The Neutrophil-to-Monocyte Ratio and Lymphocyte-to-Neutrophil Ratio at Admission Predict In-Hospital Mortality in Mexican Patients with Severe SARS-CoV-2 Infection (Covid-19)
Abstract
1. Introduction
2. Material and Methods
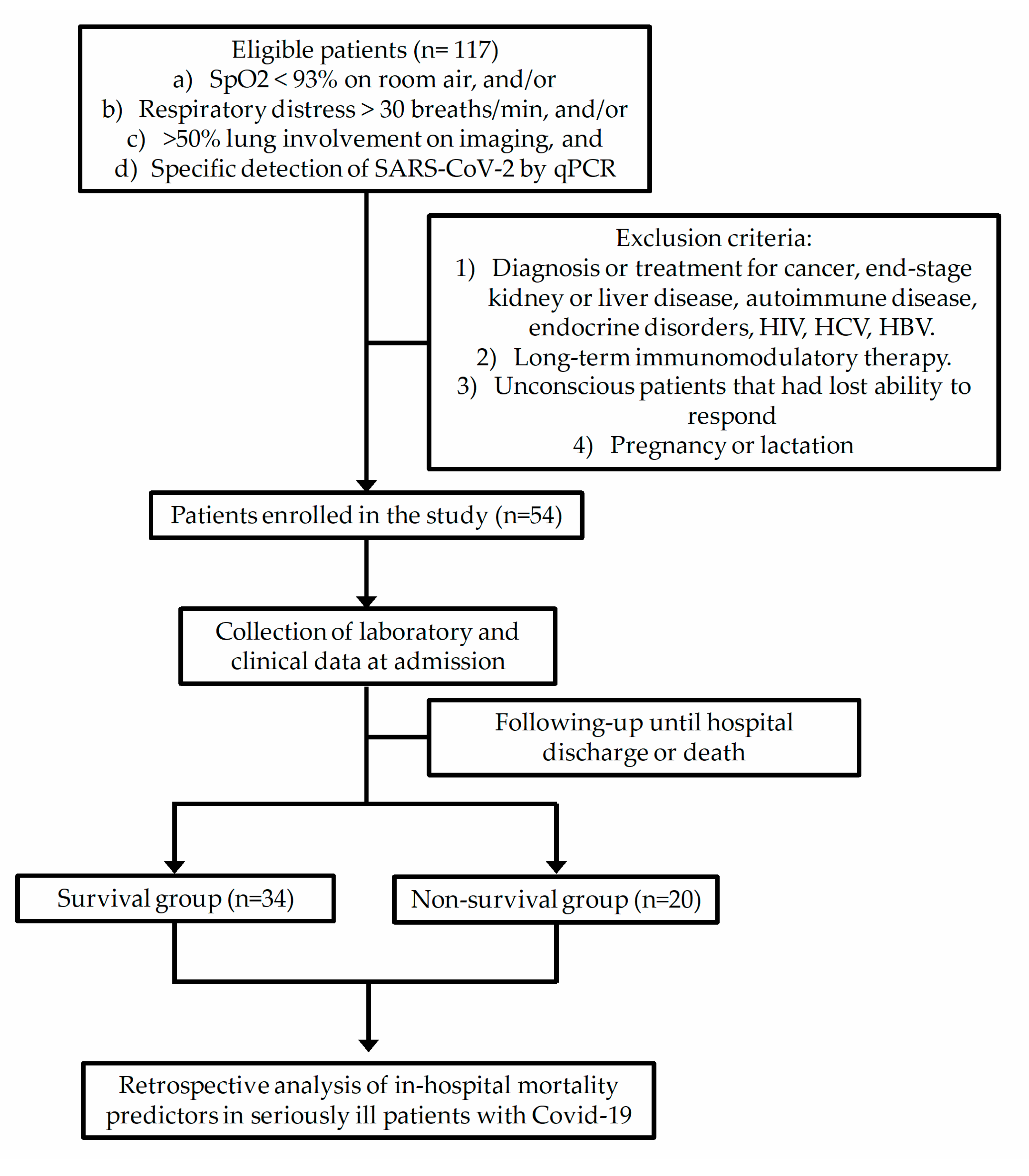
2.1. Subjects
2.2. Data Collection
2.3. Laboratory Measures
2.4. Statistics
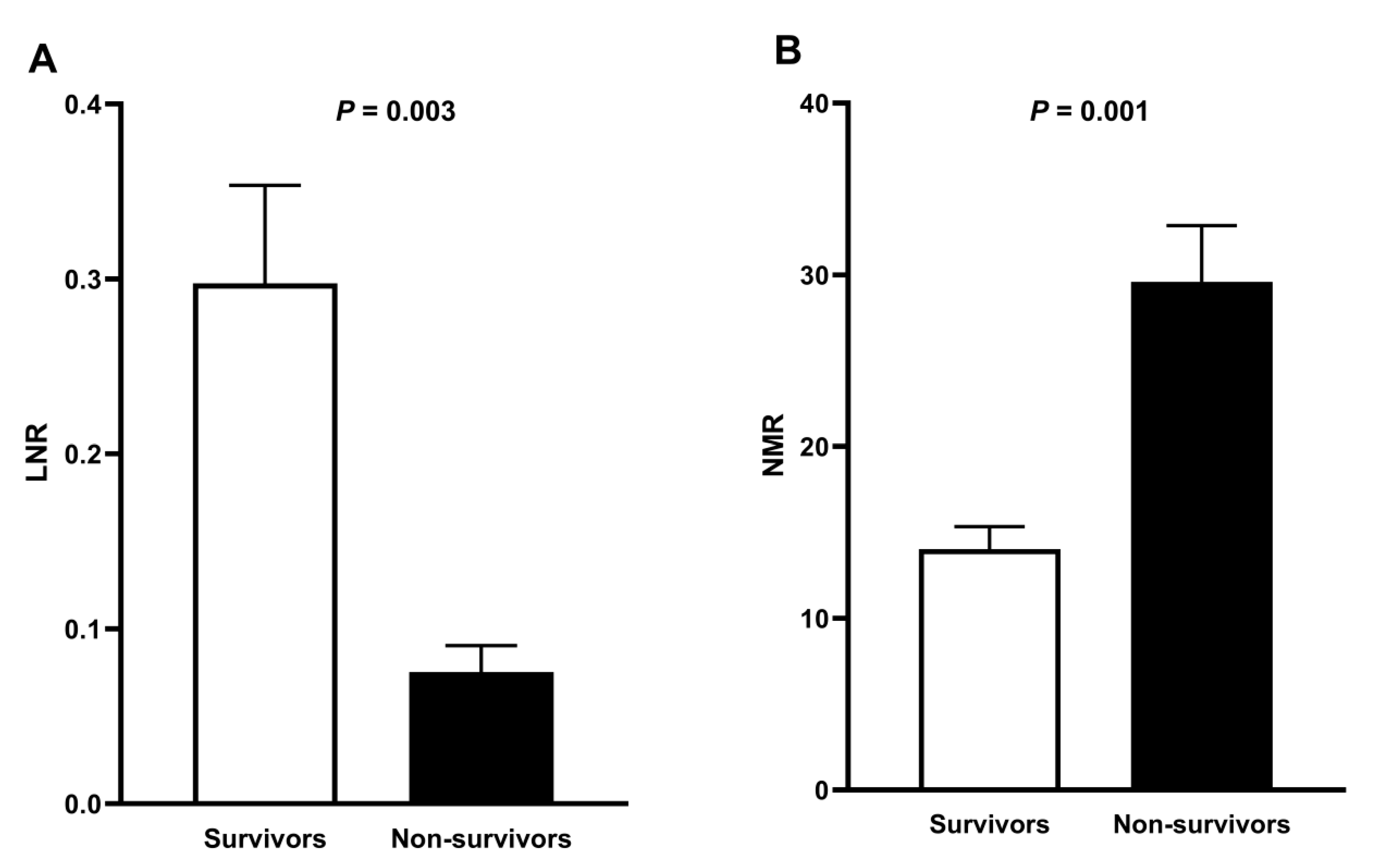
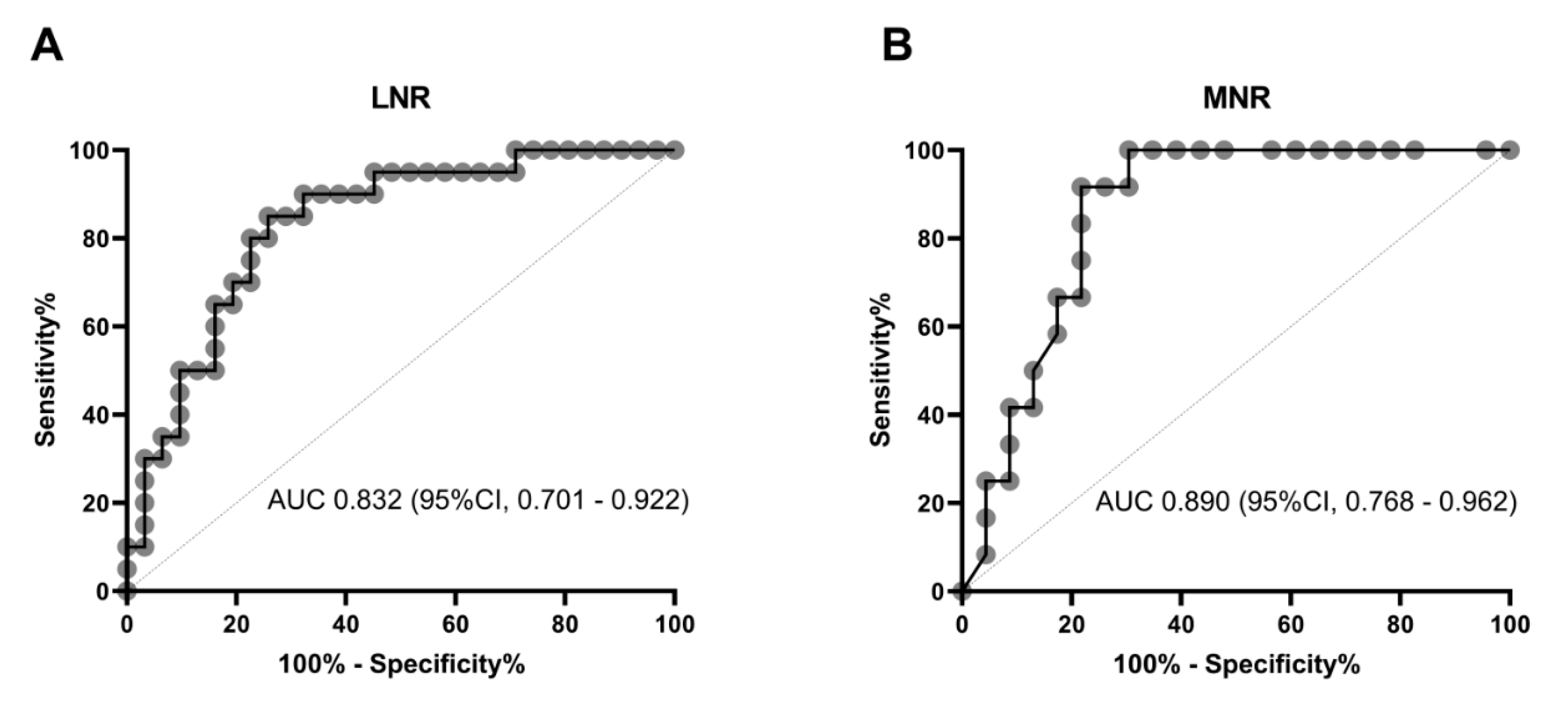
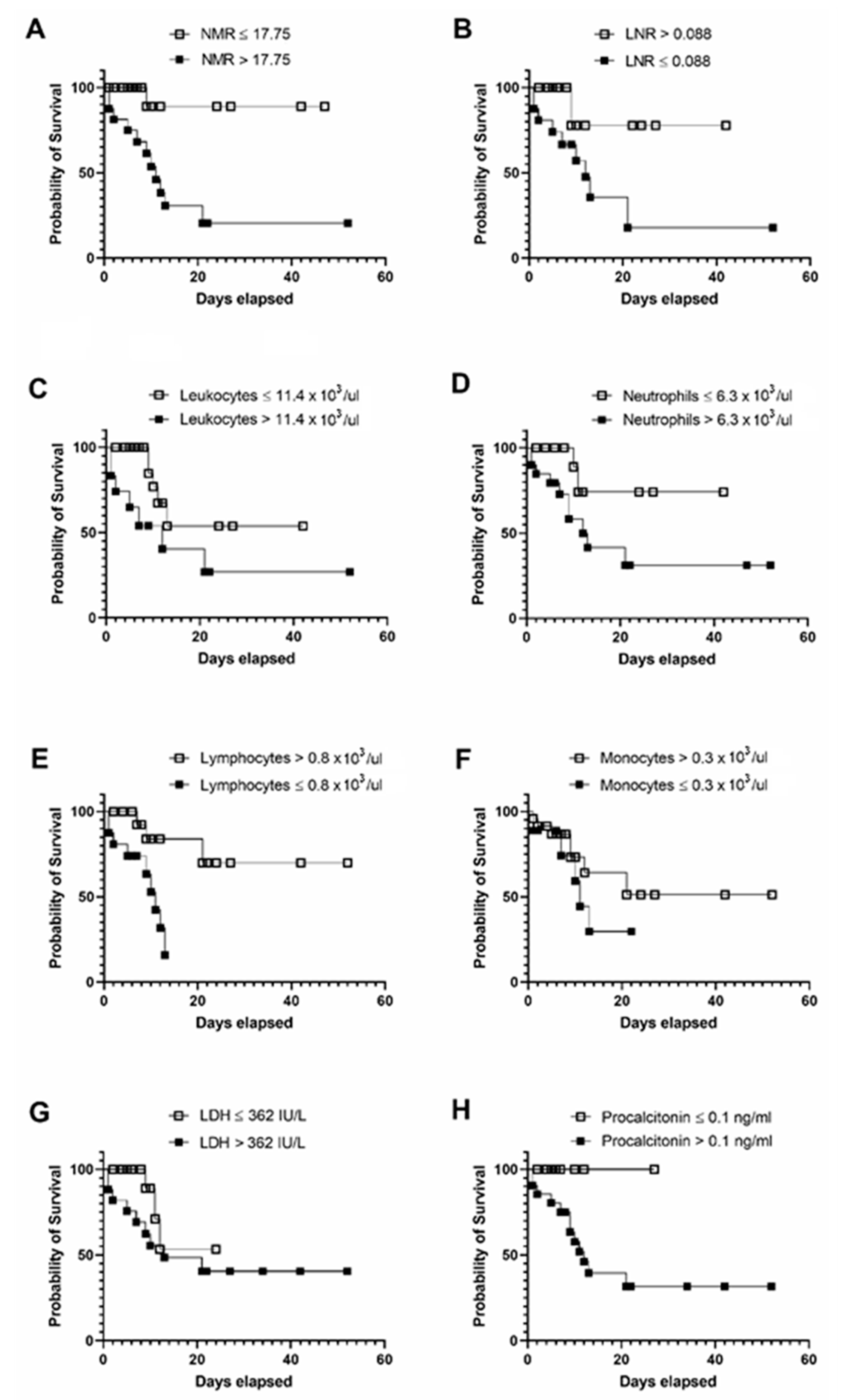
3. Results
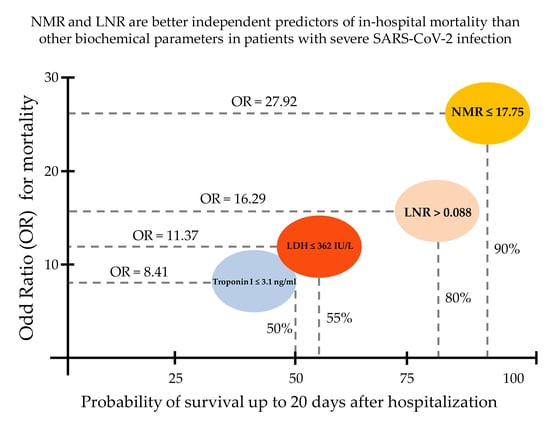
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lai, C.-C.; Shih, T.-P.; Ko, W.-C.; Tang, H.-J.; Hsueh, P.-R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. Int. J. Antimicrob. Agents 2020, 55, 105924. [Google Scholar] [CrossRef] [PubMed]
- Epidemiology Working Group for NCIP Epidemic Response, Chinese Center for Disease Control and Prevention. The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. Zhonghua Liu Xing Bing Xue Za Zhi 2020, 41, 145–151. [Google Scholar]
- Yan, Y.; Shin, W.I.; Pang, Y.X.; Meng, Y.; Lai, J.; You, C.; Zhao, H.; Lester, E.; Wu, T.; Pang, C.H. The First 75 Days of Novel Coronavirus (SARS-CoV-2) Outbreak: Recent Advances, Prevention, and Treatment. Int. J. Environ. Res. Public Health 2020, 17, 2323. [Google Scholar] [CrossRef]
- Ceylan, Z. Estimation of COVID-19 prevalence in Italy, Spain, and France. Sci. Total. Environ. 2020, 729, 138817. [Google Scholar] [CrossRef] [PubMed]
- Vandoros, S. Excess mortality during the Covid-19 pandemic: Early evidence from England and Wales. Soc. Sci. Med. 2020, 258, 113101. [Google Scholar] [CrossRef]
- Suárez, V.; Quezada, M.S.; Ruiz, S.O.; De Jesús, E.R. Epidemiology of COVID-19 in Mexico: From the 27th of February to the 30th of April 2020. Rev. Clínica Española Engl. Ed. 2020. [Google Scholar] [CrossRef]
- Friedman, J.; Calderón-Villarreal, A.; Bojorquez, I.; Hernández, C.V.; Schriger, D.L.; Hirashima, E.T. Excess Out-Of-Hospital Mortality and Declining Oxygen Saturation: The Sentinel Role of EMS Data in the COVID-19 Crisis in Tijuana, Mexico. Ann. Emerg. Med. 2020. [Google Scholar] [CrossRef]
