The Use of Antimalarial Drugs against Viral Infection
Abstract
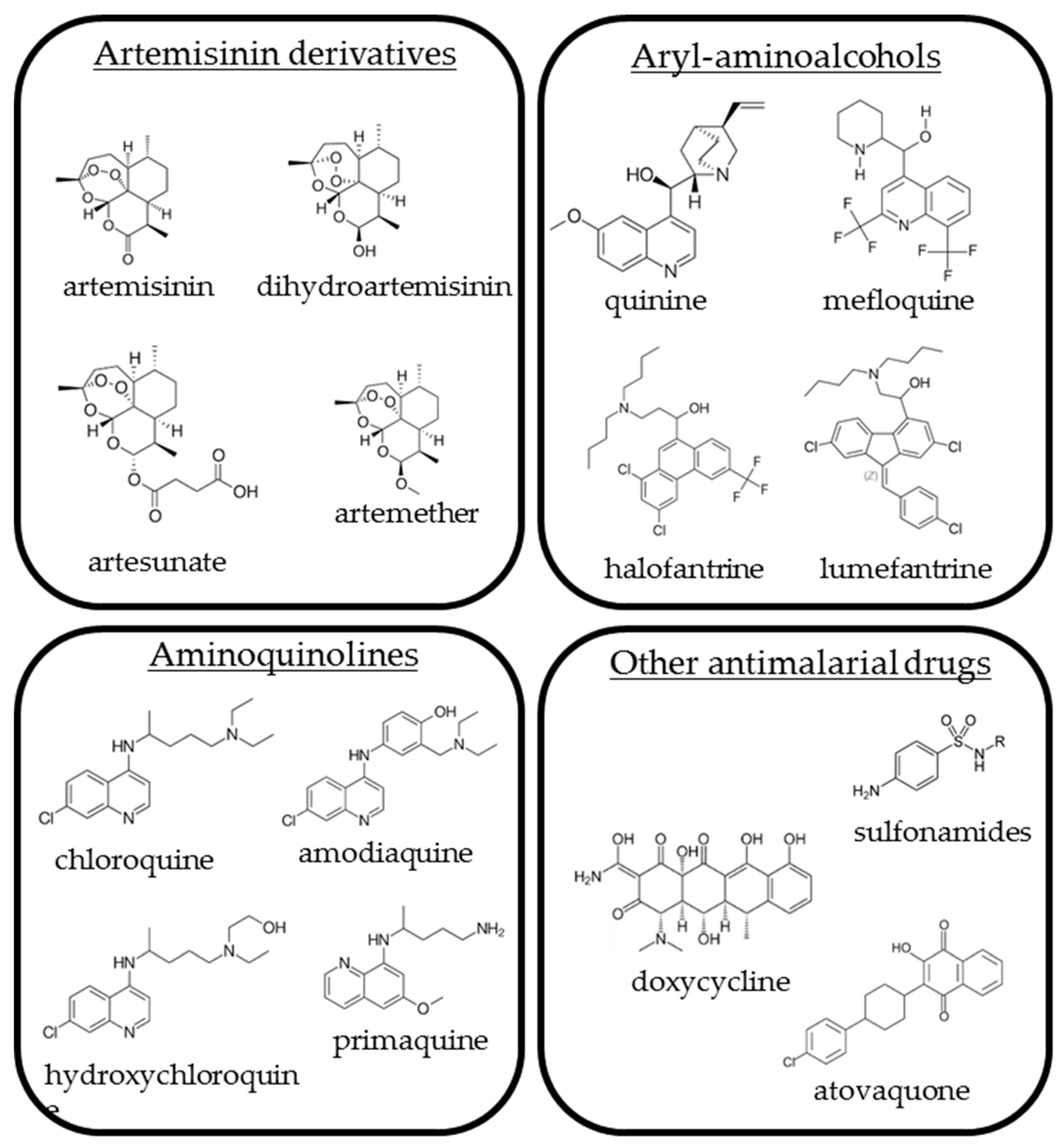
1. Introduction
2. Artemisinin Derivatives
2.1. Artemisinin
2.2. Artesunate
2.2.1. Artesunate and HCMV
2.2.2. Artesunate and Other Viruses
2.3. Other Artemisinin Derivatives
3. Aryl-Aminoalcohols
3.1. Quinine Sulfate
3.2. Mefloquine
3.2.1. Mefloquine and JCPyV
3.2.2. Mefloquine and Other Viruses
3.3. Halofantrine and Lumefantrine
4. Aminoquinolines
4.1. Chloroquine and Hydroxychloroquine and Emerging Viruses
4.2. Chloroquine and Hydroxychloroquine and HCV
4.3. Chloroquine and Hydroxychloroquine and HIV
Chloroquine and Hydroxychloroquine and Other RNA Viruses
4.4. Amodiaquine and Emerging Viruses
4.5. Primaquine
5. Other Antimalarial Drugs
5.1. Atovaquone
5.2. Antimicrobial Drugs
5.2.1. Doxycycline
5.2.2. Sulfonamides
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AQ | Amodiaquine |
| ART | Artemisinin |
| AS | Artesunate |
| CHIKV | Chikungunya virus |
| CQ | Chloroquine |
| DENV | Dengue virus |
| DHA | Dihydroartemisinin |
| EC50s | Effective concentrations |
| EBV | Epstein-Barr virus |
| HBV | Hepatitis B virus |
| HCMV | Human cytomegalovirus |
| HCV | Hepatitis C virus |
| HPV | Human papillomavirus |
| hydroxyCQ | hydroxychloroquine |
| IL | Interleukin |
| INF | Interferon |
| KSHV | Kaposi sarcoma herpesvirus |
| MQ | Mefloquine |
| RCMV | Rat cytomegalovirus |
| TNF | Tumor necrosis factor |
| VSV | Vesicular stomatitis virus |
| ZIKV | Zika virus |
References
- World Health Organization. Guidelines for the Treatment of Malaria; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Haładyj, E.; Sikora, M.; Felis-Giemza, A.; Olesińska, M. Antimalarials—Are they effective and safe in rheumatic diseases? Reumatologia 2018, 56, 164–173. [Google Scholar] [CrossRef]
- Das, A.K. Anticancer Effect of AntiMalarial Artemisinin Compounds. Ann. Med. Health Sci. Res. 2015, 5, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Wolf, R.; Tufano, M.A.; Ruocco, V.; Grimaldi, E.; Ruocco, E.; Donnarumma, G.; Baroni, A. Quinine sulfate inhibits invasion of some bacterial skin pathogens. Int. J. Dermatol. 2006, 45, 661–663. [Google Scholar] [CrossRef] [PubMed]
- Gwitira, I.; Murwira, A.; Mberikunashe, J.; Masocha, M. Spatial overlaps in the distribution of HIV/AIDS and malaria in Zimbabwe. BMC Infect. Dis. 2018, 18, 598. [Google Scholar] [CrossRef] [PubMed]
- Santana, V.O.S.; Lavezzo, L.C.; Mondini, A.; Terzian, A.C.; Bronzoni, R.V.; Rossit, A.R.; Machado, R.L.; Rahal, P.; Nogueira, M.C.; Nogueira, M.L. Concurrent Dengue and malaria in the Amazon region. Rev. Soc. Bras. Med. Trop. 2010, 43, 508–511. [Google Scholar] [CrossRef] [PubMed]
- Salam, N.; Mustafa, S.; Hafiz, A.; Chaudhary, A.A.; Deeba, F.; Parveen, S. Global prevalence and distribution of coinfection of malaria, dengue and chikungunya: A systematic review. BMC Public Health 2018, 18, 710. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y. Artemisinin-A Gift from Traditional Chinese Medicine to the World (Nobel Lecture). Angew. Chem. Int. Ed. Engl. 2016, 55, 10210–10226. [Google Scholar] [CrossRef]
- Efferth, T. Willmar Schwabe Award 2006: Antiplasmodial and antitumor activity of artemisinin—From bench to bedside. Planta Med. 2007, 73, 299–309. [Google Scholar] [CrossRef]
- Efferth, T.; Marschall, M.; Wang, X.; Huong, S.M.; Hauber, I.; Olbrich, A.; Kronschnabl, M.; Stamminger, T.; Huang, E.S. Antiviral activity of artesunate towards wild-type, recombinant, and ganciclovir-resistant human cytomegaloviruses. J. Mol. Med. 2002, 80, 233–242. [Google Scholar] [CrossRef]
- Kaptein, S.J.; Efferth, T.; Leis, M.; Rechter, S.; Auerochs, S.; Kalmer, M.; Bruggeman, C.A.; Vink, C.; Stamminger, T.; Marschall, M. The anti-malaria drug artesunate inhibits replication of cytomegalovirus in vitro and in vivo. Antivir. Res. 2006, 69, 60–69. [Google Scholar] [CrossRef]
- Shapira, M.Y.; Resnick, I.B.; Chou, S.; Neumann, A.U.; Lurain, N.S.; Stamminger, T.; Caplan, O.; Saleh, N.; Efferth, T.; Marschall, M.; et al. Artesunate as a potent antiviral agent in a patient with late drug-resistant cytomegalovirus infection after hematopoietic stem cell transplantation. Clin. Infect. Dis. 2008, 46, 1455–1457. [Google Scholar] [CrossRef] [PubMed]
- Wolf, D.G.; Shimoni, A.; Resnick, I.B.; Stamminger, T.; Neumann, A.U.; Chou, S.; Efferth, T.; Caplan, O.; Rose, J.; Nagler, A.; et al. Human cytomegalovirus kinetics following institution of artesunate after hematopoietic stem cell transplantation. Antivir. Res. 2011, 90, 183–186. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Efferth, T.; Romero, M.R.; Wolf, D.G.; Stamminger, T.; Marin, J.J.; Marschall, M. The antiviral activities of artemisinin and artesunate. Clin. Infect. Dis. 2008, 47, 804–811. [Google Scholar] [CrossRef] [PubMed]
- Efferth, T. Beyond malaria: The inhibition of viruses by artemisinin-type compounds. Biotechnol. Adv. 2018, 36, 1730–1737. [Google Scholar] [CrossRef] [PubMed]
- Flobinus, A.; Taudon, N.; Desbordes, M.; Labrosse, B.; Simon, F.; Mazeron, M.C.; Schnepf, N. Stability and antiviral activity against human cytomegalovirus of artemisinin derivatives. J. Antimicrob. Chemother. 2014, 69, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Romero, M.R.; Efferth, T.; Serrano, M.A.; Castaño, B.; Macias, R.I.; Briz, O.; Marin, J.J. Effect of artemisinin/artesunate as inhibitors of hepatitis B virus production in an “in vitro” replicative system. Antivir. Res. 2005, 68, 75–83. [Google Scholar] [CrossRef]
- Mondal, A.; Chatterji, U. Artemisinin Represses Telomerase Subunits and Induces Apoptosis in HPV-39 Infected Human Cervical Cancer Cells. J. Cell. Biochem. 2015, 116, 1968–1981. [Google Scholar] [CrossRef]
- Disbrow, G.L.; Baege, A.C.; Kierpiec, K.A.; Yuan, H.; Centeno, J.A.; Thibodeaux, C.A.; Hartmann, D.; Schlegel, R. Dihydroartemisinin is cytotoxic to papillomavirus-expressing epithelial cells in vitro and in vivo. Cancer Res. 2005, 65, 10854–10861. [Google Scholar] [CrossRef]
- Obeid, S.; Alen, J.; Nguyen, V.H.; Pham, V.C.; Meuleman, P.; Pannecouque, C.; Le, T.N.; Neyts, J.; Dehaen, W.; Paeshuyse, J. Artemisinin analogues as potent inhibitors of in vitro hepatitis C virus replication. PLoS ONE 2013, 8, e81783. [Google Scholar] [CrossRef]
- Paeshuyse, J.; Coelmont, L.; Vliegen, I.; Van hemel, J.; Vandenkerckhove, J.; Peys, E.; Sas, B.; De Clercq, E.; Neyts, J. Hemin potentiates the anti-hepatitis C virus activity of the antimalarial drug artemisinin. Biochem. Biophys. Res. Commun. 2006, 348, 139–144. [Google Scholar] [CrossRef]
