Peruvian chicha: A Focus on the Microbial Populations of This Ancient Maize-Based Fermented Beverage
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples Collection and DNA Extraction
2.2. Illumina 16S rRNA NGS
2.3. Bioinformatic Analysis
3. Results and Discussion
3.1. Chicha Samples Description
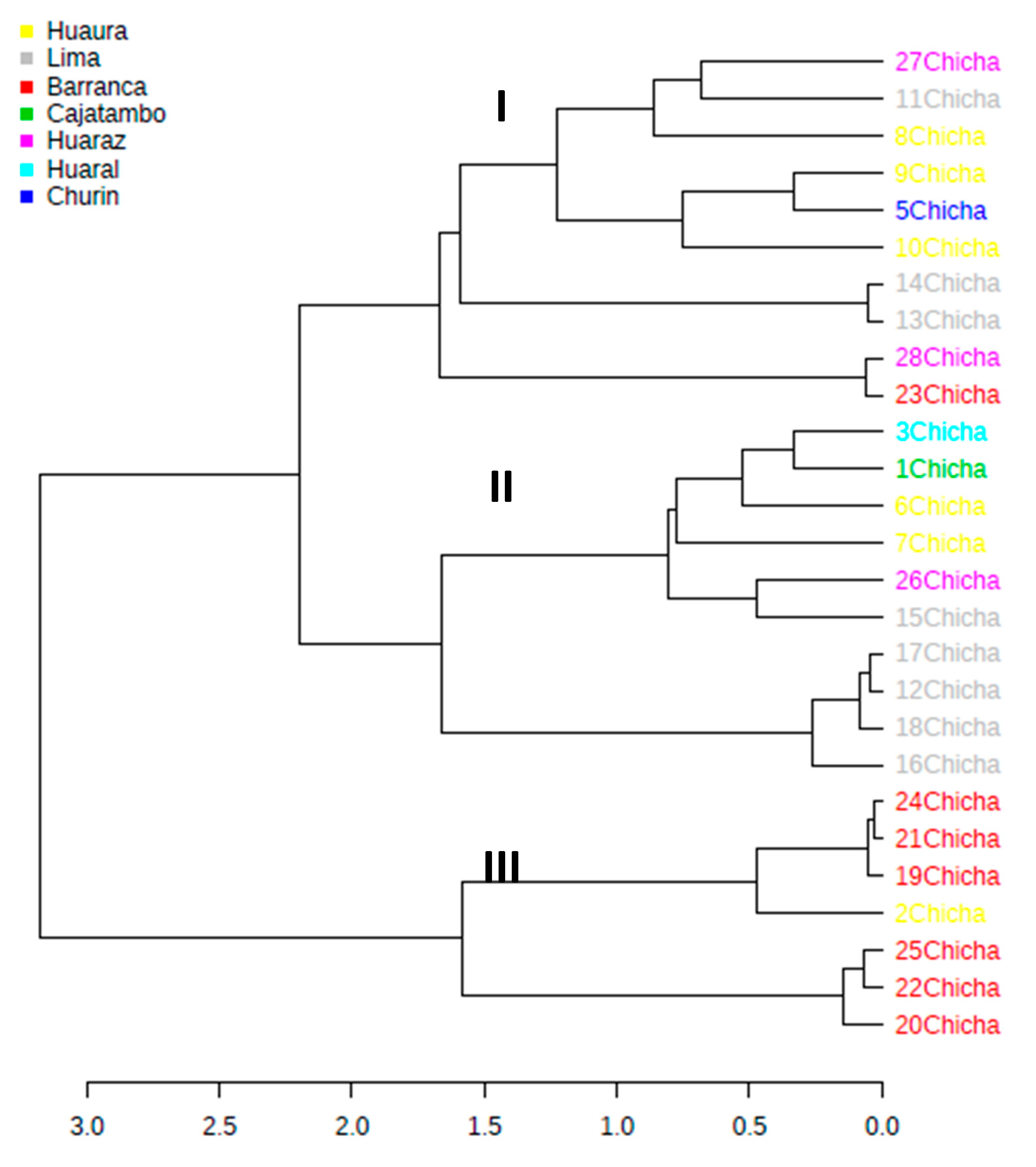
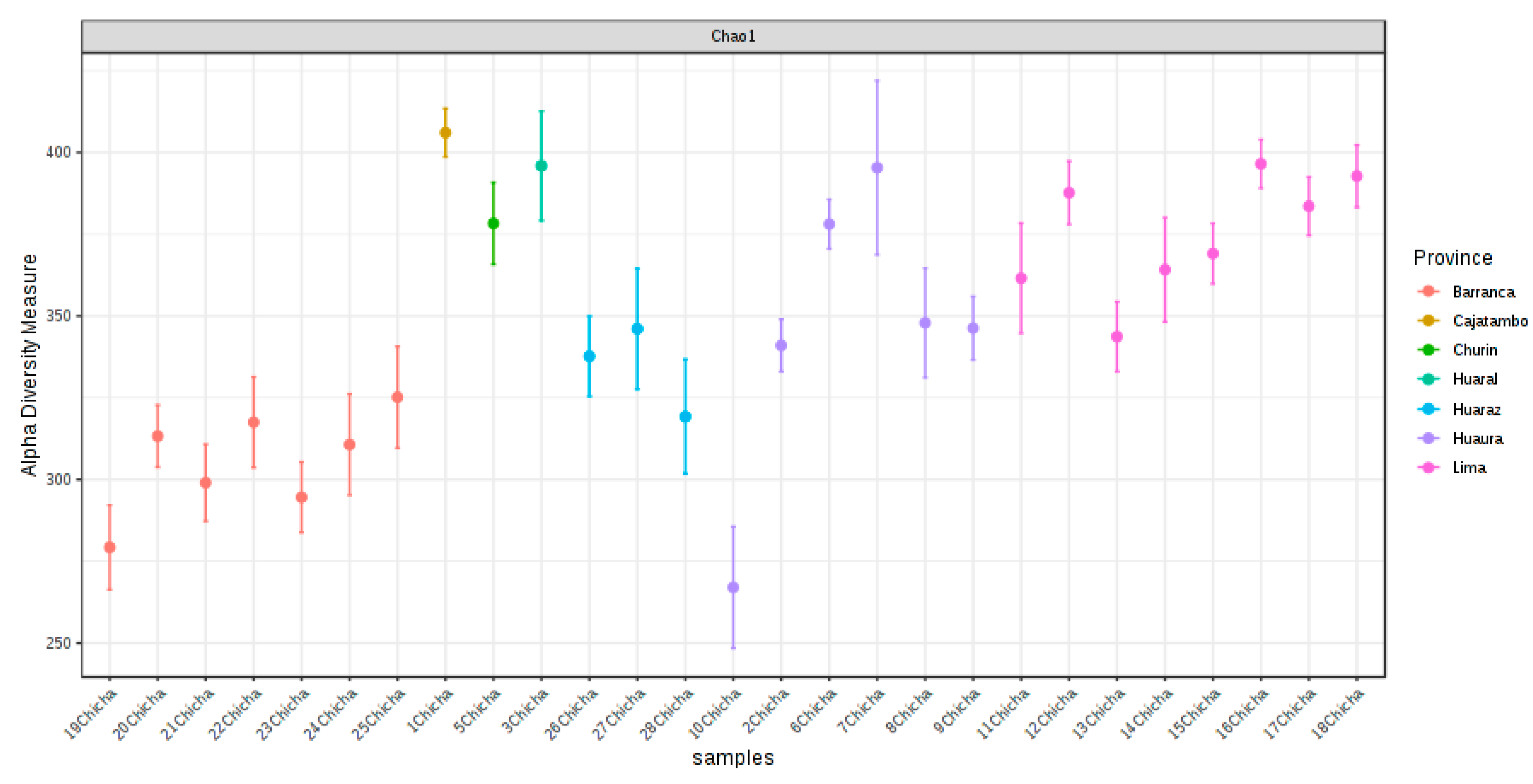
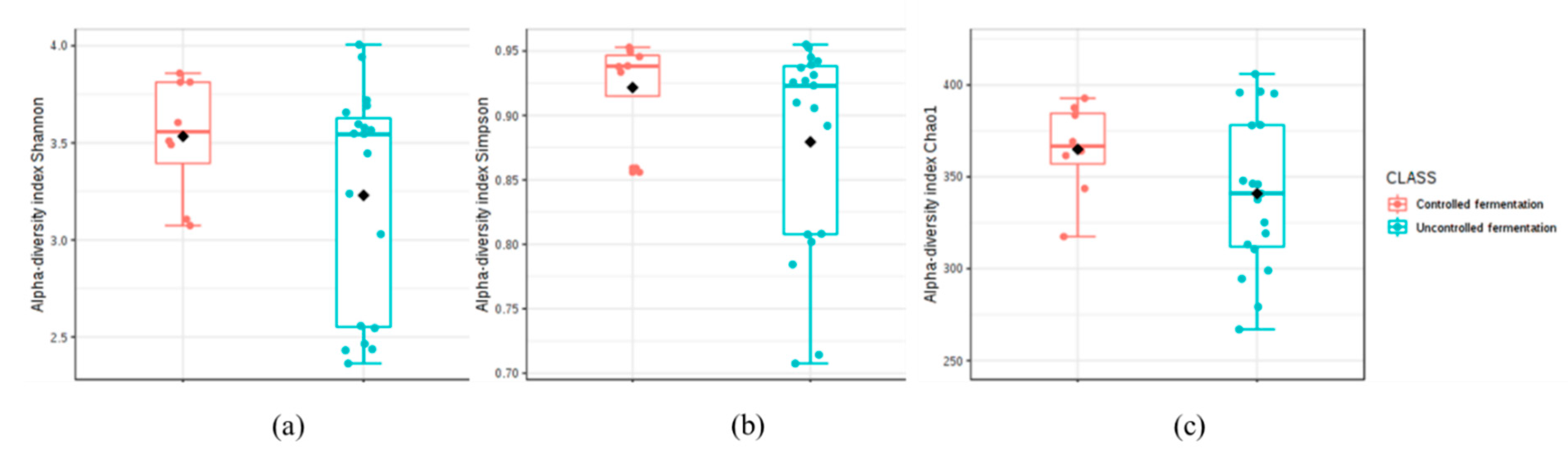
3.2. High-Throughput Sequencing (HTS) Analysis
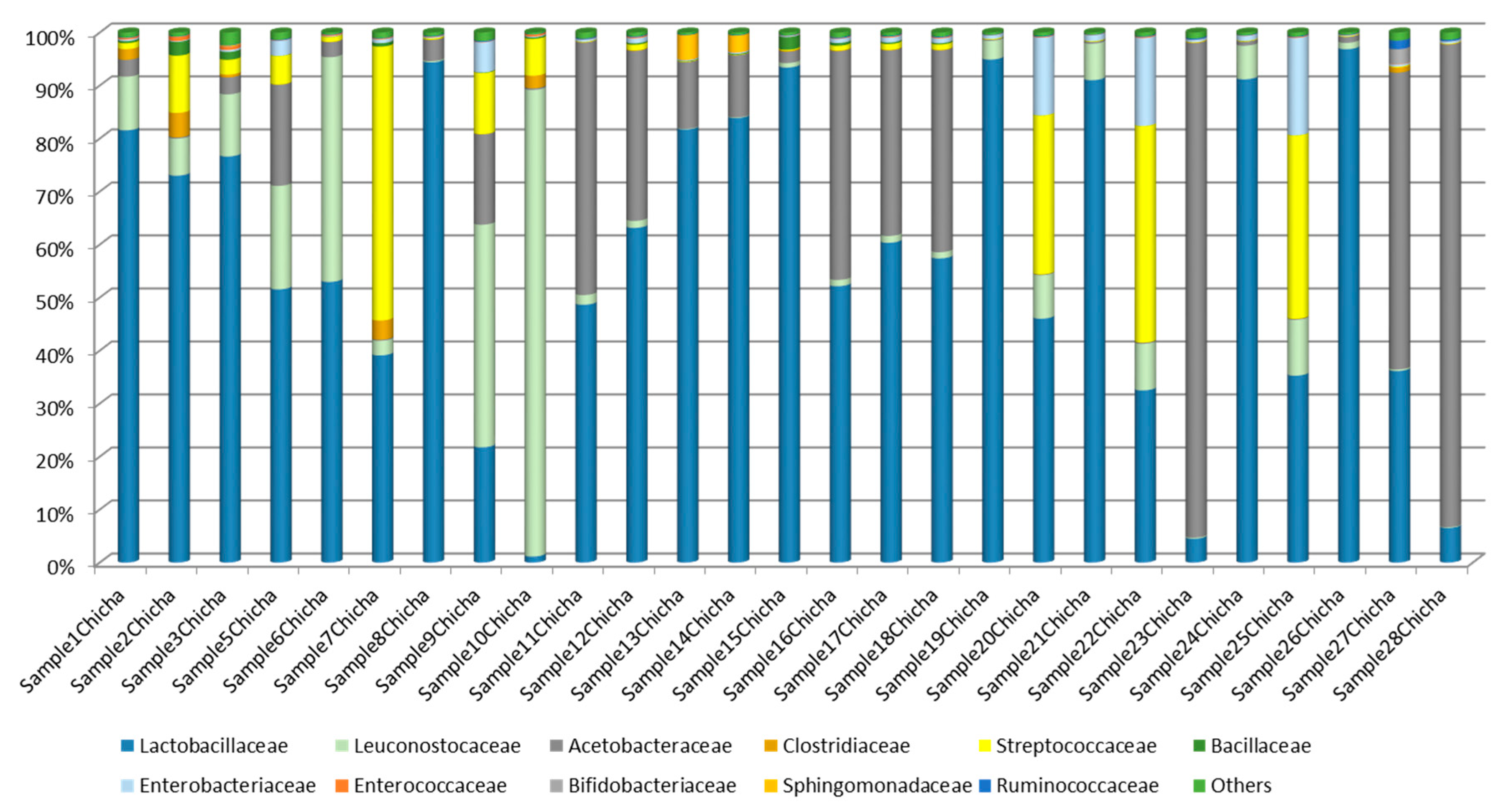
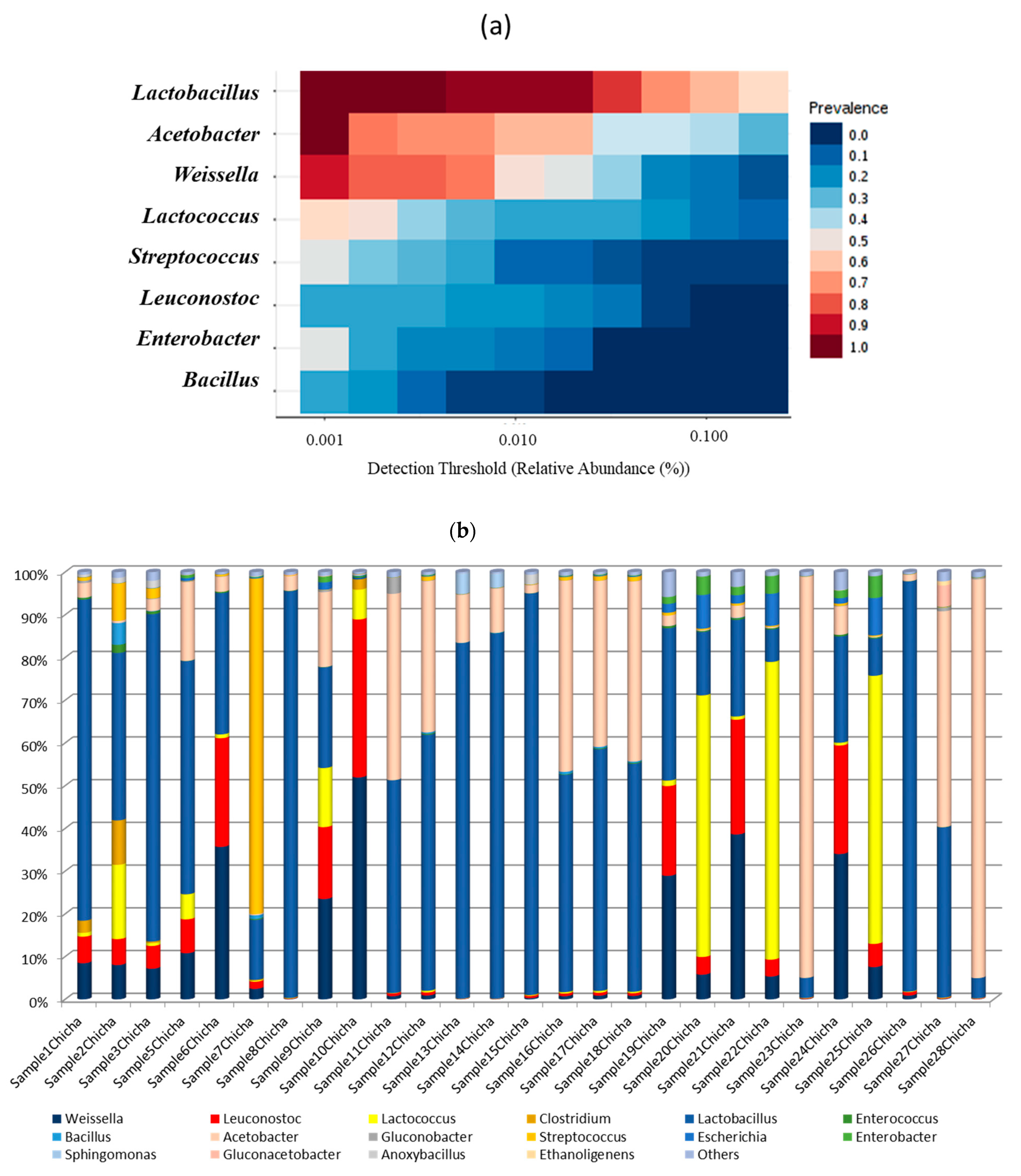
3.2.1. Abundance Profiling
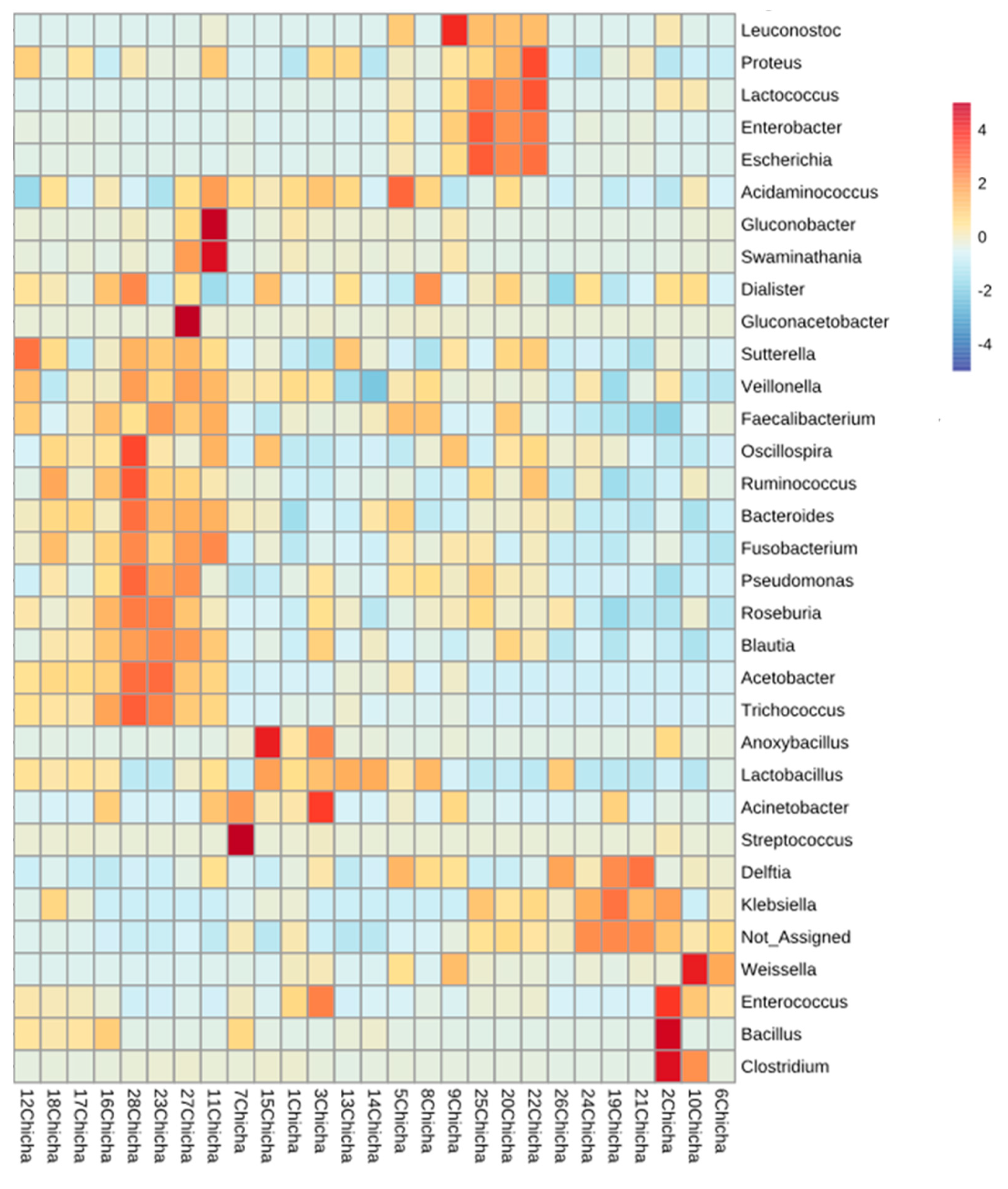
3.2.2. Bacterial Communities Profiling in Chicha Samples
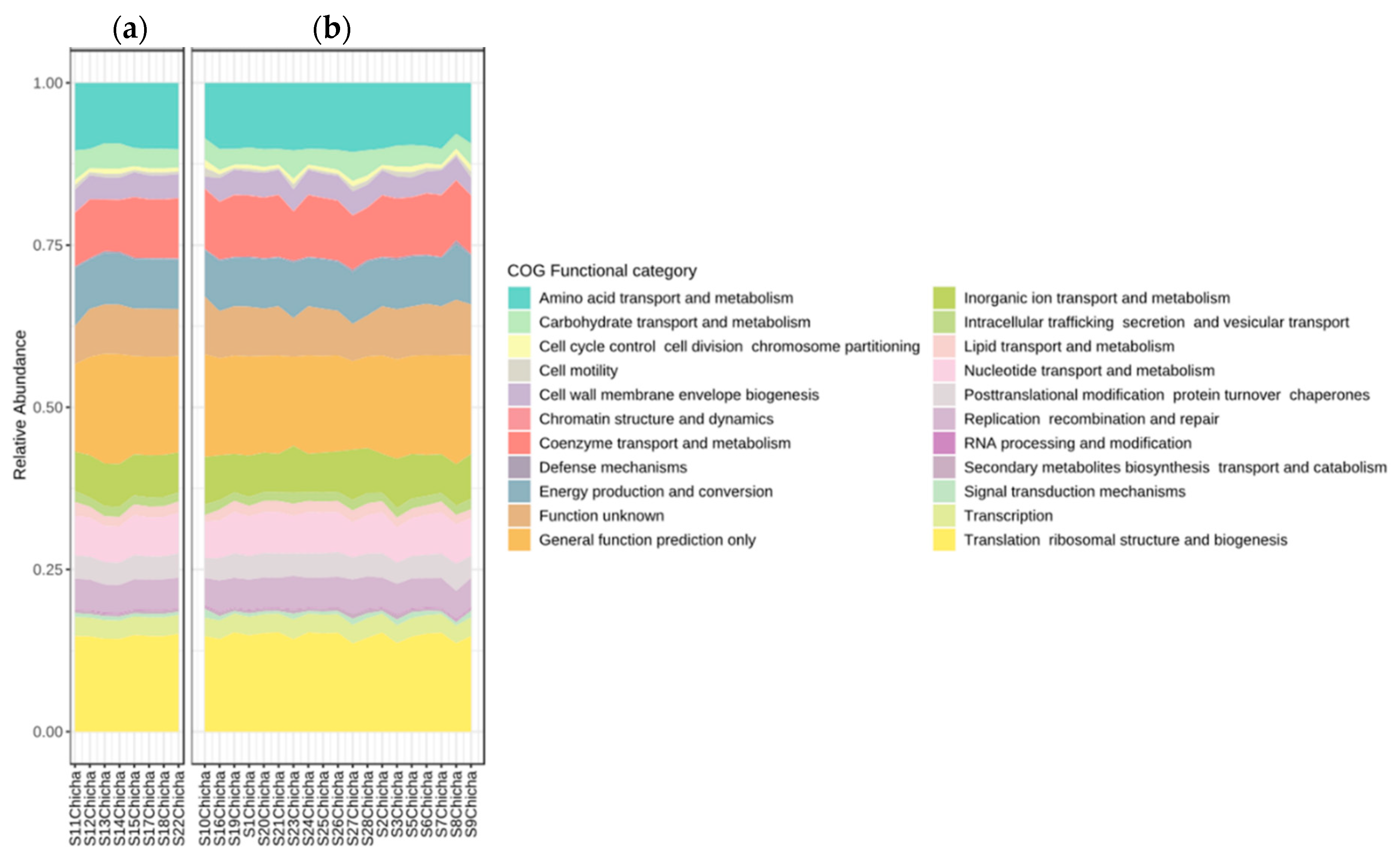
3.3. Predictions of Metabolic Potentials
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wacher, C.; Canas, A.; Bä Rzana, E.; Lappe, P.; Ulloa, M.; Owens, J.D. Microbiology of Indian and Mestizo pozol fermentations. Food Microbiol. 2000, 17, 251–256. [Google Scholar] [CrossRef]
- Blandino, A.; Al-Aseeri, M.E.; Pandiella, S.S.; Cantero, D.; Webb, C. Cereal-based fermented foods and beverages. Food Res. Int. 2003, 36, 527–543. [Google Scholar] [CrossRef]
- Osorio-Cadavid, E.; Chaves-López, C.; Tofalo, R.; Paparella, A.; Suzzi, G. Detection and identification of wild yeasts in Champús, a fermented Colombian maize beverage. Food Microbiol. 2008, 25, 771–777. [Google Scholar] [CrossRef] [PubMed]
- Delibes, R.; Barragán, A. El Consumo Ritual de Chicha en San José de Moro. In Arqueología Mochica: Nuevos Enfoques; Castillo Butters, L.J., Bernier, H., Lockard, G., Rucabado Yong, J., Eds.; Institut français d’etudes andines (IFEA) and Pontificia Universidad Catòlica del Perú (PUCP): Lima, Peru, 2008; pp. 105–117. [Google Scholar]