- Zhang, L.; Yan, X.; Fan, Q.; Liu, H.; Liu, X.; Liu, Z.; Zhang, Z. D-dimer levels on admission to predict in-hospital mortality in patients with Covid-19. J. Thromb. Haemost. 2020, 18, 1324–1329. [Google Scholar] [CrossRef]
- Lin, Z.; Long, F.; Yang, Y.; Chen, X.; Xu, L.; Yang, M. Serum ferritin as an independent risk factor for severity in COVID-19 patients. J. Infect. 2020, 81, 647–679. [Google Scholar] [CrossRef]
- Sahu, B.R.; Kampa, R.K.; Padhi, A.; Panda, A.K. C-reactive protein: A promising biomarker for poor prognosis in COVID-19 infection. Clin. Chim. Acta 2020, 509, 91–94. [Google Scholar] [CrossRef]
- Paul, P. Cardiac Troponin-I may be a predictor of complications and mortality in COVID-19 patients. Curr. Med. Res. Pract. 2020, 10, 130–131. [Google Scholar] [CrossRef] [PubMed]
- Henry, B.M.; Aggarwal, G.; Wong, J.; Benoit, S.; Vikse, J.; Plebani, M.; Lippi, G. Lactate dehydrogenase levels predict coronavirus disease 2019 (COVID-19) severity and mortality: A pooled analysis. Am. J. Emerg. Med. 2020, 38, 1722–1726. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Han, C.; Pei, S.; Yin, M.; Chen, X. Procalcitonin levels in COVID-19 patients. Int. J. Antimicrob. Agents 2020, 56, 106051. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Li, R.; Wu, X.; Zhao, Y.; Wang, T.; Zheng, Z.; Zeng, S.; Ding, X.; Nie, H. Clinical features in 52 patients with COVID-19 who have increased leukocyte count: A retrospective analysis. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 1–9. [Google Scholar] [CrossRef]
- Zhao, Y.; Nie, H.-X.; Hu, K.; Wu, X.-J.; Zhang, Y.-T.; Wang, M.-M.; Wang, T.; Zheng, Z.-S.; Li, X.-C.; Zeng, S.-L. Abnormal immunity of non-survivors with COVID-19: Predictors for mortality. Infect. Dis. Poverty 2020, 9, 1–10. [Google Scholar] [CrossRef]
- Liu, Y.; Du, X.; Chen, J.; Jin, Y.; Peng, L.; Wang, H.H.; Luo, M.; Chen, L.; Zhao, Y. Neutrophil-to-lymphocyte ratio as an independent risk factor for mortality in hospitalized patients with COVID-19. J. Infect. 2020, 81, e6–e12. [Google Scholar] [CrossRef]
- Belice, T.; Demir, I.; Yüksel, A. Role of neutrophil-lymphocyte-ratio in the mortality of males diagnosed with COVID-19. Iran. J. Microbiol. 2020, 12, 194–197. [Google Scholar] [CrossRef]
