Mardivirus Infection and Persistence in Feathers of a Chicken Model Harboring a Local Autoimmune Response
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Chickens and In Vivo Study Design
2.2. Vitiligo Scoring and Feather Sampling
2.3. DNA Preparation and Absolute Quantitation of HVT Load by qPCR in Growing Feathers and Spleens
2.4. HVT Vaccine Uptake by NDV Antibody Titration
2.5. Statistical Analysis
3. Results
3.1. Vitiligo Development Over Time and Vaccine Uptake
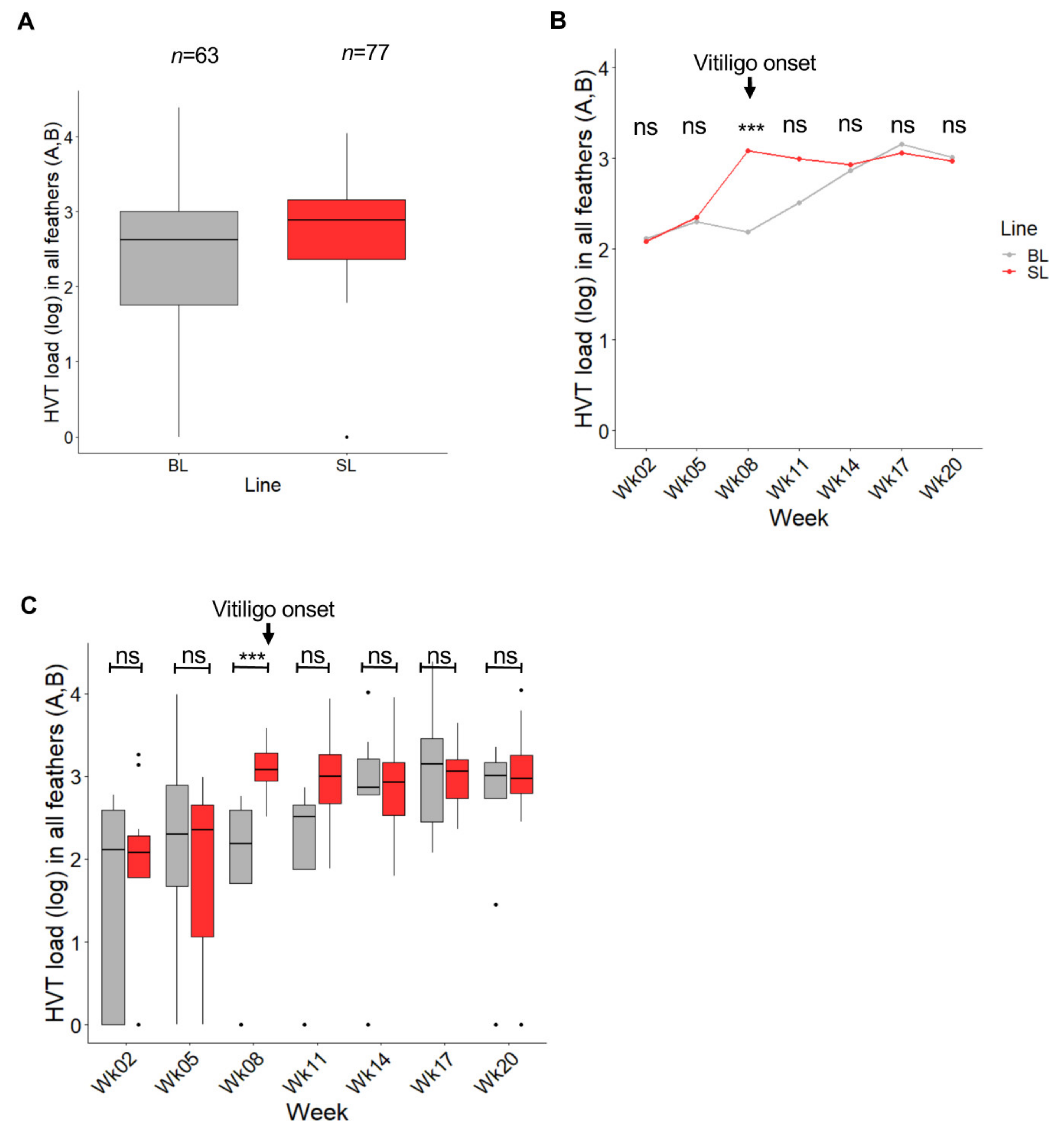
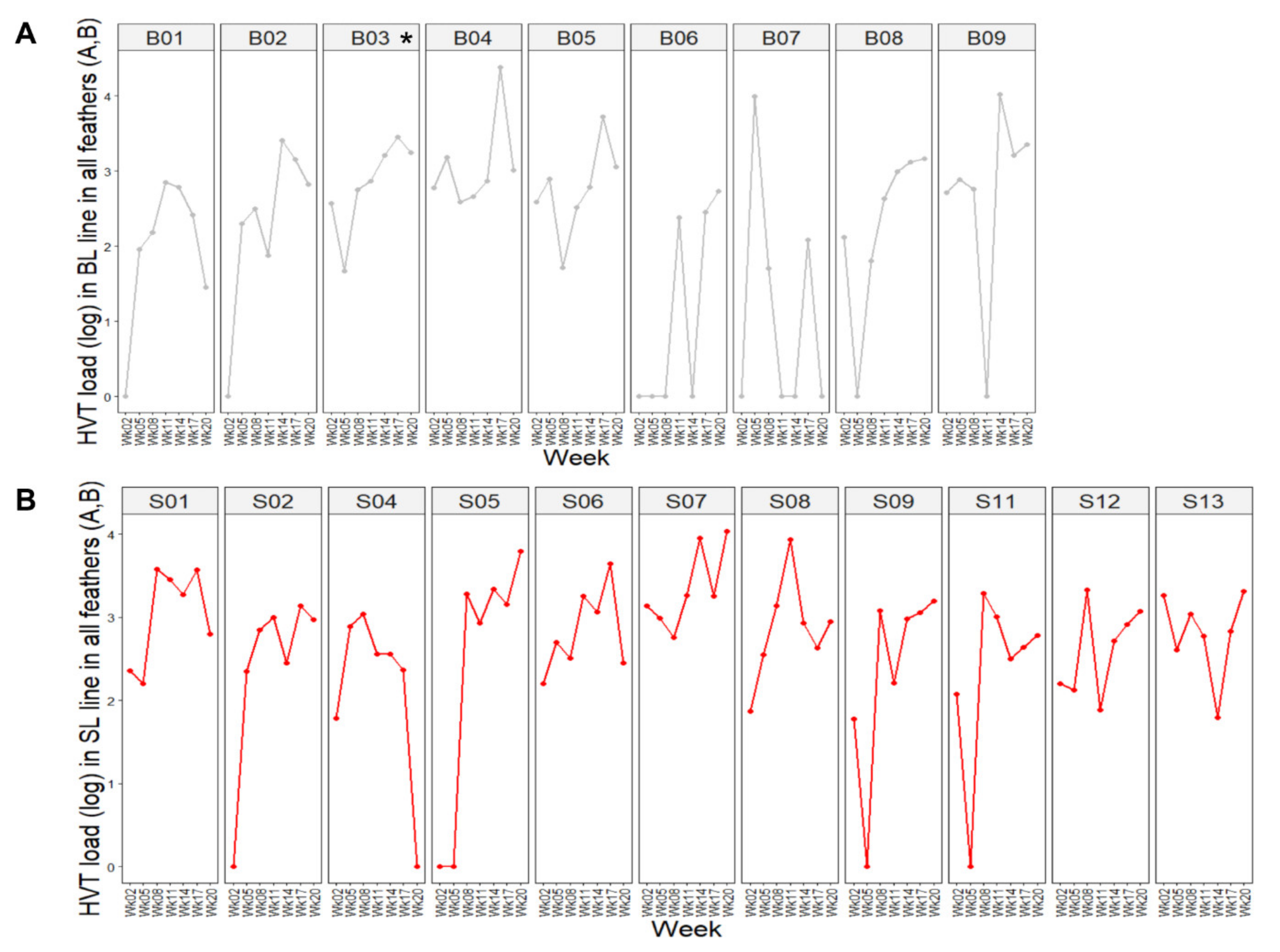
3.2. HVT Infection and Load in Feathers Collected from SL Compared to BL Chickens
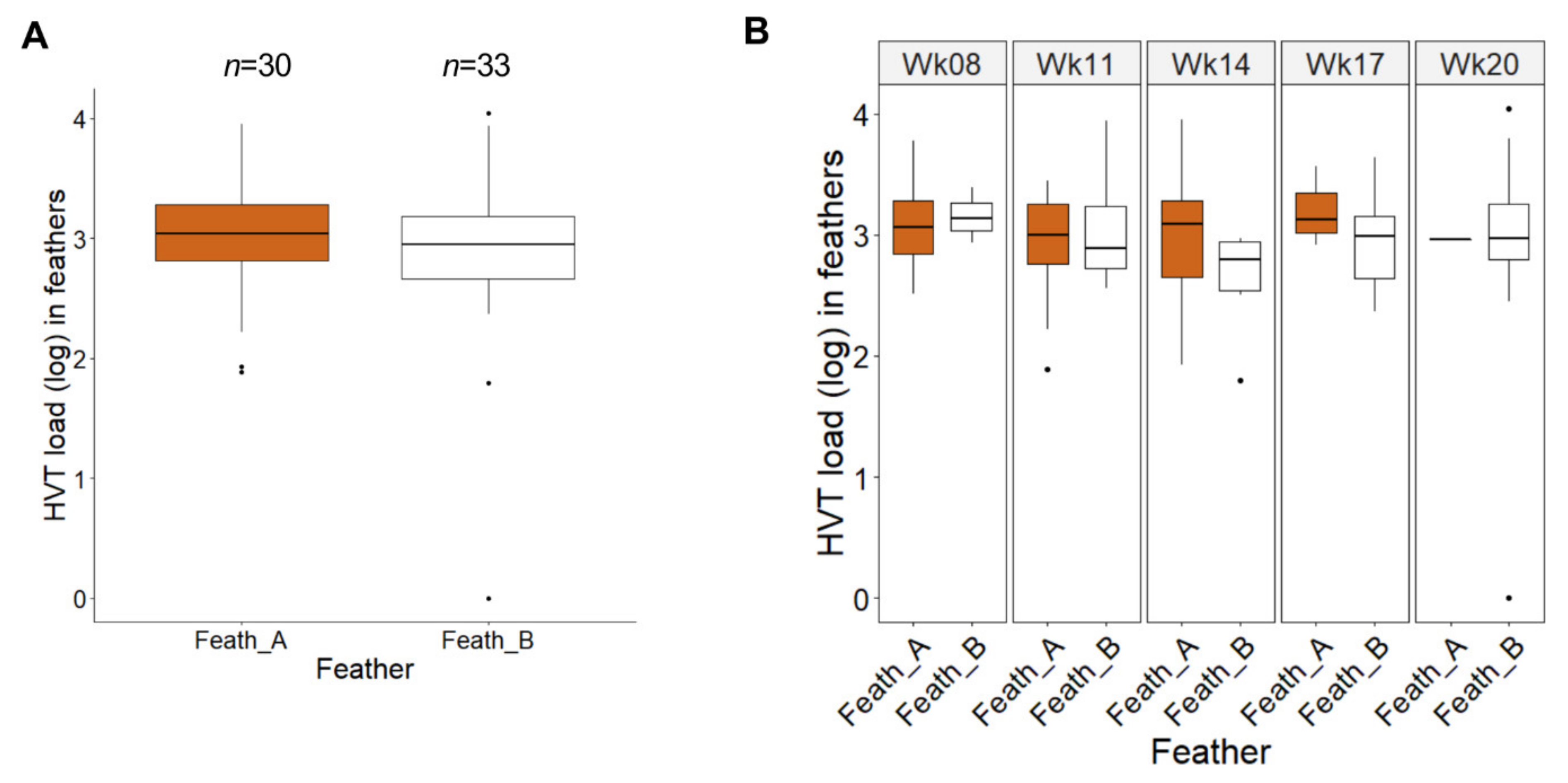
3.3. Comparison of HVT Loads in Pigmented and Non-Pigmented Feathers from SL Chickens
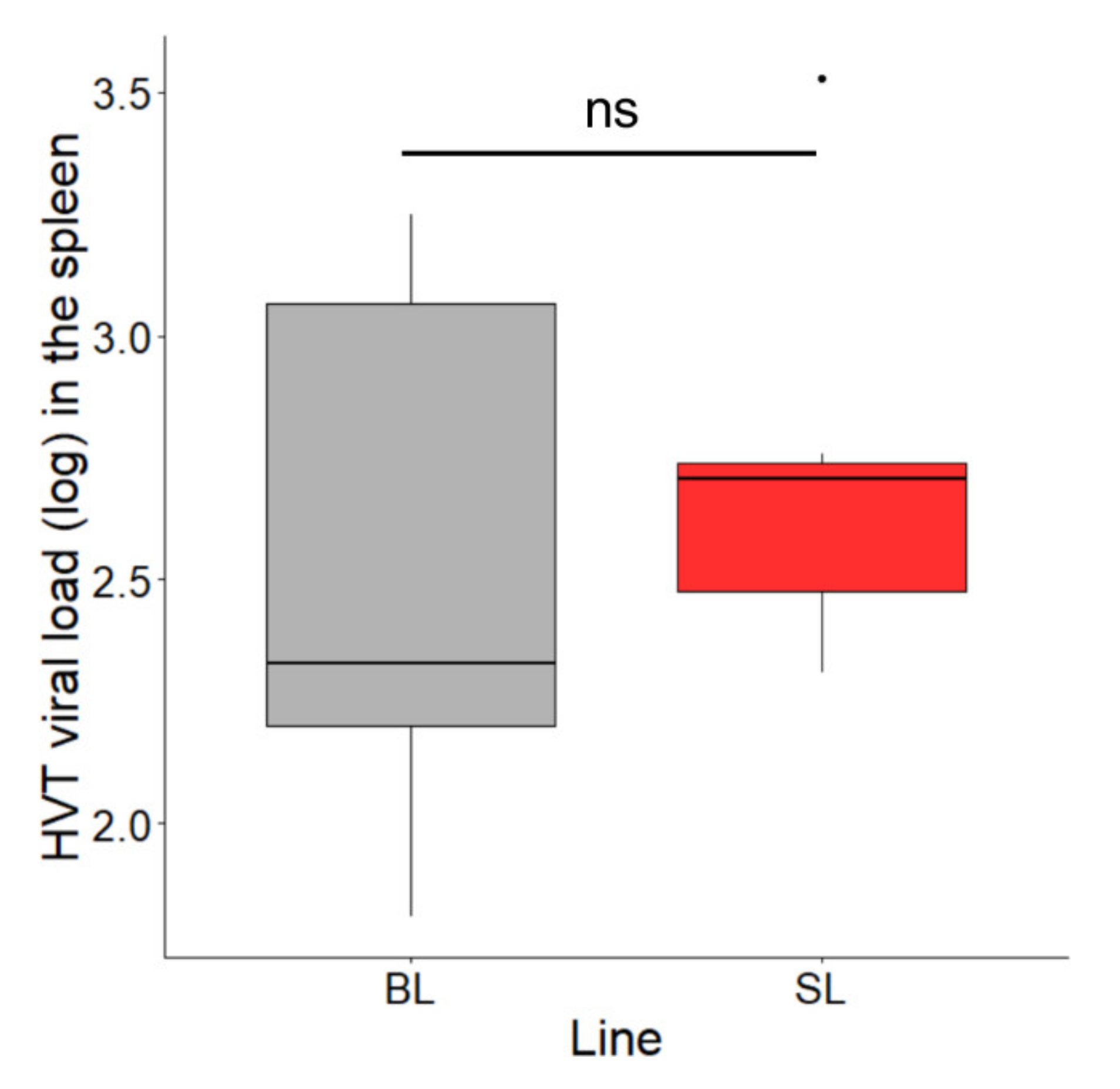
3.4. HVT Persistence in the Spleen of the Two Lines at 15.5 Months Post-Vaccination
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bublot, M.; Sharma, J.M. Vaccination against Marek’s disease. In Marek’s Disease. An Evolving Problem; Davison, F., Nair, V.K., Eds.; Elsevier Academic Press: Compton, UK, 2004; pp. 168–185. [Google Scholar]
- Reddy, S.M.; Izumiya, Y.; Lupiani, B. Marek’s disease vaccines: Current status, and strategies for improvement and development of vector vaccines. Vet. Microbiol. 2017, 206, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Darteil, R.; Bublot, M.; Laplace, E.; Bouquet, J.F.; Audonnet, J.C.; Riviere, M. Herpesvirus of turkey recombinant viruses expressing infectious bursal disease virus (IBDV) VP2 immunogen induce protection against an IBDV virulent challenge in chickens. Virology 1995, 211, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Le Gros, F.X.; Dancer, A.; Giacomini, C.; Pizzoni, L.; Bublot, M.; Graziani, M.; Prandini, F. Field efficacy trial of a novel HVT-IBD vector vaccine for 1-day-old broilers. Vaccine 2009, 27, 592–596. [Google Scholar] [CrossRef] [PubMed]
- Esaki, M.; Godoy, A.; Rosenberger, J.K.; Rosenberger, S.C.; Gardin, Y.; Yasuda, A.; Dorsey, K.M. Protection and antibody response caused by turkey herpesvirus vector Newcastle disease vaccine. Avian Dis. 2013, 57, 750–755. [Google Scholar] [CrossRef]
- Palya, V.; Tatar-Kis, T.; Mato, T.; Felfoldi, B.; Kovacs, E.; Gardin, Y. Onset and long-term duration of immunity provided by a single vaccination with a turkey herpesvirus vector ND vaccine in commercial layers. Vet. Immunol. Immunopathol. 2014, 158, 105–115. [Google Scholar] [CrossRef]
- Schat, K.A.; Nair, V. Marek’s Disease. In Disease of Poultry; Saif, Y.M., Ed.; Blackwell Publishing Ltd.: Ames, IA, USA, 2008; pp. 452–514. [Google Scholar]
- Couteaudier, M.; Denesvre, C. Marek’s disease virus and skin interactions. Vet. Res. 2014, 45, 36. [Google Scholar] [CrossRef]
- McPherson, M.C.; Cheng, H.H.; Delany, M.E. Marek’s disease herpesvirus vaccines integrate into chicken host chromosomes yet lack a virus-host phenotype associated with oncogenic transformation. Vaccine 2016, 34, 5554–5561. [Google Scholar] [CrossRef]
- Remy, S.; Le Pape, G.; Gourichon, D.; Gardin, Y.; Denesvre, C. Chickens can durably clear herpesvirus vaccine infection in feathers while still carrying vaccine-induced antibodies. Vet. Res. 2020, 51, 24. [Google Scholar] [CrossRef]