- Hayashida, F.M. Ancient beer and modern brewers: Ethnoarchaeological observations of chicha production in two regions of the North Coast of Peru. J. Anthropol. Archaeol. 2008, 27, 161–174. [Google Scholar] [CrossRef]
- Quillama, E.; Liendo, N. Aislamiento e identificación de bacterias lácticas asociadas a Chicha de Jora. Bol. Lima 1995, 100, 171–180. [Google Scholar]
- Steinkraus, K.H. Fermentation in world food processing. Compr. Rev. Food Sci. Food Saf. 2002, 1, 23–32. [Google Scholar] [CrossRef]
- Vallejo, J.A.; Miranda, P.; Flores-Félix, J.D.; Sánchez-Juanes, F.; Ageitos, J.M.; González-Buitrago, J.M.; Velázquez, E.; Villa, T.G. Atypical yeasts identified as Saccharomyces cerevisiae by MALDI-TOF MS and gene sequencing are the main responsible of fermentation of chicha, a traditional beverage from Peru. Syst. Appl. Microbiol. 2013, 36, 560–564. [Google Scholar] [CrossRef]
- Elizaquível, P.; Pérez-Cataluña, A.; Yépez, A.; Aristimuño, C.; Jiménez, E.; Cocconcelli, P.S.; Vignolo, G.; Aznar, R. Pyrosequencing vs. culture-dependent approaches to analyze lactic acid bacteria associated to chicha, a traditional maize-based fermented beverage from Northwestern Argentina. Int. J. Food Microbiol. 2015, 198, 9–18. [Google Scholar] [CrossRef]
- Freire, A.L.; Zapata, S.; Mosquera, J.; Mejia, M.L.; Trueba, G. Bacteria associated with human saliva are major microbial components of Ecuadorian indigenous beers (chicha). PeerJ 2016, 4, e1962. [Google Scholar] [CrossRef]
- Mendoza, L.M.; Neef, A.; Vignolo, G.; Belloch, C. Yeast diversity during the fermentation of Andean chicha: A comparison of high-throughput sequencing and culture-dependent approaches. Food Microbiol. 2017, 67, 1–10. [Google Scholar] [CrossRef]