- Fillebeen, C.; Rivas-Estilla, A.M.; Bisaillon, M.; Ponka, P.; Muckenthaler, M.; Hentze, M.W.; Koromilas, A.E.; Pantopoulos, K. Iron inactivates the RNA polymerase NS5B and suppresses subgenomic replication of hepatitis C Virus. J. Biol. Chem. 2005, 280, 9049–9057. [Google Scholar] [CrossRef] [PubMed]
- Oguariri, R.M.; Adelsberger, J.W.; Baseler, M.W.; Imamichi, T. Evaluation of the effect of pyrimethamine, an anti-malarial drug, on HIV-1 replication. Virus Res. 2010, 153, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Zeng, A.H.; Ou, Y.Y.; Guo, M.M.; Dai, X.; Zhou, D.Z.; Chen, R. Human embryonic lung fibroblasts treated with artesunate exhibit reduced rates of proliferation and human cytomegalovirus infection in vitro. J. Thorac. Dis. 2015, 7, 1151–1157. [Google Scholar] [CrossRef] [PubMed]
- Schnepf, N.; Corvo, J.; Pors, M.J.; Mazeron, M.C. Antiviral activity of ganciclovir and artesunate towards human cytomegalovirus in astrocytoma cells. Antivir. Res. 2011, 89, 186–188. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Forman, M.; Mott, B.T.; Venkatadri, R.; Posner, G.H.; Arav-Boger, R. Unique and highly selective anticytomegalovirus activities of artemisinin-derived dimer diphenyl phosphate stem from combination of dimer unit and a diphenyl phosphate moiety. Antimicrob. Agents Chemother. 2013, 57, 4208–4214. [Google Scholar] [CrossRef] [PubMed]
- Chou, S.; Marousek, G.; Auerochs, S.; Stamminger, T.; Milbradt, J.; Marschall, M. The unique antiviral activity of artesunate is broadly effective against human cytomegaloviruses including therapy-resistant mutants. Antivir. Res. 2011, 92, 364–368. [Google Scholar] [CrossRef] [PubMed]
- Reiter, C.; Fröhlich, T.; Zeino, M.; Marschall, M.; Bahsi, H.; Leidenberger, M.; Friedrich, O.; Kappes, B.; Hampel, F.; Efferth, T.; et al. New efficient artemisinin derived agents against human leukemia cells, human cytomegalovirus and Plasmodium falciparum: 2nd generation 1,2,4-trioxane-ferrocene hybrids. Eur. J. Med. Chem. 2015, 97, 164–172. [Google Scholar] [CrossRef]
- Hutterer, C.; Niemann, I.; Milbradt, J.; Fröhlich, T.; Reiter, C.; Kadioglu, O.; Bahsi, H.; Zeitträger, I.; Wagner, S.; Einsiedel, J.; et al. The broad-spectrum antiinfective drug artesunate interferes with the canonical nuclear factor kappa B (NF-κB) pathway by targeting RelA/p65. Antivir. Res. 2015, 124, 101–109. [Google Scholar] [CrossRef]
- He, R.; Mott, B.T.; Rosenthal, A.S.; Genna, D.T.; Posner, G.H.; Arav-Boger, R. An artemisinin-derived dimer has highly potent anti-cytomegalovirus (CMV) and anti-cancer activities. PLoS ONE 2011, 6, e24334. [Google Scholar] [CrossRef]
- Reiter, C.; Fröhlich, T.; Gruber, L.; Hutterer, C.; Marschall, M.; Voigtländer, C.; Friedrich, O.; Kappes, B.; Efferth, T.; Tsogoeva, S.B. Highly potent artemisinin-derived dimers and trimers: Synthesis and evaluation of their antimalarial, antileukemia and antiviral activities. Bioorg. Med. Chem. 2015, 23, 5452–5458. [Google Scholar] [CrossRef]
- Morère, L.; Andouard, D.; Labrousse, F.; Saade, F.; Calliste, C.A.; Cotin, S.; Aubard, Y.; Rawlinson, W.D.; Esclaire, F.; Hantz, S.; et al. Ex vivo model of congenital cytomegalovirus infection and new combination therapies. Placenta 2015, 36, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Drouot, E.; Piret, J.; Boivin, G. Artesunate demonstrates in vitro synergism with several antiviral agents against human cytomegalovirus. Antivir. Ther. 2016, 21, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Kapoor, A.; He, R.; Venkatadri, R.; Forman, M.; Posner, G.H.; Arav-Boger, R. In vitro combination of anti-cytomegalovirus compounds acting through different targets: Role of the slope parameter and insights into mechanisms of Action. Antimicrob. Agents Chemother. 2014, 58, 986–994. [Google Scholar] [CrossRef] [PubMed]
- Canivet, C.; Menasria, R.; Rhéaume, C.; Piret, J.; Boivin, G. Valacyclovir combined with artesunate or rapamycin improves the outcome of herpes simplex virus encephalitis in mice compared to antiviral therapy alone. Antivir. Res. 2015, 123, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Borges, M.C.; Castro, L.A.; Fonseca, B.A. Chloroquine use improves dengue-related symptoms. Mem. Inst. Oswaldo. Cruz. 2013, 108, 596–599. [Google Scholar] [CrossRef]
- De Lamballerie, X.; Boisson, V.; Reynier, J.C.; Enault, S.; Charrel, R.N.; Flahault, A.; Roques, P.; Le Grand, R. On chikungunya acute infection and chloroquine treatment. Vector Borne Zoonotic Dis. 2008, 8, 837–839. [Google Scholar] [CrossRef]
- Paton, N.I.; Lee, L.; Xu, Y.; Ooi, E.E.; Cheung, Y.B.; Archuleta, S.; Wong, G.; Wilder-Smith, A.; Smith, A.W. Chloroquine for influenza prevention: A randomised, double-blind, placebo controlled trial. Lancet Infect. Dis. 2011, 11, 677–683. [Google Scholar] [CrossRef]
- Sperber, K.; Louie, M.; Kraus, T.; Proner, J.; Sapira, E.; Lin, S.; Stecher, V.; Mayer, L. Hydroxychloroquine treatment of patients with human immunodeficiency virus type 1. Clin. Ther. 1995, 17, 622–636. [Google Scholar] [CrossRef]
- Sperber, K.; Chiang, G.; Chen, H.; Ross, W.; Chusid, E.; Gonchar, M.; Chow, R.; Liriano, O. Comparison of hydroxychloroquine with zidovudine in asymptomatic patients infected with human immunodeficiency virus type 1. Clin. Ther. 1997, 19, 913–923. [Google Scholar] [CrossRef]
- Paton, N.I.; Goodall, R.L.; Dunn, D.T.; Franzen, S.; Collaco-Moraes, Y.; Gazzard, B.G.; Williams, I.G.; Fisher, M.J.; Winston, A.; Fox, J.; et al. Effects of hydroxychloroquine on immune activation and disease progression among HIV-infected patients not receiving antiretroviral therapy: A randomized controlled trial. JAMA 2012, 308, 353–361. [Google Scholar] [CrossRef]
- Jacobson, J.M.; Bosinger, S.E.; Kang, M.; Belaunzaran-Zamudio, P.; Matining, R.M.; Wilson, C.C.; Flexner, C.; Clagett, B.; Plants, J.; Read, S.; et al. The Effect of Chloroquine on Immune Activation and Interferon Signatures Associated with HIV-1. AIDS Res. Hum. Retrovir. 2016, 32, 636–647. [Google Scholar] [CrossRef] [PubMed]
- Murray, S.M.; Down, C.M.; Boulware, D.R.; Stauffer, W.M.; Cavert, W.P.; Schacker, T.W.; Brenchley, J.M.; Douek, D.C. Reduction of immune activation with chloroquine therapy during chronic HIV infection. J. Virol. 2010, 84, 12082–12086. [Google Scholar] [CrossRef] [PubMed]