- Grenader, T.; Pavel, M.E.; Ruszniewski, P.B.; Ćwikła, J.B.; Phan, A.T.; Raderer, M.; Sedláčková, E.; Cadiot, G.; Wolin, E.M.; Capdevila, J.; et al. Prognostic value of the neutrophil/lymphocyte ratio in enteropancreatic neuroendocrine tumors. Anti-Cancer Drugs 2020, 31, 216–222. [Google Scholar] [CrossRef]
- Ventriglia, J.; Petrillo, A.; Alváro, M.H.; Laterza, M.M.; Savastano, B.; Gambardella, V.; Tirino, G.; Pompella, L.; Diana, A.; Iovino, F.; et al. Neutrophil to Lymphocyte Ratio as a Predictor of Poor Prognosis in Metastatic Pancreatic Cancer Patients Treated with Nab-Paclitaxel plus Gemcitabine: A Propensity Score Analysis. Gastroenterol. Res. Pract. 2018, 2018, 2373868. [Google Scholar] [CrossRef] [PubMed]
- Howard, R.; Kanetsky, P.A.; Egan, K.M. Exploring the prognostic value of the neutrophil-to-lymphocyte ratio in cancer. Sci. Rep. 2019, 9, 19670. [Google Scholar] [CrossRef] [PubMed]
- Corman, V.M.; Landt, O.; Kaiser, M.; Molenkamp, R.; Meijer, A.; Chu, D.K.; Bleicker, T.; Brünink, S.; Schneider, J.; Schmidt, M.L.; et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Eurosurveillance 2020, 25, 2000045. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, S.; Liu, J.; Liang, B.; Wang, X.; Wang, H.; Li, W.; Tong, Q.; Yi, J.; Zhao, L.; et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine 2020, 55, 102763. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Li, F.; Wang, X.; Yan, J.; Zhu, F.; Tang, S.; Deng, Y.; Wang, H.; Chen, R.; Yu, Z.; et al. Neutrophil to lymphocyte ratio as prognostic and predictive factor in patients with coronavirus disease 2019: A retrospective cross-sectional study. J. Med Virol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Basbus, L.; I Lapidus, M.; Martingano, I.; Puga, M.C.; Pollán, J. Neutrophil to lymphocyte ratio as a prognostic marker in COVID-19. Medicina (B Aires) 2020, 80 (Suppl. 3), 31–36. [Google Scholar] [PubMed]
- Tatum, D.; Taghavi, S.; Houghton, A.; Stover, J.; Toraih, E.; Duchesne, J. Neutrophil-to-Lymphocyte Ratio and Outcomes in Louisiana Covid-19 Patients. Shock 2020. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-J.; Cao, Y.-Y.; Tan, G.; Dong, X.; Wang, B.-C.; Lin, J.; Yan, Y.-Q.; Liu, G.-H.; Akdis, M.; Akdis, C.A.; et al. Clinical, radiological and laboratory characteristics and risk factors for severity and mortality of 289 hospitalized COVID-19 patients. Allergy 2020. [Google Scholar] [CrossRef]