- Puerari, C.; Magalhães-Guedes, K.T.; Schwan, R.F. Physicochemical and microbiological characterization of chicha, a rice-based fermented beverage produced by Umutina Brazilian Amerindians. Food Microbiol. 2015, 46, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Resende, L.V.; Pinheiro, L.K.; Miguel, M.G.d.C.P.; Ramos, C.L.; Vilela, D.M.; Schwan, R.F. Microbial community and physicochemical dynamics during the production of ‘Chicha’, a traditional beverage of Indigenous people of Brazil. World J. Microbiol. Biotechnol. 2018, 34, 46. [Google Scholar] [CrossRef] [PubMed]
- Fontana, C.; Bassi, D.; López, C.; Pisacane, V.; Otero, M.C.; Puglisi, E.; Rebecchi, A.; Cocconcelli, P.S.; Vignolo, G. Microbial ecology involved in the ripening of naturally fermented llama meat sausages. A focus on lactobacilli diversity. Int. J. Food Microbiol. 2016, 236, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef]
- McDonald, D.; Price, M.N.; Goodrich, J.; Nawrocki, E.P.; Desantis, T.Z.; Probst, A.; Andersen, G.L.; Knight, R.; Hugenholtz, P. An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. ISME J. 2012, 6, 610–618. [Google Scholar] [CrossRef]
- Pérez-Losada, M.; Cabezas, P.; Castro-Nallar, E.; Crandall, K.A. Pathogen typing in the genomics era: MLST and the future of molecular epidemiology. Infect. Genet. Evol. 2013, 16, 38–53. [Google Scholar] [CrossRef]
- Westcott, S.L.; Schloss, P.D. OptiClust, an Improved Method for Assigning Amplicon-Based Sequence Data to Operational Taxonomic Units. mSphere 2017, 2, e00073-17. [Google Scholar] [CrossRef]