- Routy, J.P.; Angel, J.B.; Patel, M.; Kanagaratham, C.; Radzioch, D.; Kema, I.; Gilmore, N.; Ancuta, P.; Singer, J.; Jenabian, M.A. Assessment of chloroquine as a modulator of immune activation to improve CD4 recovery in immune nonresponding HIV-infected patients receiving antiretroviral therapy. HIV Med. 2015, 16, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Laurens, M.B.; Mungwira, R.G.; Nyirenda, O.M.; Divala, T.H.; Kanjala, M.; Muwalo, F.; Mkandawire, F.A.; Tsirizani, L.; Nyangulu, W.; Mwinjiwa, E.; et al. TSCQ study: A randomized, controlled, open-label trial of daily trimethoprim-sulfamethoxazole or weekly chloroquine among adults on antiretroviral therapy in Malawi: Study protocol for a randomized controlled trial. Trials 2016, 17, 322. [Google Scholar] [CrossRef] [PubMed]
- Lau, P.K.; Woods, M.L.; Ratanjee, S.K.; John, G.T. Artesunate is ineffective in controlling valganciclovir-resistant cytomegalovirus infection. Clin. Infect. Dis. 2011, 52, 279. [Google Scholar] [CrossRef][Green Version]
- Stuehler, C.; Stüssi, G.; Halter, J.; Nowakowska, J.; Schibli, A.; Battegay, M.; Dirks, J.; Passweg, J.; Heim, D.; Rovo, A.; et al. Combination therapy for multidrug-resistant cytomegalovirus disease. Transpl. Infect. Dis. 2015, 17, 751–755. [Google Scholar] [CrossRef]
- Germi, R.; Mariette, C.; Alain, S.; Lupo, J.; Thiebaut, A.; Brion, J.P.; Epaulard, O.; Saint Raymond, C.; Malvezzi, P.; Morand, P. Success and failure of artesunate treatment in five transplant recipients with disease caused by drug-resistant cytomegalovirus. Antivir. Res. 2014, 101, 57–61. [Google Scholar] [CrossRef]
- Gantt, S.; Huang, M.L.; Magaret, A.; Bunts, L.; Selke, S.; Wald, A.; Rosenthal, P.J.; Dorsey, G.; Casper, C. An artesunate-containing antimalarial treatment regimen did not suppress cytomegalovirus viremia. J. Clin. Virol. 2013, 58, 276–278. [Google Scholar] [CrossRef]
- Auerochs, S.; Korn, K.; Marschall, M. A reporter system for Epstein-Barr virus (EBV) lytic replication: Anti-EBV activity of the broad anti-herpesviral drug artesunate. J. Virol. Methods 2011, 173, 334–339. [Google Scholar] [CrossRef]
- Milbradt, J.; Auerochs, S.; Korn, K.; Marschall, M. Sensitivity of human herpesvirus 6 and other human herpesviruses to the broad-spectrum antiinfective drug artesunate. J. Clin. Virol. 2009, 46, 24–28. [Google Scholar] [CrossRef]
- Hakacova, N.; Klingel, K.; Kandolf, R.; Engdahl, E.; Fogdell-Hahn, A.; Higgins, T. First therapeutic use of Artesunate in treatment of human herpesvirus 6B myocarditis in a child. J. Clin. Virol. 2013, 57, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Naesens, L.; Bonnafous, P.; Agut, H.; De Clercq, E. Antiviral activity of diverse classes of broad-acting agents and natural compounds in HHV-6-infected lymphoblasts. J. Clin. Virol. 2006, 37 (Suppl. S1), S69–S75. [Google Scholar] [CrossRef]
- Sharma, B.N.; Marschall, M.; Rinaldo, C.H. Antiviral effects of artesunate on JC polyomavirus replication in COS-7 cells. Antimicrob. Agents Chemother. 2014, 58, 6724–6734. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.N.; Marschall, M.; Henriksen, S.; Rinaldo, C.H. Antiviral effects of artesunate on polyomavirus BK replication in primary human kidney cells. Antimicrob. Agents Chemother. 2014, 58, 279–289. [Google Scholar] [CrossRef]
- Cook, L. Polyomaviruses. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef]
- Dai, R.; Xiao, X.; Peng, F.; Li, M.; Gong, G. Artesunate, an anti-malarial drug, has a potential to inhibit HCV replication. Virus Genes 2016, 52, 22–28. [Google Scholar] [CrossRef]
- Gignoux, E.; Azman, A.S.; de Smet, M.; Azuma, P.; Massaquoi, M.; Job, D.; Tiffany, A.; Petrucci, R.; Sterk, E.; Potet, J.; et al. Effect of Artesunate-Amodiaquine on Mortality Related to Ebola Virus Disease. N. Engl. J. Med. 2016, 374, 23–32. [Google Scholar] [CrossRef]
- Lee, J.S.; Adhikari, N.K.J.; Kwon, H.Y.; Teo, K.; Siemieniuk, R.; Lamontagne, F.; Chan, A.; Mishra, S.; Murthy, S.; Kiiza, P.; et al. Anti-Ebola therapy for patients with Ebola virus disease: A systematic review. BMC Infect. Dis. 2019, 19, 376. [Google Scholar] [CrossRef]
- Garbern, S.C.; Yam, D.; Aluisio, A.R.; Cho, D.K.; Kennedy, S.B.; Massaquoi, M.; Sahr, F.; Perera, S.M.; Levine, A.C.; Liu, T. Effect of Mass Artesunate-Amodiaquine Distribution on Mortality of Patients With Ebola Virus Disease During West African Outbreak. Open Forum Infect. Dis. 2019, 6, ofz250. [Google Scholar] [CrossRef]
- Arav-Boger, R.; He, R.; Chiou, C.J.; Liu, J.; Woodard, L.; Rosenthal, A.; Jones-Brando, L.; Forman, M.; Posner, G. Artemisinin-derived dimers have greatly improved anti-cytomegalovirus activity compared to artemisinin monomers. PLoS ONE 2010, 5, e10370. [Google Scholar] [CrossRef]
- Mott, B.T.; He, R.; Chen, X.; Fox, J.M.; Civin, C.I.; Arav-Boger, R.; Posner, G.H. Artemisinin-derived dimer phosphate esters as potent anti-cytomegalovirus (anti-CMV) and anti-cancer agents: A structure-activity study. Bioorg. Med. Chem. 2013, 21, 3702–3707. [Google Scholar] [CrossRef]
- Blazquez, A.G.; Fernandez-Dolon, M.; Sanchez-Vicente, L.; Maestre, A.D.; Gomez-San Miguel, A.B.; Alvarez, M.; Serrano, M.A.; Jansen, H.; Efferth, T.; Marin, J.J.; et al. Novel artemisinin derivatives with potential usefulness against liver/colon cancer and viral hepatitis. Bioorg. Med. Chem. 2013, 21, 4432–4441. [Google Scholar] [CrossRef]
- Barger-Kamate, B.; Forman, M.; Sangare, C.O.; Haidara, A.S.; Maiga, H.; Vaidya, D.; Djimde, A.; Arav-Boger, R. Effect of artemether-lumefantrine (Coartem) on cytomegalovirus urine viral load during and following treatment for malaria in children. J. Clin. Virol. 2016, 77, 40–45. [Google Scholar] [CrossRef][Green Version]
- Achan, J.; Talisuna, A.O.; Erhart, A.; Yeka, A.; Tibenderana, J.K.; Baliraine, F.N.; Rosenthal, P.J.; D’Alessandro, U. Quinine, an old anti-malarial drug in a modern world: Role in the treatment of malaria. Malar. J. 2011, 10, 144. [Google Scholar] [CrossRef]
- Malakar, S.; Sreelatha, L.; Dechtawewat, T.; Noisakran, S.; Yenchitsomanus, P.T.; Chu, J.J.H.; Limjindaporn, T. Drug repurposing of quinine as antiviral against dengue virus infection. Virus Res. 2018, 255, 171–178. [Google Scholar] [CrossRef]
- Seeler, A.O.; Graessle, O.; Ott, W.H. Effect of quinine on influenza virus infections in mice. J. Infect. Dis. 1946, 79, 156–158. [Google Scholar] [CrossRef]
- Wolf, R.; Baroni, A.; Greco, R.; Corrado, F.; Ruocco, E.; Tufano, M.A.; Ruocco, V. Quinine sulfate and HSV replication. Dermatol. Online J. 2003, 9, 3. [Google Scholar]