- Islam, A.; Cheetham, B.F.; Mahony, T.J.; Young, P.L.; Walkden-Brown, S.W. Absolute quantitation of Marek’s disease virus and Herpesvirus of turkeys in chicken lymphocyte, feather tip and dust samples using real-time PCR. J. Virol. Methods 2006, 132, 127–134. [Google Scholar] [CrossRef]
- Walkden-Brown, S.; Islam, A.; Groves, P.; Rubite, A.; Sharpe, S.M.; Burgess, S. Development, application, and results of routine monitoring of Marek’s disease virus in broiler house dust using real-time quantitative PCR. Avian Dis. 2013, 57, 544–554. [Google Scholar] [CrossRef]
- Denesvre, C.; Dumarest, M.; Rémy, S.; Gourichon, D.; Eloit, M. Chicken skin virome analyzed by high-throughput sequencing shows a composition highly different from human skin. Virus Genes 2015, 5, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Cho, B.R.; Kenzy, S.G. Horizontal transmission of turkey herpesvirus to chickens. 3. Transmission in three different lines of chickens. Poult. Sci. 1975, 54, 109–115. [Google Scholar] [CrossRef]
- Cho, B.R.; Kenzy, S.G. Horizontal transmission of turkey herpesvirus to chickens. 2. Some factors affecting transmission. Poult. Sci. 1973, 52, 608–613. [Google Scholar] [CrossRef] [PubMed]
- Witter, R.L.; Burgoyne, G.H.; Burmester, B.R. Survival of Marek’s disease agent in litter and droppings. Avian Dis. 1968, 12, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Erf, G.F. Apoptosis in feathers of Smyth line chickens with autoimmune vitiligo. J. Autoimmun. 2004, 22, 21–30. [Google Scholar] [CrossRef]
- Erf, G.P. Autoimmune Diseases of Poultry. In Avian Immunology, 2nd ed.; Schat, K.A., Kaspers, B., Kaiser, P., Eds.; Elsevier Academic Press: London, UK, 2014; pp. 315–332. [Google Scholar]