- Gray, M.A.; Pratte, Z.A.; Kellogg, C.A. Comparison of DNA preservation methods for environmental bacterial community samples. FEMS Microbiol. Ecol. 2013, 83, 468–477. [Google Scholar] [CrossRef]
- Kato, H.; Cáceres, A.G.; Mimori, T.; Ishimaru, Y.; Sayed, A.S.M.; Fujita, M.; Iwata, H.; Uezato, H.; Velez, L.N.; Gomez, E.A.L.; et al. Use of FTA Cards for Direct Sampling of Patients’ Lesions in the Ecological Study of Cutaneous Leishmaniasis. J. Clin. Microbiol. 2010, 48, 3661–3665. [Google Scholar] [CrossRef]
- Keeler, S.P.; Ferro, P.J.; Brown, J.D.; Fang, X.; El-Attrache, J.; Poulson, R.; Jackwood, M.W.; Stallknecht, D.E. Use of FTA® Sampling Cards for Molecular Detection of Avian Influenza Virus in Wild Birds. Avian Dis. 2012, 56, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Holt, J.C.; Purple, K.E.; Gerhold, R. Use of FTA technology for detection of Trichomonas gallinae. Vet. Parasitol. 2015, 212, 396–399. [Google Scholar] [CrossRef] [PubMed]
- Fábio Faria-Oliveira, R.H.S.D.; Fernanda Godoy-Santos, F.B.P.; Hygor Mezadri, I.M.C.; Brandão, R.L. The Role of Yeast and Lactic Acid Bacteria in the Production of Fermented Beverages in South America. In Food Production and Industry; Intech: London UK, 2016. [Google Scholar]
- Abriouel, H.; Ben Omar, N.; López, R.L.; Martínez-Cañamero, M.; Keleke, S.; Gálvez, A. Culture-independent analysis of the microbial composition of the African traditional fermented foods poto poto and dégué by using three different DNA extraction methods. Int. J. Food Microbiol. 2006, 111, 228–233. [Google Scholar] [CrossRef] [PubMed]
- De Vuyst, L.; Van Kerrebroeck, S.; Harth, H.; Huys, G.; Daniel, H.M.; Weckx, S. Microbial ecology of sourdough fermentations: Diverse or uniform? Food Microbiol. 2014, 37, 11–29. [Google Scholar] [CrossRef] [PubMed]