- Baroni, A.; Paoletti, I.; Ruocco, E.; Ayala, F.; Corrado, F.; Wolf, R.; Tufano, M.A.; Donnarumma, G. Antiviral effects of quinine sulfate on HSV-1 HaCat cells infected: Analysis of the molecular mechanisms involved. J. Dermatol. Sci. 2007, 47, 253–255. [Google Scholar] [CrossRef] [PubMed]
- Marois, I.; Cloutier, A.; Meunier, I.; Weingartl, H.M.; Cantin, A.M.; Richter, M.V. Inhibition of influenza virus replication by targeting broad host cell pathways. PLoS ONE 2014, 9, e110631. [Google Scholar] [CrossRef] [PubMed]
- Brickelmaier, M.; Lugovskoy, A.; Kartikeyan, R.; Reviriego-Mendoza, M.M.; Allaire, N.; Simon, K.; Frisque, R.J.; Gorelik, L. Identification and characterization of mefloquine efficacy against JC virus in vitro. Antimicrob. Agents Chemother. 2009, 53, 1840–1849. [Google Scholar] [CrossRef] [PubMed]
- Kishida, S.; Tanaka, K. Mefloquine treatment in a patient suffering from progressive multifocal leukoencephalopathy after umbilical cord blood transplant. Intern. Med. 2010, 49, 2509–2513. [Google Scholar] [CrossRef] [PubMed]
- Gofton, T.E.; Al-Khotani, A.; O’Farrell, B.; Ang, L.C.; McLachlan, R.S. Mefloquine in the treatment of progressive multifocal leukoencephalopathy. J. Neurol. Neurosurg. Psychiatry 2011, 82, 452–455. [Google Scholar] [CrossRef] [PubMed]
- Beppu, M.; Kawamoto, M.; Nukuzuma, S.; Kohara, N. Mefloquine improved progressive multifocal leukoencephalopathy in a patient with systemic lupus erythematosus. Intern. Med. 2012, 51, 1245–1247. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Z.; Akaza, M.; Numasawa, Y.; Ishihara, S.; Tomimitsu, H.; Nakamichi, K.; Saijo, M.; Morio, T.; Shimizu, N.; Sanjo, N.; et al. Failure of mefloquine therapy in progressive multifocal leukoencephalopathy: Report of two Japanese patients without human immunodeficiency virus infection. J. Neurol. Sci. 2013, 324, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Ramadhani, P.; Bramantono, B.; Sedana, M.P. A Chronic Lymphocytic Leukemia Patient with Progressive Multifocal Leukoencephalopathy Caused by John Cunningham Virus. Acta Med. Indones. 2018, 50, 151–158. [Google Scholar] [PubMed]
- Nishiyama, S.; Misu, T.; Shishido-Hara, Y.; Nakamichi, K.; Saijo, M.; Takai, Y.; Takei, K.; Yamamoto, N.; Kuroda, H.; Saito, R.; et al. Fingolimod-associated PML with mild IRIS in MS: A clinicopathologic study. Neurol. Neuroimmunol. NeuroInflamm. 2018, 5, e415. [Google Scholar] [CrossRef] [PubMed]
- Ishii, J.; Shishido-Hara, Y.; Kawamoto, M.; Fujiwara, S.; Imai, Y.; Nakamichi, K.; Kohara, N. A Punctate Magnetic Resonance Imaging Pattern in a Patient with Systemic Lupus Erythematosus Is an Early Sign of Progressive Multifocal Leukoencephalopathy: A Clinicopathological Study. Intern. Med. 2018, 57, 2727–2734. [Google Scholar] [CrossRef] [PubMed]
- Dubey, D.; Zhang, Y.; Graves, D.; DeSena, A.D.; Frohman, E.; Greenberg, B. Use of interleukin-2 for management of natalizumab-associated progressive multifocal leukoencephalopathy: Case report and review of literature. Ther. Adv. Neurol. Disord. 2016, 9, 211–215. [Google Scholar] [CrossRef]
- Sano, Y.; Nakano, Y.; Omoto, M.; Takao, M.; Ikeda, E.; Oga, A.; Nakamichi, K.; Saijo, M.; Maoka, T.; Sano, H.; et al. Rituximab-associated progressive multifocal leukoencephalopathy derived from non-Hodgkin lymphoma: Neuropathological findings and results of mefloquine treatment. Intern. Med. 2015, 54, 965–970. [Google Scholar] [CrossRef]
- Garrote, H.; de la Fuente, A.; Oña, R.; Rodríguez, I.; Echevarría, J.E.; Sepúlveda, J.M.; García, J.F. Long-term survival in a patient with progressive multifocal leukoencephalopathy after therapy with rituximab, fludarabine and cyclophosphamide for chronic lymphocytic leukemia. Exp. Hematol. Oncol. 2015, 4, 8. [Google Scholar] [CrossRef][Green Version]
- Yoshida, T.; Kawamoto, M.; Togo, M.; Kohara, N.; Ito, T.; Nakamichi, K.; Saijo, M.; Mizuno, T. Progressive multifocal leukoencephalopathy developing after liver transplantation showing marked neurological symptom improvement and arrest of further deterioration of imaging findings: A case report. J. Neurol. Sci. 2015, 359, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, M.; Nosaki, Y.; Matsui, K.; Terao, S.; Kuwayama, M.; Tateyama, H.; Yoshida, M.; Hashizume, Y. Efficacy of mefloquine to progressive multifocal leukoencephalopathy initially presented with parkinsonism. Clin. Neurol. Neurosurg. 2012, 114, 728–731. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.W.; Jung, K.H.; Lee, S.T.; Moon, J.; Lim, J.A.; Byun, J.I.; Park, K.I.; Lee, S.K.; Chu, K. Mefloquine improved progressive multifocal leukoencephalopathy in a patient with immunoglobulin A nephropathy. J. Clin. Neurosci. 2014, 21, 1661–1664. [Google Scholar] [CrossRef] [PubMed]
- Gourineni, V.C.; Juvet, T.; Kumar, Y.; Bordea, D.; Sena, K.N. Progressive multifocal leukoencephalopathy in a 62-year-old immunocompetent woman. Case Rep. Neurol. Med. 2014, 2014, 549271. [Google Scholar] [CrossRef]
- Shirai, S.; Yabe, I.; Kano, T.; Shimizu, Y.; Sasamori, T.; Sato, K.; Hirotani, M.; Nonaka, T.; Takahashi, I.; Matsushima, M.; et al. Usefulness of 11C-methionine-positron emission tomography for the diagnosis of progressive multifocal leukoencephalopathy. J. Neurol. 2014, 261, 2314–2318. [Google Scholar] [CrossRef][Green Version]
- Mitsikostas, D.D.; Mastorodemos, V.; Tsagournizakis, M.; Kodounis, A.; Tsagkaropoulos, A.; Konitsiotis, S.; Toulas, P.; Papadimitriou, A.; Papadimitriou, D.; Tavernarakis, A.; et al. Natalizumab-related progressive multifocal leukoencephalopathy in Greece. Mult. Scler. Relat. Disord. 2014, 3, 203–210. [Google Scholar] [CrossRef]
- Sanchez-Quintana, A.; Breña-Atienza, J.; Marrero-Santos, C.; Alvarez-Acosta, L. Late relapse of progressive multifocal leucoencephalopathy postallogenic transplant in a young patient with CLL. BMJ Case Rep. 2013, 2013. [Google Scholar] [CrossRef]
- Schröder, A.; Lee, D.H.; Hellwig, K.; Lukas, C.; Linker, R.A.; Gold, R. Successful management of natalizumab-associated progressive multifocal leukoencephalopathy and immune reconstitution syndrome in a patient with multiple sclerosis. Arch. Neurol. 2010, 67, 1391–1394. [Google Scholar] [CrossRef]
- McGuire, J.L.; Fridman, V.; Wüthrich, C.; Koralnik, I.J.; Jacobs, D. Progressive multifocal leukoencephalopathy associated with isolated CD8+ T-lymphocyte deficiency mimicking tumefactive MS. J. Neurovirol. 2011, 17, 500–503. [Google Scholar] [CrossRef][Green Version]