- Erf, G.F.; Le Poole, I.C. Animal Models. In Vitiligo; Picardo, M., Taïeb, A., Eds.; Springer: Cham, Switzeland, 2019. [Google Scholar]
- Smyth, J.R.J. The Smyth line chicken: A model for autoimmune amelanosis. Crit. Rev. Poult. Biol. 1989, 2, 1–19. [Google Scholar]
- Boissy, R.E.; Gecks, S.; Smyth, J.R., Jr.; Nordlund, J.J. Ocular pathology in the minimally depigmented subline of the vitiliginous Smyth chicken. Pigment Cell Res. 1988, 1, 303–314. [Google Scholar] [CrossRef]
- Wang, X.; Erf, G.F. Melanocyte-specific cell mediated immune response in vitiliginous Smyth line chickens. J. Autoimmun. 2003, 21, 149–160. [Google Scholar] [CrossRef]
- Shi, F.; Erf, G.F. IFN-gamma, IL-21, and IL-10 co-expression in evolving autoimmune vitiligo lesions of Smyth line chickens. J. Invest. Dermatol. 2012, 132, 642–649. [Google Scholar] [CrossRef]
- Erf, G.F.; Trejo-Skalli, A.V.; Smyth, J.R., Jr. T cells in regenerating feathers of Smyth line chickens with vitiligo. Clin. Immunol. Immunopathol. 1995, 76, 120–126. [Google Scholar] [CrossRef]
- Wang, X. The Role of T Cell-mediated Immunity in Smyth Line Autoimmune Vitiligo. Ph.D. Thesis, University of Arkansas, Fayetteville, AR, USA, 1995. [Google Scholar]
- Erf, G.F.; Bersi, T.K.; Wang, X.; Sreekumar, G.P.; Smyth, J.R., Jr. Herpesvirus connection in the expression of autoimmune vitiligo in Smyth line chickens. Pigment Cell Res. 2001, 14, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Sreekumar, G.P.; Erf, G.F.; Smyth, J.R., Jr. 5-azacytidine treatment induces autoimmune vitiligo in parental control strains of the Smyth line chicken model for autoimmune vitiligo. Clin. Immunol. Immunopathol. 