- Schoustra, S.E.; Kasase, C.; Toarta, C.; Kassen, R.; Poulain, A.J. Microbial Community Structure of Three Traditional Zambian Fermented Products: Mabisi, Chibwantu and Munkoyo. PLoS ONE 2013, 8, e63948. [Google Scholar] [CrossRef]
- Chaves-Lopez, C.; Serio, A.; Delgado-Ospina, J.; Rossi, C.; Grande-Tovar, C.D.; Paparella, A. Exploring the Bacterial Microbiota of Colombian Fermented Maize Dough “Masa Agria” (Maiz Añejo). Front. Microbiol. 2016, 7, 1168. [Google Scholar] [CrossRef]
- Pérez-Cataluña, A.; Elizaquível, P.; Carrasco, P.; Espinosa, J.; Reyes, D.; Wacher, C.; Aznar, R. Diversity and dynamics of lactic acid bacteria in Atole agrio, a traditional maize-based fermented beverage from South-Eastern Mexico, analysed by high throughput sequencing and culturing. Antonie Leeuwenhoek 2018, 111, 385–399. [Google Scholar] [CrossRef]
- De Roos, J.; De Vuyst, L. Acetic acid bacteria in fermented foods and beverages. Curr. Opin. Biotechnol. 2018, 49, 115–119. [Google Scholar] [CrossRef]
- Jung, J.Y.; Lee, S.H.; Kim, J.M.; Park, M.S.; Bae, J.W.; Hahn, Y.; Madsen, E.L.; Jeon, C.O. Metagenomic analysis of kimchi, a traditional Korean fermented food. Appl. Environ. Microbiol. 2011, 77, 2264–2274. [Google Scholar] [CrossRef]
- Fusco, V.; Quero, G.M.; Cho, G.-S.; Kabisch, J.; Meske, D.; Neve, H.; Bockelmann, W.; Franz, C.M.A.P. The genus Weissella: Taxonomy, ecology and biotechnological potential. Front. Microbiol. 2015, 6, 155. [Google Scholar] [CrossRef]
- Cao, Y.; Fanning, S.; Proos, S.; Jordan, K.; Srikumar, S. A Review on the Applications of Next Generation Sequencing Technologies as Applied to Food-Related Microbiome Studies. Front. Microbiol. 2017, 8, 1829. [Google Scholar] [CrossRef] [PubMed]