- Pallin, M.; O’Sullivan, C.; Dodd, J.D.; McCreery, K.; Brett, F.; Farrell, M.; O’Brien, D.; Hall, W.W.; Tubridy, N.J.; Keane, M.P. A case of progressive multifocal leukoencephalopathy in a patient with sarcoidosis. QJM 2012, 105, 1011–1016. [Google Scholar] [CrossRef][Green Version]
- Christakis, P.G.; Okin, D.; Huttner, A.J.; Baehring, J.M. Progressive multifocal leukoencephalopathy in an immunocompetent patient. J. Neurol. Sci. 2013, 326, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Lindå, H.; von Heijne, A. Presymptomatic diagnosis with MRI and adequate treatment ameliorate the outcome after natalizumab-associated progressive multifocal leukoencephalopathy. Front. Neurol. 2013, 4, 11. [Google Scholar] [CrossRef] [PubMed]
- Epperla, N.; Medina-Flores, R.; Mazza, J.J.; Yale, S.H. Mirtazapine and mefloquine therapy for non-AIDS-related progressive multifocal leukoencephalopathy. WMJ 2014, 113, 242–245. [Google Scholar] [PubMed]
- Kurmann, R.; Weisstanner, C.; Kardas, P.; Hirsch, H.H.; Wiest, R.; Lämmle, B.; Furrer, H.; Du Pasquier, R.; Bassetti, C.L.; Sturzenegger, M.; et al. Progressive multifocal leukoencephalopathy in common variable immunodeficiency: Mitigated course under mirtazapine and mefloquine. J. Neurovirol. 2015, 21, 694–701. [Google Scholar] [CrossRef]
- Balak, D.M.W.; Hajdarbegovic, E.; Bramer, W.M.; Neumann, H.A.M.; Thio, H.B. Progressive multifocal leukoencephalopathy associated with fumaric acid esters treatment in psoriasis patients. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 1475–1482. [Google Scholar] [CrossRef]
- Yoshida, H.; Ohshima, K.; Toda, J.; Kusakabe, S.; Masaie, H.; Yagi, T.; Ishikawa, J. Significant improvement following combination treatment with mefloquine and mirtazapine in a patient with progressive multifocal leukoencephalopathy after allogeneic peripheral blood stem cell transplantation. Int. J. Hematol. 2014, 99, 95–99. [Google Scholar] [CrossRef]
- Calic, Z.; Cappelen-Smith, C.; Hodgkinson, S.J.; McDougall, A.; Cuganesan, R.; Brew, B.J. Treatment of progressive multifocal leukoencephalopathy-immune reconstitution inflammatory syndrome with intravenous immunoglobulin in a patient with multiple sclerosis treated with fingolimod after discontinuation of natalizumab. J. Clin. Neurosci. 2015, 22, 598–600. [Google Scholar] [CrossRef]
- Silverio, K.A.; Patel, S.A. Progressive Multifocal Leukoencephalopathy with Negative JC Virus PCR following Treatment of Follicular Lymphoma: Implications for Biologics in the Era of Targeted Cancer Therapy. Case Rep. Oncol. Med. 2015, 2015, 534529. [Google Scholar] [CrossRef]
- Fabis-Pedrini, M.J.; Xu, W.; Burton, J.; Carroll, W.M.; Kermode, A.G. Asymptomatic progressive multifocal leukoencephalopathy during natalizumab therapy with treatment. J. Clin. Neurosci. 2016, 25, 145–147. [Google Scholar] [CrossRef]
- Ikeda, J.; Matsushima, A.; Ishii, W.; Goto, T.; Takahashi, K.; Nakamichi, K.; Saijo, M.; Sekijima, Y.; Ikeda, S.I. Brain Biopsy Is More Reliable than the DNA test for JC Virus in Cerebrospinal Fluid for the Diagnosis of Progressive Multifocal Leukoencephalopathy. Intern. Med. 2017, 56, 1231–1234. [Google Scholar] [CrossRef][Green Version]
- Nambirajan, A.; Suri, V.; Kataria, V.; Sharma, M.C.; Goyal, V. Progressive multifocal leukoencephalopathy in a 44-year old male with idiopathic CD4+ T-lymphocytopenia treated with mirtazapine and mefloquine. Neurol. India 2017, 65, 1061–1064. [Google Scholar] [CrossRef]
- Ishikawa, Y.; Kasuya, T.; Ishikawa, J.; Fujiwara, M.; Kita, Y. A case of developing progressive multifocal leukoencephalopathy while using rituximab and mycophenolate mofetil in refractory systemic lupus erythematosus. Ther. Clin. Risk Manag. 2018, 14, 1149–1153. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, K.M.; Das, S.; Strong, M.; Mirsattari, S.M.; Leung, A.; Steven, D.; Hammond, R. Diagnosis of Inclusion. Can. J. Neurol. Sci. 2015, 42, 138–143. [Google Scholar] [CrossRef][Green Version]
- Nishigori, R.; Warabi, Y.; Shishido-Hara, Y.; Nakamichi, K.; Nakata, Y.; Komori, T.; Isozaki, E. Inflammatory Cerebellar PML with a CD4/CD8 ratio of 2.9 Showed a Favorable Prognosis in a Patient with Rheumatoid Arthritis: A Case Report. Intern. Med. 2019. [Google Scholar] [CrossRef]
- AlTahan, A.M.; Berger, T.; AlOrainy, I.A.; AlTahan, H. Progressive Multifocal Leukoencephalopathy in the Absence of Typical Radiological Changes: Can We Make a Diagnosis? Am. J. Case Rep. 2019, 20, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Harel, A.; Horng, S.; Gustafson, T.; Ramineni, A.; Farber, R.S.; Fabian, M. Successful treatment of progressive multifocal leukoencephalopathy with recombinant interleukin-7 and maraviroc in a patient with idiopathic CD4 lymphocytopenia. J. Neurovirol. 2018, 24, 652–655. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wright, C.; Flores, A. Asymptomatic progressive multifocal leukoencephalopathy: A case report and review of the literature. J. Med. Case Rep. 2018, 12, 187. [Google Scholar] [CrossRef] [PubMed]
- Soleimani-Meigooni, D.N.; Schwetye, K.E.; Angeles, M.R.; Ryschkewitsch, C.F.; Major, E.O.; Dang, X.; Koralnik, I.J.; Schmidt, R.E.; Clifford, D.B.; Kuhlmann, F.M.; et al. JC virus granule cell neuronopathy in the setting of chronic lymphopenia treated with recombinant interleukin-7. J. Neurovirol. 2017, 23, 141–146. [Google Scholar] [CrossRef]
- Berntsson, S.G.; Katsarogiannis, E.; Lourenço, F.; Moraes-Fontes, M.F. Progressive Multifocal Leukoencephalopathy and Systemic Lupus Erythematosus: Focus on Etiology. Case Rep. Neurol. 2016, 8, 59–65. [Google Scholar] [CrossRef]
- Di Pauli, F.; Berger, T.; Walder, A.; Maier, H.; Rhomberg, P.; Uprimny, C.; Steurer, M.; Stockhammer, G. Progressive multifocal leukoencephalopathy complicating untreated chronic lymphatic leukemia: Case report and review of the literature. J. Clin. Virol. 2014, 60, 424–427. [Google Scholar] [CrossRef]
- Ueno, T.; Sato, N.; Kon, T.; Haga, R.; Nunomura, J.I.; Nakamichi, K.; Saijo, M.; Tomiyama, M. Progressive multifocal leukoencephalopathy associated with thymoma with immunodeficiency: A case report and literature review. BMC Neurol. 2018, 18, 37. [Google Scholar] [CrossRef] [PubMed]