- Gormez, S.; Ekicibasi, E.; Degirmencioglu, A.; Paudel, A.; Erdim, R.; Gumusel, H.K.; Eroglu, E.; Tanboga, I.H.; Dagdelen, S.; Sariguzel, N.; et al. Association between renin–angiotensin–aldosterone system inhibitor treatment, neutrophil–lymphocyte ratio, D-Dimer and clinical severity of COVID-19 in hospitalized patients: A multicenter, observational study. J. Hum. Hypertens. 2020. [Google Scholar] [CrossRef]
- Zhang, H.; Cao, X.; Kong, M.; Mao, X.; Huang, L.; He, P.; Pan, S.; Li, J.; Lu, Z. Clinical and hematological characteristics of 88 patients with COVID-19. Int. J. Lab. Hematol. 2020. [Google Scholar] [CrossRef]
- Kolaczkowska, E.; Kubes, P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 2013, 13, 159–175. [Google Scholar] [CrossRef]
- Hemmat, N.; Derakhshani, A.; Baghi, H.B.; Silvestris, N.; Baradaran, B.; De Summa, S. Neutrophils, Crucial, or Harmful Immune Cells Involved in Coronavirus Infection: A Bioinformatics Study. Front. Genet. 2020, 11, 641. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Li, S.; Sun, Q.; Zhu, J.; Chen, B.; Xiong, M.; Cao, G. Immune-Inflammatory Parameters in COVID-19 Cases: A Systematic Review and Meta-Analysis. Front. Med. 2020, 7, 301. [Google Scholar] [CrossRef] [PubMed]
- Chaplin, D.D. Overview of the immune response. J. Allergy Clin. Immunol. 2010, 125, S3–S23. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, S.; Yamaguchi, T.; Nomura, T.; Ono, M. Regulatory T cells and immune tolerance. Cell 2008, 133, 775–787. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.; Verhagen, J.; Blaser, K.; Akdis, M.; Akdis, C.A. Mechanisms of immune suppression by interleukin-10 and transforming growth factor-beta: The role of T regulatory cells. Immunology 2006, 117, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Ziegler-Heitbrock, L.; Ancuta, P.; Crowe, S.; Dalod, M.; Grau, V.; Hart, D.N.; Leenen, P.J.M.; Liu, Y.-J.; MacPherson, G.; Randolph, G.J.; et al. Nomenclature of monocytes and dendritic cells in blood. Blood 2010, 116, e74–e80. [Google Scholar] [CrossRef]