- Langille, M.G.I.; Zaneveld, J.; Caporaso, J.G.; McDonald, D.; Knights, D.; Reyes, J.A.; Clemente, J.C.; Burkepile, D.E.; Vega Thurber, R.L.; Knight, R.; et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotechnol. 2013, 31, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Goto, S.; Sato, Y.; Furumichi, M.; Tanabe, M. KEGG for integration and interpretation of large-scale molecular data sets. Nucleic Acids Res. 2011, 40, D109–D114. [Google Scholar] [CrossRef] [PubMed]
| Sample | Province | “Chicheria” | Region | Fermentation (days) | Additives | Note |
|---|---|---|---|---|---|---|
| 1chicha | Cajatambo | Cajatambo | Lima | 2 | Yellow maize, sugar, “Chancaca” | Uncontrolled fermentation |
| 2chicha | Huaura | “La Parada” Market | Lima | 2 | “Chancaca”, cinnamon (Cinnamomum verum), cloves (Syzygium aromaticum), barley (Hordeum vulgare), sugar | Uncontrolled fermentation |
| 3chicha | Huaral | “La Parada” Market | Lima | 3–4 | Sugar, barley, wheat flour, quinoa (Chenopodium quinoa), fava beans, cinnamon, cloves | Uncontrolled fermentation |
| 5chicha | Churin | Churin Central market | Lima | 2 | Barley, “Chancaca” | Uncontrolled fermentation |
| 6chicha | Huaura | Huaura | Lima | 1 | Fava beans, barley, wheat flour, quinoa, orange banana, “Chancaca” | Uncontrolled fermentation |
| 7chicha | Huaura | Huaura | Lima | 3 | Sugar, cinnamon, cloves | Uncontrolled fermentation |
| 8chicha | Huaura | Huaura | Lima | 4 | Quinoa, barley, wheat, “Chancaca” | Uncontrolled fermentation |
| 9chicha | Huaura | Huaura | Lima | 2 | Quinoa, roasted barley, wheat, “Chancaca” | Uncontrolled fermentation |