- Ikegawa, S.; Fujii, N.; Tadokoro, K.; Sato, K.; Iwamoto, M.; Matsuda, M.; Inomata, T.; Sugiura, H.; Asano, T.; Yoshida, S.; et al. Progressive multifocal leukoencephalopathy after T-cell replete HLA-haploidentical transplantation with post-transplantation cyclophosphamide graft-versus-host disease prophylaxis. Transpl. Infect. Dis. 2018, 20, e12850. [Google Scholar] [CrossRef] [PubMed]
- Lutz, M.; Schulze, A.B.; Rebber, E.; Wiebe, S.; Zoubi, T.; Grauer, O.M.; Keßler, T.; Kerkhoff, A.; Lenz, G.; Berdel, W.E. Progressive Multifocal Leukoencephalopathy after Ibrutinib Therapy for Chronic Lymphocytic Leukemia. Cancer Res. Treat. 2017, 49, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Wüthrich, C.; Popescu, B.F.; Gheuens, S.; Marvi, M.; Ziman, R.; Denq, S.P.; Tham, M.; Norton, E.; Parisi, J.E.; Dang, X.; et al. Natalizumab-associated progressive multifocal leukoencephalopathy in a patient with multiple sclerosis: A postmortem study. J. Neuropathol. Exp. Neurol. 2013, 72, 1043–1051. [Google Scholar] [CrossRef]
- Kalisch, A.; Wilhelm, M.; Erbguth, F.; Birkmann, J. Progressive multifocal leukoencephalopathy in patients with a hematological malignancy: Review of therapeutic options. Chemotherapy 2014, 60, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Meister, S.; Benecke, R.; König, F.B.; Großmann, A.; Zettl, U.K.; Winkelmann, A. Progressive multifocal leukoencephalopathy in a patient with pre-clinical primary biliary cirrhosis. Clin. Neurol. Neurosurg. 2014, 123, 45–49. [Google Scholar] [CrossRef]
- Motte, J.; Kneiphof, J.; Straßburger-Krogias, K.; Klasing, A.; Adams, O.; Haghikia, A.; Gold, R. Detection of JC virus archetype in cerebrospinal fluid in a MS patient with dimethylfumarate treatment without lymphopenia or signs of PML. J. Neurol. 2018, 265, 1880–1882. [Google Scholar] [CrossRef]
- Zucker, B.E.; Stacpoole, S.R.L. Progressive multifocal leukoencephalopathy in the absence of immunosuppression. J. Neurovirol. 2018, 24, 119–122. [Google Scholar] [CrossRef]
- Sanjo, N.; Kina, S.; Shishido-Hara, Y.; Nose, Y.; Ishibashi, S.; Fukuda, T.; Maehara, T.; Eishi, Y.; Mizusawa, H.; Yokota, T. Progressive Multifocal Leukoencephalopathy with Balanced CD4/CD8 T-Cell Infiltration and Good Response to Mefloquine Treatment. Intern. Med. 2016, 55, 1631–1635. [Google Scholar] [CrossRef]
- Hervás, J.V.; Presas-Rodríguez, S.; Crespo-Cuevas, A.M.; Canento, T.; Lozano-Sánchez, M.; Massuet-Vilamajó, A.; Ramo-Tello, C. Progressive multifocal leukoencephalopathy associated to natalizumab extended dosing regimen. Neurodegener. Dis. Manag. 2015, 5, 399–402. [Google Scholar] [CrossRef]
- Mikita, K.; Maeda, T.; Fujikura, Y.; Kozaki, Y.; Hara, Y.; Kanoh, S.; Kishida, S.; Saijo, M.; Nakamichi, K.; Kawana, A. Does anti-JCV therapy improve the prognosis of AIDS-related PML? Clin. Neurol. Neurosurg. 2013, 115, 1853–1854. [Google Scholar] [CrossRef] [PubMed]
- Young, B.E.; Yeo, T.R.; Lim, H.T.; Vong, K.Y.; Tan, K.; Lye, D.C.; Lee, C.C. Progressive Multifocal Leukoencephalopathy with Immune Reconstitution Inflammatory Syndrome (PML-IRIS): Two case reports of successful treatment with mefloquine and a review of the literature. Ann. Acad. Med. Singap. 2012, 41, 620–624. [Google Scholar] [PubMed]
- Adachi, E.; Koibuchi, T.; Imai, K.; Kikuchi, T.; Koga, M.; Nakamura, H.; Miura, T.; Iwamoto, A.; Fujii, T. Favourable outcome of progressive multifocal leukoencephalopathy with mefloquine treatment in combination with antiretroviral therapy in an HIV-infected patient. Int. J. STD AIDS 2012, 23, 603–605. [Google Scholar] [CrossRef] [PubMed]
- Naito, K.; Ueno, H.; Sekine, M.; Kanemitsu, M.; Ohshita, T.; Nakamura, T.; Yamawaki, T.; Matsumoto, M. Akinetic mutism caused by HIV-associated progressive multifocal leukoencephalopathy was successfully treated with mefloquine: A serial multimodal MRI Study. Intern. Med. 2012, 51, 205–209. [Google Scholar] [CrossRef][Green Version]
- Kawakami, T.; Sakai, K.; Mimura, Y.; Senoo, Y.; Hirabayashi, Y.; Nakazawa, H.; Koshihara, H.; Oguchi, K.; Takei, Y.; Ohara, S.; et al. Development of primary central nervous system lymphoma associated with human immunodeficiency virus and JC virus infection. J. Clin. Exp. Hematop. 2014, 54, 211–217. [Google Scholar] [CrossRef]
- Moenster, R.P.; Jett, R.A. Mirtazapine and mefloquine therapy for progressive multifocal leukoencephalopathy in a patient infected with human immunodeficiency virus. Am. J. Health Syst. Pharm. 2012, 69, 496–498. [Google Scholar] [CrossRef]
- Iannetta, M.; Bellizzi, A.; Lo Menzo, S.; Anzivino, E.; D’Abramo, A.; Oliva, A.; D’Agostino, C.; d’Ettorre, G.; Pietropaolo, V.; Vullo, V.; et al. HIV-associated progressive multifocal leukoencephalopathy: Longitudinal study of JC virus non-coding control region rearrangements and host immunity. J. Neurovirol. 2013, 19, 274–279. [Google Scholar] [CrossRef]
- Clifford, D.B.; Nath, A.; Cinque, P.; Brew, B.J.; Zivadinov, R.; Gorelik, L.; Zhao, Z.; Duda, P. A study of mefloquine treatment for progressive multifocal leukoencephalopathy: Results and exploration of predictors of PML outcomes. J. Neurovirol. 2013, 19, 351–358. [Google Scholar] [CrossRef]
- Barrows, N.J.; Campos, R.K.; Powell, S.T.; Prasanth, K.R.; Schott-Lerner, G.; Soto-Acosta, R.; Galarza-Muñoz, G.; McGrath, E.L.; Urrabaz-Garza, R.; Gao, J.; et al. A Screen of FDA-Approved Drugs for Inhibitors of Zika Virus Infection. Cell Host Microbe 2016, 20, 259–270. [Google Scholar] [CrossRef]
- Balasubramanian, A.; Teramoto, T.; Kulkarni, A.A.; Bhattacharjee, A.K.; Padmanabhan, R. Antiviral activities of selected antimalarials against dengue virus type 2 and Zika virus. Antivir. Res 2017, 137, 141–150. [Google Scholar] [CrossRef]
- Sun, W.; He, S.; Martínez-Romero, C.; Kouznetsova, J.; Tawa, G.; Xu, M.; Shinn, P.; Fisher, E.; Long, Y.; Motabar, O.; et al. Synergistic drug combination effectively blocks Ebola virus infection. Antivir. Res. 2017, 137, 165–172. [Google Scholar] [CrossRef]
- Nevin, R.L. A serious nightmare: Psychiatric and neurologic adverse reactions to mefloquine are serious adverse reactions. Pharmacol. Res. Perspect. 2017, 5. [Google Scholar] [CrossRef] [PubMed]
- Mazzon, M.; Ortega-Prieto, A.M.; Imrie, D.; Luft, C.; Hess, L.; Czieso, S.; Grove, J.; Skelton, J.K.; Farleigh, L.; Bugert, J.J.; et al. Identification of Broad-Spectrum Antiviral Compounds by Targeting Viral Entry. Viruses 2019, 11, 176. [Google Scholar] [CrossRef] [PubMed]