- Devêvre, E.F.; Renovato-Martins, M.; Clément, K.; Sautès-Fridman, C.; Cremer, I.; Poitou, C. Profiling of the Three Circulating Monocyte Subpopulations in Human Obesity. J. Immunol. 2015, 194, 3917–3923. [Google Scholar] [CrossRef]
- Mukherjee, R.; Barman, P.K.; Thatoi, P.K.; Tripathy, R.; Das, B.K.; Ravindran, B. Non-Classical monocytes display inflammatory features: Validation in Sepsis and Systemic Lupus Erythematous. Sci. Rep. 2015, 5, 13886. [Google Scholar] [CrossRef]
- Grün, J.L.; Manjarrez-Reyna, A.N.; Gómez-Arauz, A.Y.; León-Cabrera, S.; Rückert, F.; Fragoso, J.M.; Bueno-Hernández, N.; Islas-Andrade, S.; Melendez, G.; Escobedo, G. High-Density Lipoprotein Reduction Differentially Modulates to Classical and Nonclassical Monocyte Subpopulations in Metabolic Syndrome Patients and in LPS-Stimulated Primary Human Monocytes In Vitro. J. Immunol. Res. 2018, 2018, 2737040. [Google Scholar] [CrossRef]
- Gatti, A.; Radrizzani, D.; Viganò, P.; Mazzone, A.; Brando, B. Decrease of non-classical and intermediate monocyte subsets in severe acute SARS-CoV -2 infection. Cytom. Part A 2020, 97, 887–890. [Google Scholar] [CrossRef]
- Duque, G.A.; Descoteaux, A. Macrophage Cytokines: Involvement in Immunity and Infectious Diseases. Front. Immunol. 2014, 5, 491. [Google Scholar] [CrossRef]
- Sanyaolu, A.; Okorie, C.; Marinkovic, A.; Patidar, R.; Younis, K.; Desai, P.; Hosein, Z.; Padda, I.; Mangat, J.; Altaf, M. Comorbidity and its Impact on Patients with COVID-19. SN Compr. Clin. Med. 2020, 2, 1069–1076. [Google Scholar] [CrossRef] [PubMed]

| Parameters | Survivors (n = 34) | Non-Survivors (n = 20) | p Value |
|---|---|---|---|
| Gender (W/M) | 21/13 | 5/15 | 0.002 * |
| Age (years) | 54.06 ± 12.43 | 62.9 ± 14.18 | 0.020 * |
| BMI (kg/m2) | 28.24 ± 4.60 | 27.88 ± 4.05 | 0.903 |
| Obesity prevalence (%) | 52.17 | 41.66 | 0.277 |
| T2D prevalence (%) | 43.47 | 75.00 | 0.037 * |
| Hypertension prevalence (%) | 17.39 | 58.33 | 0.006 * |
| Coronary heart disease (%) | 8.69 | 33.33 | 0.033 * |
| IMV (%) | 30.43 | 83.33 | 0.001 * |
| Time to extubation (days) | 2.43 ± 0.79 | 3.66 ± 0.82 | 0.167 |