1996, 81, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Sreekumar, G.P.; Smyth, J.R., Jr.; Erf, G.F. Immune response to sheep red blood cells in two Smyth line populations homozygous for different major histocompatibility complex haplotypes. Poult. Sci. 1995, 74, 951–956. [Google Scholar] [CrossRef]
- Brunner, E.; Domhof, S.; Langer, F. Nonparametric Analysis of Longitudinal Data in Factorial Experiments; Wiley: New York, NY, USA, 2002. [Google Scholar]
- Noguchi, K.; Gel, Y.R.; Brunner, E.; Konietschke, F. nparLD: An R Software Package for the Nonparametric Analysis of Longitudinal Data in Factorial Experiments. J. Stat. Softw. 2012, 50, 1–23. [Google Scholar] [CrossRef]
- Abdul-Careem, M.F.; Hunter, D.B.; Shanmuganathan, S.; Haghighi, H.R.; Read, L.; Heidari, M.; Sharif, S. Cellular and cytokine responses in feathers of chickens vaccinated against Marek’s disease. Vet. Immunol. Immunopathol. 2008, 126, 362–366. [Google Scholar] [CrossRef] [PubMed]
- Shresta, S.; Smyth, J.R., Jr.; Erf, G.F. Profiles of pulp infiltrating lymphocytes at various times throughout feather regeneration in Smyth line chickens with vitiligo. Autoimmunity 1997, 25, 193–201. [Google Scholar] [CrossRef]
- Laddha, N.C.; Dwivedi, M.; Mansuri, M.S.; Gani, A.R.; Ansarullah, M.; Ramachandran, A.V.; Dalai, S.; Begum, R. Vitiligo: Interplay between oxidative stress and immune system. Exp. Dermatol. 2013, 22, 245–250. [Google Scholar] [CrossRef]
- Picardo, M.; Dell’Anna, M.L. Oxidative Stress and Intrinsic Defects. In Vitiligo; Picardo, M., Taieb, A., Eds.; Springer: Cham, Switzerland, 2019. [Google Scholar]