- Al-Bari, M.A. Chloroquine analogues in drug discovery: New directions of uses, mechanisms of actions and toxic manifestations from malaria to multifarious diseases. J. Antimicrob. Chemother. 2015, 70, 1608–1621. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, Y.; Yamamoto, S.; Homma, M.; Ishida, N. Effect of chloroquine on the growth of animal viruses. Arch. Gesamte Virusforsch. 1972, 36, 93–104. [Google Scholar] [CrossRef]
- Inglot, A.D. Comparison of the antiviral activity in vitro of some non-steroidal anti-inflammatory drugs. J. Gen. Virol. 1969, 4, 203–214. [Google Scholar] [CrossRef]
- Khan, M.; Santhosh, S.R.; Tiwari, M.; Lakshmana Rao, P.V.; Parida, M. Assessment of in vitro prophylactic and therapeutic efficacy of chloroquine against Chikungunya virus in vero cells. J. Med. Virol. 2010, 82, 817–824. [Google Scholar] [CrossRef]
- Sourisseau, M.; Schilte, C.; Casartelli, N.; Trouillet, C.; Guivel-Benhassine, F.; Rudnicka, D.; Sol-Foulon, N.; Le Roux, K.; Prevost, M.C.; Fsihi, H.; et al. Characterization of reemerging chikungunya virus. PLoS Pathog. 2007, 3, e89. [Google Scholar] [CrossRef]
- Han, Y.; Mesplède, T.; Xu, H.; Quan, Y.; Wainberg, M.A. The antimalarial drug amodiaquine possesses anti-ZIKA virus activities. J. Med. Virol. 2018, 90, 796–802. [Google Scholar] [CrossRef]
- Delvecchio, R.; Higa, L.M.; Pezzuto, P.; Valadão, A.L.; Garcez, P.P.; Monteiro, F.L.; Loiola, E.C.; Dias, A.A.; Silva, F.J.; Aliota, M.T.; et al. Chloroquine, an Endocytosis Blocking Agent, Inhibits Zika Virus Infection in Different Cell Models. Viruses 2016, 8, 322. [Google Scholar] [CrossRef]
- Dowall, S.D.; Bosworth, A.; Watson, R.; Bewley, K.; Taylor, I.; Rayner, E.; Hunter, L.; Pearson, G.; Easterbrook, L.; Pitman, J.; et al. Chloroquine inhibited Ebola virus replication in vitro but failed to protect against infection and disease in the in vivo guinea pig model. J. Gen. Virol. 2015, 96, 3484–3492. [Google Scholar] [CrossRef] [PubMed]
- Madrid, P.B.; Chopra, S.; Manger, I.D.; Gilfillan, L.; Keepers, T.R.; Shurtleff, A.C.; Green, C.E.; Iyer, L.V.; Dilks, H.H.; Davey, R.A.; et al. A systematic screen of FDA-approved drugs for inhibitors of biological threat agents. PLoS ONE 2013, 8, e60579. [Google Scholar] [CrossRef] [PubMed]
- Akpovwa, H. Chloroquine could be used for the treatment of filoviral infections and other viral infections that emerge or emerged from viruses requiring an acidic pH for infectivity. Cell Biochem. Funct. 2016, 34, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Vincent, M.J.; Bergeron, E.; Benjannet, S.; Erickson, B.R.; Rollin, P.E.; Ksiazek, T.G.; Seidah, N.G.; Nichol, S.T. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol. J. 2005, 2, 69. [Google Scholar] [CrossRef] [PubMed]
- Keyaerts, E.; Li, S.; Vijgen, L.; Rysman, E.; Verbeeck, J.; Van Ranst, M.; Maes, P. Antiviral activity of chloroquine against human coronavirus OC43 infection in newborn mice. Antimicrob. Agents Chemother. 2009, 53, 3416–3421. [Google Scholar] [CrossRef] [PubMed]
- Keyaerts, E.; Vijgen, L.; Maes, P.; Neyts, J.; Van Ranst, M. In vitro inhibition of severe acute respiratory syndrome coronavirus by chloroquine. Biochem. Biophys. Res. Commun. 2004, 323, 264–268. [Google Scholar] [CrossRef]
- Blanchard, E.; Belouzard, S.; Goueslain, L.; Wakita, T.; Dubuisson, J.; Wychowski, C.; Rouillé, Y. Hepatitis C virus entry depends on clathrin-mediated endocytosis. J. Virol. 2006, 80, 6964–6972. [Google Scholar] [CrossRef]
- Mizui, T.; Yamashina, S.; Tanida, I.; Takei, Y.; Ueno, T.; Sakamoto, N.; Ikejima, K.; Kitamura, T.; Enomoto, N.; Sakai, T.; et al. Inhibition of hepatitis C virus replication by chloroquine targeting virus-associated autophagy. J. Gastroenterol. 2010, 45, 195–203. [Google Scholar] [CrossRef]
- Al-Bari, M.A.A. Targeting endosomal acidification by chloroquine analogs as a promising strategy for the treatment of emerging viral diseases. Pharmacol. Res. Perspect. 2017, 5, e00293. [Google Scholar] [CrossRef]
- Tsai, W.P.; Nara, P.L.; Kung, H.F.; Oroszlan, S. Inhibition of human immunodeficiency virus infectivity by chloroquine. AIDS Res. Hum. Retrovir. 1990, 6, 481–489. [Google Scholar] [CrossRef]
- Sperber, K.; Kalb, T.H.; Stecher, V.J.; Banerjee, R.; Mayer, L. Inhibition of human immunodeficiency virus type 1 replication by hydroxychloroquine in T cells and monocytes. AIDS Res. Hum. Retrovir. 1993, 9, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Chiang, G.; Sassaroli, M.; Louie, M.; Chen, H.; Stecher, V.J.; Sperber, K. Inhibition of HIV-1 replication by hydroxychloroquine: Mechanism of action and comparison with zidovudine. Clin. Ther. 1996, 18, 1080–1092. [Google Scholar] [CrossRef]
- Savarino, A.; Gennero, L.; Sperber, K.; Boelaert, J.R. The anti-HIV-1 activity of chloroquine. J. Clin. Virol. 2001, 20, 131–135. [Google Scholar] [CrossRef]
- Boelaert, J.R.; Piette, J.; Sperber, K. The potential place of chloroquine in the treatment of HIV-1-infected patients. J. Clin. Virol. 2001, 20, 137–140. [Google Scholar] [CrossRef]
- Savarino, A.; Lucia, M.B.; Rastrelli, E.; Rutella, S.; Golotta, C.; Morra, E.; Tamburrini, E.; Perno, C.F.; Boelaert, J.R.; Sperber, K.; et al. Anti-HIV effects of chloroquine: Inhibition of viral particle glycosylation and synergism with protease inhibitors. J. Acquir. Immune. Defic. Syndr. 2004, 35, 223–232. [Google Scholar] [CrossRef]
- Naarding, M.A.; Baan, E.; Pollakis, G.; Paxton, W.A. Effect of chloroquine on reducing HIV-1 replication in vitro and the DC-SIGN mediated transfer of virus to CD4+ T-lymphocytes. Retrovirology 2007, 4, 6. [Google Scholar] [CrossRef]
- Martinson, J.A.; Roman-Gonzalez, A.; Tenorio, A.R.; Montoya, C.J.; Gichinga, C.N.; Rugeles, M.T.; Tomai, M.; Krieg, A.M.; Ghanekar, S.; Baum, L.L.; et al. Dendritic cells from HIV-1 infected individuals are less responsive to toll-like receptor (TLR) ligands. Cell. Immunol. 2007, 250, 75–84. [Google Scholar] [CrossRef]
- Ooi, E.E.; Chew, J.S.; Loh, J.P.; Chua, R.C. In vitro inhibition of human influenza A virus replication by chloroquine. Virol. J. 2006, 3, 39. [Google Scholar] [CrossRef]
- Lin, H.Y.; Yang, Y.T.; Yu, S.L.; Hsiao, K.N.; Liu, C.C.; Sia, C.; Chow, Y.H. Caveolar endocytosis is required for human PSGL-1-mediated enterovirus 71 infection. J. Virol. 2013, 87, 9064–9076. [Google Scholar] [CrossRef]
- Tan, Y.W.; Yam, W.K.; Sun, J.; Chu, J.J.H. An evaluation of Chloroquine as a broad-acting antiviral against Hand, Foot and Mouth Disease. Antivir. Res. 2018, 149, 143–149. [Google Scholar] [CrossRef]