| Inpatient days (days) | 15.65 ± 3.13 | 8.41 ± 1.66 | 0.060 |
| Drug regimen | Aziythromycin, ceftriaxone, enoxaparin sodium, dexamethasone, and acetaminophen | - | |
| Parameters | Survivors (n = 34) | Non-Survivors (n = 20) | p Value |
|---|---|---|---|
| Glucose (mg/dL) | 148.19 ± 92.13 | 148.16 ± 60.76 | 0.999 |
| Urea (mg/dL) | 42.05 ± 37.06 | 91.07 ± 77.16 | 0.004 * |
| Creatinine (mg/dL) | 0.995 ± 1.18 | 1.85 ± 2.79 | 0.133 |
| Uric Acid (mg/dL) | 5.65 ± 2.81 | 7.39 ± 4.09 | 0.097 |
| Total Cholesterol (mg/dL) | 151.77 ± 34.98 | 126.06 ± 20.85 | 0.010 * |
| Triglycerides (mg/dL) | 166.04 ± 67.93 | 169.75 ± 54.89 | 0.853 |
| HDL (mg/dL) | 34.30 ± 10.88 | 23.92 ± 12.09 | 0.015 * |
| LDL (mg/dL) | 95.30 ± 30.14 | 70.38 ± 18.69 | 0.012 * |
| Total bilirubin (mg/dL) | 0.684 ± 0.401 | 0.804 ± 0.315 | 0.349 |
| Direct bilirubin (mg/dL) | 0.185 ± 0.133 | 0.323 ± 0.215 | 0.012 * |
| Indirect bilirubin (mg/dL) | 0.492 ± 0.254 | 0.498 ± 0.153 | 0.939 |
| ALT (IU/L) | 35.42 ± 26.29 | 41.47 ± 28.92 | 0.465 |
| AST (IU/L) | 34.74 ± 22.86 | 59 ± 47.74 | 0.021 * |
| ALP (IU/L) | 91.00 ± 27.22 | 125.29 ± 120.86 | 0.134 |
| GGT (IU/L) | 69.54 ± 43.07 | 124.33 ± 101.26 | 0.015 * |
| Total Protein (mg/dL) | 6.59 ± 0.525 | 6.31 ± 0.627 | 0.099 |
| Albumin (mg/dL) | 3.59 ± 0.479 | 2.94 ± 0.409 | 0.001 * |
| LDH (IU/L) | 320.63 ± 132.11 | 475.63 ± 195.83 | 0.001 * |
| Amylase (IU/L) | 46.1 ± 35.39 | 56.83 ± 26.93 | 0.429 |
| Lipase (IU/L) | 116.00 ± 304.00 | 52.36 ± 52.27 | 0.502 |
| CPK (IU/L) | 101.52 ± 97.49 | 699.57 ± 1937.82 | 0.114 |
| CK-MB (IU/L) | 23.80 ± 11.90 | 44.43 ± 59.39 | 0.099 |
| Phosphorus (mg/dL) | 3.89 ± 2.01 | 4.25 ± 1.67 | 0.516 |
| Magnesium (mg/dL) | 2.74 ± 3.53 | 2.42 ± 0.629 | 0.698 |
| Sodium (mEq/L) | 136.00 ± 6.56 | 138.53 ± 6.31 | 0.182 |
| Potassium (mEq/L) | 5.56 ± 6.67 | 4.47 ± 0.719 | 0.484 |
| Chlorine (mEq/L) | 100.28 ± 7.19 | 101.84 ± 6.49 | 0.441 |
| Calcium (mg/dL) | 8.63 ± 0.686 | 8.14 ± 1.20 | 0.076 |
| Parameters | Survivors (n = 34) | Non-Survivors (n = 20) | p Value |
|---|---|---|---|
| Prothrombine time (s) | 11.97 ± 2.49 | 12.96 ± 1.55 | 0.237 |
| INR | 0.995 ± 0.246 | 1.10 ± 0.167 | 0.216 |