- Bencherit, D.; Rémy, S.; Le Vern, Y.; Vychodil, T.; Bertzbach, L.D.; Kaufer, B.B.; Denesvre, C.; Trapp-Fragnet, L. Induction of DNA damages upon Marek’s disease virus infection: Implication in viral replication and pathogenesis. J. Virol. 2017, 91, 1–36. [Google Scholar] [CrossRef]
- Boodhoo, N.; Kamble, N.; Kaufer, B.B.; Behboudi, S. Replication of Marek’s Disease Virus is dependent on synthesis of de novo Fatty Acid and Prostaglandin E2. J. Virol. 2019, 93. [Google Scholar] [CrossRef]
- Hooks, J.J.; Chin, M.S.; Srinivasan, K.; Momma, Y.; Hooper, L.C.; Nagineni, C.N.; Chan, C.C.; Detrick, B. Human cytomegalovirus induced cyclooxygenase-2 in human retinal pigment epithelial cells augments viral replication through a prostaglandin pathway. Microbes Infect. 2006, 8, 2236–2244. [Google Scholar] [CrossRef]
- Moriuchi, M.; Inoue, H.; Moriuchi, H. Reciprocal interactions between human T-lymphotropic virus type 1 and prostaglandins: Implications for viral transmission. J. Virol. 2001, 75, 192–198. [Google Scholar] [CrossRef]
- Abbas, A.K.; Lichtman, A.H.; Pillai, S. Cellular and Molecular Immunology; Elsevier Saunders: Philadelphia, PA, USA, 2012. [Google Scholar]
- Kang, S.; Brown, H.M.; Hwang, S. Direct Antiviral Mechanisms of Interferon-Gamma. Immune Netw. 2018, 18, e33. [Google Scholar] [CrossRef] [PubMed]
- Shakya, A.K.; O’Callaghan, D.J.; Kim, S.K. Interferon Gamma Inhibits Varicella-Zoster Virus Replication in a Cell Line-Dependent Manner. J. Virol. 2019, 93. [Google Scholar] [CrossRef]
- Xing, Z.; Schat, K.A. Inhibitory effects of nitric oxide and gamma interferon on in vitro and in vivo replication of Marek’s disease virus. J. Virol. 2000, 74, 3605–3612. [Google Scholar] [CrossRef] [PubMed]