| 10chicha | Huaura | Huaura | Lima | 2 | Sugar, roasted maize (“cancha serrana”) banana peel | Uncontrolled fermentation |
| 11chicha | Lima | Restaurant “Caleta del Búho” | Lima | 3–4 | “Chancaca” | Controlled fermentation |
| 12chicha | Lima | Lima | Lima | 5 | “Chancaca”, fava beans, quinoa, barley | Controlled fermentation |
| 13chicha | Lima | Rimac | Lima | 4 | Sugar, barley, fava beans | Controlled fermentation |
| 14chicha | Lima | Restaurant “Olla internacional” | Lima | 7 | Sugar | Controlled fermentation |
| 15chicha | Lima | Restaurant “Costumbres Arequipeñas” | Lima | 3 | Chancaca, quinoa, fava beans | Controlled fermentation |
| 16chicha | Lima | Restaurant “Tradiciones Arequipeñas” | Lima | 3 | Sugar, barley, fava beans, quinoa | Uncontrolled fermentation |
| 17chicha | Lima | Restaurant “Semilla de Dioses” | Lima | 4 | Sugar, barley, fava beans, quinoa, Chenopodium pallidicaule | Controlled fermentation |
| 18chicha | Lima | Restaurant “Sabor Andino” | Lima | 3 | Sugar, Chenopodium pallidicaule, fava beans, maca (Lepidium meyenii) | Controlled fermentation |
| 19chicha | Barranca | Barranca | Lima | 15 | “Chancaca”, quinoa, maize amiláceo (Zea mays ssp amiláceo) | Uncontrolled fermentation |
| 20chicha | Barranca | Barranca | Lima | 3 | Quinoa, cinnamon, cloves, “Chancaca” | Uncontrolled fermentation |
| 21chicha | Barranca | Barranca | Lima | 2 | “Chancaca”, sugar, herbs | Uncontrolled fermentation |
| 22chicha | Barranca | Barranca | Lima | 4 | White maize, quinoa, “Chancaca”, apple | Controlled fermentation |
| 23chicha | Barranca | Barranca | Lima | 3 | Fava beans, quinoa, banana, cinnamon, “Chancaca”, cloves | Uncontrolled fermentation |