| Thrombin time (s) | 16.76 ± 1.61 | 17.78 ± 1.42 | 0.085 |
| aPTT (s) | 26.18 ± 6.99 | 26.36 ± 5.68 | 0.941 |
| Fibrinogen (mg/dL) | 637.23 ± 222.29 | 703.75 ± 206.92 | 0.400 |
| D-dimer (Ug/L) | 975.33 ± 477.81 | 8260.33 ± 13354.39 | 0.017 * |
| Ferritin (ng/mL) | 522.62 ± 451.93 | 937.00 ± 415.09 | 0.012 * |
| CRP (mg/L) | 129.43 ± 90.10 | 221.07 ± 108.32 | 0.014 * |
| Troponin I (ng/mL) | 37.68 ± 63.57 | 68.458 ± 112.73 | 0.338 |
| Myoglobine (ng/L) | 94.49 ± 107.76 | 254.38 ± 353.69 | 0.089 |
| Procalcitonin (ng/mL) | 0.220 ± 0.256 | 2.01 ± 4.07 | 0.037 * |
| Parameters | Survivors (n = 34) | Non-Survivors (n = 20) | p Value |
|---|---|---|---|
| Leukocytes (×103/μL) | 13.48 ± 25.44 | 13.19 ± 6.34 | 0.960 |
| Neutrophil percentage (%) | 73.95 ± 17.19 | 84.72 ± 18.89 | 0.041 * |
| Lymphocyte percentage (%) | 16.79 ± 11.35 | 7.48 ± 5.65 | 0.001 * |
| Monocyte percentage (%) | 5.96 ± 2.54 | 3.35 ± 1.42 | 0.001 * |
| Band cells (%) | 0.000 ± 0.000 | 0.08 ± 0.358 | 0.217 |
| Eosinophil percentage (%) | 0.739 ± 0.974 | 0.110 ± 0.281 | 0.007 * |
| Basophil percentage (%) | 0.429 ± 1.23 | 0.085 ± 0.123 | 0.221 |
| Neutrophils (×103/μL) | 7.39 ± 4.44 | 11.93 ± 5.99 | 0.003 * |
| Lymphocytes (×103/μL) | 2.09 ± 4.40 | 0.750 ± 0.426 | 0.181 |
| Monocytes (×103/μL) | 0.519 ± 0.256 | 0.450 ± 0.276 | 0.364 |
| Eosinophils (×103/μL) | 0.096 ± 0.272 | 0.015 ± 0.049 | 0.193 |
| Basophils (×103/μL) | 0.339 ± 1.87 | 0.000 ± 0.000 | 0.423 |
| Erythrocyte (×106/μL) | 4.71 ± 0.893 | 4.75 ± 1.09 | 0.884 |
| Hemoglobin (g/dL) | 14.17 ± 2.56 | 14.42 ± 3.11 | 0.756 |
| Hematocrit (%) | 42.40 ± 7.58 | 42.82 ± 9.44 | 0.863 |
| MCV (fL) | 90.54 ± 5.88 | 91.35 ± 4.15 | 0.597 |
| MCH (pg) | 30.34 ± 2.73 | 30.42 ± 1.64 | 0.905 |
| RDW (%) | 15.07 ± 3.38 | 14.72 ± 2.07 | 0.685 |
| Platelets (×103/μL) | 266.61 ± 111.11 | 240.25 ± 113.83 | 0.416 |
| Parameters | AUC | CI 95% |
|---|---|---|
| Total leukocyte count | 0.702 | 0.557–0.822 |
| Neutrophil count | 0.746 | 0.605–0.857 |
| Lymphocyte count | 0.735 | 0.593–0.849 |
| Monocyte count | 0.605 | 0.458–0.739 |
| D-dimer | 0.730 | 0.548–0.869 |
| Ferritin | 0.777 | 0.601–0.901 |
| CRP | 0.750 | 0.569–0.884 |