- Bertzbach, L.D.; Harlin, O.; Hartle, S.; Fehler, F.; Vychodil, T.; Kaufer, B.B.; Kaspers, B. IFNalpha and IFNgamma Impede Marek’s Disease Progression. Viruses 2019, 11, 1103. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Careem, M.F.; Read, L.R.; Parvizi, P.; Thanthrige-Don, N.; Sharif, S. Marek’s disease virus-induced expression of cytokine genes in feathers of genetically defined chickens. Dev. Comp. Immunol. 2009, 33, 618–623. [Google Scholar] [CrossRef]
- Haq, K.; Wootton, S.K.; Barjesteh, N.; Golovan, S.; Bendall, A.; Sharif, S. Effects of interferon-gamma knockdown on vaccine-induced immunity against Marek’s disease in chickens. Can. J. Vet. Res. 2015, 79, 1–7. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erf, G.F.; Le Pape, G.; Rémy, S.; Denesvre, C. Mardivirus Infection and Persistence in Feathers of a Chicken Model Harboring a Local Autoimmune Response. Microorganisms 2020, 8, 1613. https://doi.org/10.3390/microorganisms8101613
Erf GF, Le Pape G, Rémy S, Denesvre C. Mardivirus Infection and Persistence in Feathers of a Chicken Model Harboring a Local Autoimmune Response. Microorganisms. 2020; 8(10):1613. https://doi.org/10.3390/microorganisms8101613
Chicago/Turabian StyleErf, Gisela F., Gilles Le Pape, Sylvie Rémy, and Caroline Denesvre. 2020. "Mardivirus Infection and Persistence in Feathers of a Chicken Model Harboring a Local Autoimmune Response" Microorganisms 8, no. 10: 1613. https://doi.org/10.3390/microorganisms8101613
APA StyleErf, G. F., Le Pape, G., Rémy, S., & Denesvre, C. (2020). Mardivirus Infection and Persistence in Feathers of a Chicken Model Harboring a Local Autoimmune Response. Microorganisms, 8(10), 1613. https://doi.org/10.3390/microorganisms8101613