| 24chicha | Barranca | Barranca | Lima | 3 | “Chancaca”, cinnamon, fava beans, cloves | Uncontrolled fermentation |
| 25chicha | Barranca | Barranca | Lima | 4 | “Chancaca” | Uncontrolled fermentation |
| 26chicha | Huaraz | Huaraz Market | Ancash | 3 | Fava beans, quinoa, “Chancaca” | Uncontrolled fermentation |
| 27chicha | Huaraz | Huaraz Market | Ancash | 2 | Fava, white maize, “Chancaca” | Uncontrolled fermentation |
| 28chicha | Huaraz | Huaraz Market | Ancash | 2 | “Chancaca”, quinoa | Uncontrolled fermentation |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bassi, D.; Orrù, L.; Cabanillas Vasquez, J.; Cocconcelli, P.S.; Fontana, C. Peruvian chicha: A Focus on the Microbial Populations of This Ancient Maize-Based Fermented Beverage. Microorganisms 2020, 8, 93. https://doi.org/10.3390/microorganisms8010093
Bassi D, Orrù L, Cabanillas Vasquez J, Cocconcelli PS, Fontana C. Peruvian chicha: A Focus on the Microbial Populations of This Ancient Maize-Based Fermented Beverage. Microorganisms. 2020; 8(1):93. https://doi.org/10.3390/microorganisms8010093
Chicago/Turabian StyleBassi, Daniela, Luigi Orrù, Jeison Cabanillas Vasquez, Pier Sandro Cocconcelli, and Cecilia Fontana. 2020. "Peruvian chicha: A Focus on the Microbial Populations of This Ancient Maize-Based Fermented Beverage" Microorganisms 8, no. 1: 93. https://doi.org/10.3390/microorganisms8010093
APA StyleBassi, D., Orrù, L., Cabanillas Vasquez, J., Cocconcelli, P. S., & Fontana, C. (2020). Peruvian chicha: A Focus on the Microbial Populations of This Ancient Maize-Based Fermented Beverage. Microorganisms, 8(1), 93. https://doi.org/10.3390/microorganisms8010093