| Troponin I | 0.656 | 0.464–0.816 |
| LDH | 0.758 | 0.618–0.867 |
| Procalcitonin | 0.826 | 0.682–0.924 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rizo-Téllez, S.A.; Méndez-García, L.A.; Flores-Rebollo, C.; Alba-Flores, F.; Alcántara-Suárez, R.; Manjarrez-Reyna, A.N.; Baltazar-López, N.; Hernández-Guzmán, V.A.; León-Pedroza, J.I.; Zapata-Arenas, R.; et al. The Neutrophil-to-Monocyte Ratio and Lymphocyte-to-Neutrophil Ratio at Admission Predict In-Hospital Mortality in Mexican Patients with Severe SARS-CoV-2 Infection (Covid-19). Microorganisms 2020, 8, 1560. https://doi.org/10.3390/microorganisms8101560
Rizo-Téllez SA, Méndez-García LA, Flores-Rebollo C, Alba-Flores F, Alcántara-Suárez R, Manjarrez-Reyna AN, Baltazar-López N, Hernández-Guzmán VA, León-Pedroza JI, Zapata-Arenas R, et al. The Neutrophil-to-Monocyte Ratio and Lymphocyte-to-Neutrophil Ratio at Admission Predict In-Hospital Mortality in Mexican Patients with Severe SARS-CoV-2 Infection (Covid-19). Microorganisms. 2020; 8(10):1560. https://doi.org/10.3390/microorganisms8101560
Chicago/Turabian StyleRizo-Téllez, Salma A., Lucia A. Méndez-García, Cruz Flores-Rebollo, Fernando Alba-Flores, Raúl Alcántara-Suárez, Aarón N. Manjarrez-Reyna, Neyla Baltazar-López, Verónica A. Hernández-Guzmán, José I. León-Pedroza, Rogelio Zapata-Arenas, and et al. 2020. "The Neutrophil-to-Monocyte Ratio and Lymphocyte-to-Neutrophil Ratio at Admission Predict In-Hospital Mortality in Mexican Patients with Severe SARS-CoV-2 Infection (Covid-19)" Microorganisms 8, no. 10: 1560. https://doi.org/10.3390/microorganisms8101560
APA StyleRizo-Téllez, S. A., Méndez-García, L. A., Flores-Rebollo, C., Alba-Flores, F., Alcántara-Suárez, R., Manjarrez-Reyna, A. N., Baltazar-López, N., Hernández-Guzmán, V. A., León-Pedroza, J. I., Zapata-Arenas, R., González-Chávez, A., Hernández-Ruíz, J., Carrillo-Ruíz, J. D., Serrano-Loyola, R., Guerrero-Avendaño, G. M. L., & Escobedo, G. (2020). The Neutrophil-to-Monocyte Ratio and Lymphocyte-to-Neutrophil Ratio at Admission Predict In-Hospital Mortality in Mexican Patients with Severe SARS-CoV-2 Infection (Covid-19). Microorganisms, 8(10), 1560. https://doi.org/10.3390/microorganisms8101560