Boer, L.; Zaat, S.A.J.; Dijkstra, R.J.B.; Van Dam, G.M.; Van Der Mei, H.C.; Busscher, H.J. Persistence of a bioluminescent Staphylococcus aureus strain on and around degradable and non-degradable surgical meshes in a murine model. Acta Biomater. 2012, 8, 3991–3996. [Google Scholar] [CrossRef]
- Rais, B.; Köster, M.; Rahim, M.I.; Pils, M.; Seitz, J.; Hauser, H.; Wirth, D.; Mueller, P.P. Evaluation of the inflammatory potential of implant materials in a mouse model by bioluminescent imaging of intravenously injected bone marrow cells. J. Biomed. Mater. Res. Part A 2016, 104, 2149–2158. [Google Scholar] [CrossRef]
- Kesavalu, L.; Holt, S.C.; Ebersole, J.L. Virulence of a polymicrobic complex, Treponema denticola and Porphyromonas gingivalis, in a murine model. Oral Microbiol. Immunol. 1998, 13, 373–377. [Google Scholar] [CrossRef]
- Polak, D.; Shapira, L.; Weiss, E.I.; Houri-Haddad, Y. The role of coaggregation between Porphyromonas gingivalis and Fusobacterium nucleatum on the host response to mixed infection. J. Clin. Periodontol. 2012, 39, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Feuille, F.; Ebersole, J.L.; Kesavalu, L.; Stepfen, M.J.; Holt, S.C. Mixed infection with Porphyromonas gingivalis and Fusobacterium nucleatum in a murine lesion model: Potential synergistic effects on virulence. Infect. Immun. 1996, 64, 2094–2100. [Google Scholar] [CrossRef] [PubMed]
- Ebersole, J.L.; Feuille, F.; Kesavalu, L.; Holt, S.C. Host modulation of tissue destruction caused by periodontopathogens: Effects on a mixed microbial infection composed of Porphyromonas gingivalisand Fusobacterium nucleatum. Microb. Pathog. 1997, 23, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Polak, D.; Wilensky, A.; Shapira, L.; Halabi, A.; Goldstein, D.; Weiss, E.I.; Houri-Haddad, Y. Mouse model of experimental periodontitis induced by Porphyromonas gingivalis/Fusobacterium nucleatum infection: Bone loss and host response. J. Clin. Periodontol. 2009, 36, 406–410. [Google Scholar] [CrossRef] [PubMed]
- Kesavalu, L.; Sathishkumar, S.; Bakthavatchalu, V.; Matthews, C.; Dawson, D.; Steffen, M.; Ebersole, J.L. Rat Model of Polymicrobial Infection, Immunity, and Alveolar Bone Resorption in Periodontal Disease. Infect. Immun. 2007, 75, 1704–1712. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahim, M.I.; Winkel, A.; Lienenklaus, S.; Stumpp, N.S.; Szafrański, S.P.; Kommerein, N.; Willbold, E.; Reifenrath, J.; Mueller, P.P.; Eisenburger, M.; et al. Non-Invasive Luciferase Imaging of Type I Interferon Induction in a Transgenic Mouse Model of Biomaterial Associated Bacterial Infections: Microbial Specificity and Inter-Bacterial Species Interactions. Microorganisms 2020, 8, 1624. https://doi.org/10.3390/microorganisms8101624
Rahim MI, Winkel A, Lienenklaus S, Stumpp NS, Szafrański SP, Kommerein N, Willbold E, Reifenrath J, Mueller PP, Eisenburger M, et al. Non-Invasive Luciferase Imaging of Type I Interferon Induction in a Transgenic Mouse Model of Biomaterial Associated Bacterial Infections: Microbial Specificity and Inter-Bacterial Species Interactions. Microorganisms. 2020; 8(10):1624. https://doi.org/10.3390/microorganisms8101624
Chicago/Turabian StyleRahim, Muhammad Imran, Andreas Winkel, Stefan Lienenklaus, Nico S. Stumpp, Szymon P. Szafrański, Nadine Kommerein, Elmar Willbold, Janin Reifenrath, Peter P. Mueller, Michael Eisenburger, and et al. 2020. "Non-Invasive Luciferase Imaging of Type I Interferon Induction in a Transgenic Mouse Model of Biomaterial Associated Bacterial Infections: Microbial Specificity and Inter-Bacterial Species Interactions" Microorganisms 8, no. 10: 1624. https://doi.org/10.3390/microorganisms8101624
APA StyleRahim, M. I., Winkel, A., Lienenklaus, S., Stumpp, N. S., Szafrański, S. P., Kommerein, N., Willbold, E., Reifenrath, J., Mueller, P. P., Eisenburger, M., & Stiesch, M. (2020). Non-Invasive Luciferase Imaging of Type I Interferon Induction in a Transgenic Mouse Model of Biomaterial Associated Bacterial Infections: Microbial Specificity and Inter-Bacterial Species Interactions. Microorganisms, 8(10), 1624. https://doi.org/10.3390/microorganisms8101624