- Boonyasuppayakorn, S.; Reichert, E.D.; Manzano, M.; Nagarajan, K.; Padmanabhan, R. Amodiaquine, an antimalarial drug, inhibits dengue virus type 2 replication and infectivity. Antivir. Res. 2014, 106, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Baba, M.; Toyama, M.; Sakakibara, N.; Okamoto, M.; Arima, N.; Saijo, M. Establishment of an antiviral assay system and identification of severe fever with thrombocytopenia syndrome virus inhibitors. Antivir. Chem. Chemother. 2017, 25, 83–89. [Google Scholar] [CrossRef][Green Version]
- Burdick, J.R.; Durand, D.P. Primaquine diphosphate: Inhibition of Newcastle disease virus replication. Antimicrob. Agents Chemother. 1974, 6, 460–464. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nixon, G.L.; Moss, D.M.; Shone, A.E.; Lalloo, D.G.; Fisher, N.; O’Neill, P.M.; Ward, S.A.; Biagini, G.A. Antimalarial pharmacology and therapeutics of atovaquone. J. Antimicrob. Chemother. 2013, 68, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Cifuentes Kottkamp, A.; De Jesus, E.; Grande, R.; Brown, J.A.; Jacobs, A.R.; Lim, J.K.; Stapleford, K.A. Atovaquone Inhibits Arbovirus Replication through the Depletion of Intracellular Nucleotides. J. Virol. 2019, 93. [Google Scholar] [CrossRef] [PubMed]
- Castelli, F.; Odolini, S.; Autino, B.; Foca, E.; Russo, R. Malaria Prophylaxis: A Comprehensive Review. Pharmaceuticals 2010, 3, 3212–3239. [Google Scholar] [CrossRef]
- Rothan, H.A.; Mohamed, Z.; Paydar, M.; Rahman, N.A.; Yusof, R. Inhibitory effect of doxycycline against dengue virus replication in vitro. Arch. Virol. 2014, 159, 711–718. [Google Scholar] [CrossRef]
- Rothan, H.A.; Bahrani, H.; Mohamed, Z.; Teoh, T.C.; Shankar, E.M.; Rahman, N.A.; Yusof, R. A combination of doxycycline and ribavirin alleviated chikungunya infection. PLoS ONE 2015, 10, e0126360. [Google Scholar] [CrossRef]
- Wu, Z.C.; Wang, X.; Wei, J.C.; Li, B.B.; Shao, D.H.; Li, Y.M.; Liu, K.; Shi, Y.Y.; Zhou, B.; Qiu, Y.F.; et al. Antiviral activity of doxycycline against vesicular stomatitis virus in vitro. FEMS Microbiol. Lett. 2015, 362. [Google Scholar] [CrossRef][Green Version]
- Ng, H.H.; Narasaraju, T.; Phoon, M.C.; Sim, M.K.; Seet, J.E.; Chow, V.T. Doxycycline treatment attenuates acute lung injury in mice infected with virulent influenza H3N2 virus: Involvement of matrix metalloproteinases. Exp. Mol. Pathol. 2012, 92, 287–295. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, S.; Liu, Z.; Li, Y.; Tian, J.; Hu, X. A highly sensitive and selective assay of doxycycline by dualwavelength overlapping resonance Rayleigh scattering. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 124, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Polat, K.Y.; Tosun, M.S.; Ertekin, V.; Aydinli, B.; Emre, S. Brucella infection with pancytopenia after pediatric liver transplantation. Transpl. Infect. Dis. 2012, 14, 326–329. [Google Scholar] [CrossRef] [PubMed]
- Walter, M.S.; Frank, M.J.; Satué, M.; Monjo, M.; Rønold, H.J.; Lyngstadaas, S.P.; Haugen, H.J. Bioactive implant surface with electrochemically bound doxycycline promotes bone formation markers in vitro and in vivo. Dent. Mater. 2014, 30, 200–214. [Google Scholar] [CrossRef] [PubMed]
- Drugs.com. Doxycycline. 1 May 2018. Available online: http://www.drugs.com/pro/doxycycline.html (accessed on 15 December 2019).
- Briolant, S.; Wurtz, N.; Zettor, A.; Rogier, C.; Pradines, B. Susceptibility of Plasmodium falciparum isolates to doxycycline is associated with pftetQ sequence polymorphisms and pftetQ and pfmdt copy numbers. J. Infect. Dis. 2010, 201, 153–159. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, X.; Li, L.; Li, C. Doxycycline inhibits proliferation and induces apoptosis of both human papillomavirus positive and negative cervical cancer cell lines. Can. J. Physiol. Pharmacol. 2016, 94, 526–533. [Google Scholar] [CrossRef]
- Yang, J.M.; Chen, Y.F.; Tu, Y.Y.; Yen, K.R.; Yang, Y.L. Combinatorial computational approaches to identify tetracycline derivatives as flavivirus inhibitors. PLoS ONE 2007, 2, e428. [Google Scholar] [CrossRef]
- Bhattacharjee, M.K. Antimetabolites: Antibiotics That Inhibit Nucleotide Synthesis; Springer International Publishing: Basel, Switzerland, 2016; pp. 95–108. [Google Scholar]
- Supuran, C.T. Special Issue: Sulfonamides. Molecules 2017, 22, 1642. [Google Scholar] [CrossRef]
- Green, M.D.; van Eijk, A.M.; van Ter Kuile, F.O.; Ayisi, J.G.; Parise, M.E.; Kager, P.A.; Nahlen, B.L.; Steketee, R.; Nettey, H. Pharmacokinetics of sulfadoxine-pyrimethamine in HIV-infected and uninfected pregnant women in Western Kenya. J. Infect. Dis. 2007, 196, 1403–1408. [Google Scholar] [CrossRef][Green Version]
- Li, N.; Thompson, S.; Schultz, D.C.; Zhu, W.; Jiang, H.; Luo, C.; Lieberman, P.M. Discovery of selective inhibitors against EBNA1 via high throughput in silico virtual screening. PLoS ONE 2010, 5, e10126. [Google Scholar] [CrossRef]
- Angius, F.; Piras, E.; Uda, S.; Madeddu, C.; Serpe, R.; Bigi, R.; Chen, W.; Dittmer, D.P.; Pompei, R.; Ingianni, A. Antimicrobial sulfonamides clear latent Kaposi sarcoma herpesvirus infection and impair MDM2-p53 complex formation. J. Antibiot. 2017, 70, 962–966. [Google Scholar] [CrossRef]
- Caselli, E.; Galvan, M.; Santoni, F.; Alvarez, S.; de Lera, A.R.; Ivanova, D.; Gronemeyer, H.; Caruso, A.; Guidoboni, M.; Cassai, E.; et al. Retinoic acid analogues inhibit human herpesvirus 8 replication. Antivir. Ther. 2008, 13, 199–209. [Google Scholar] [PubMed]
- Krug, L.T.; Pozharskaya, V.P.; Yu, Y.; Inoue, N.; Offermann, M.K. Inhibition of infection and replication of human herpesvirus 8 in microvascular endothelial cells by alpha interferon and phosphonoformic acid. J. Virol. 2004, 78, 8359–8371. [Google Scholar] [CrossRef] [PubMed]
- Tselis, A. Evidence for viral etiology of multiple sclerosis. Semin. Neurol. 2011, 31, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Buckwold, V.E.; Beer, B.E.; Donis, R.O. Bovine viral diarrhea virus as a surrogate model of hepatitis C virus for the evaluation of antiviral agents. Antivir. Res. 2003, 60, 1–15. [Google Scholar] [CrossRef]
- Romero, M.R.; Serrano, M.A.; Vallejo, M.; Efferth, T.; Alvarez, M.; Marin, J.J. Antiviral effect of artemisinin from Artemisia annua against a model member of the Flaviviridae family, the bovine viral diarrhoea virus (BVDV). Planta Med. 2006, 72, 1169–1174. [Google Scholar] [CrossRef]
| Virus Family | Virus Species | Drug | Trial Number/Reference | Outcome |
|---|---|---|---|---|
| Herpesviridae | HCMV | Artesunate | NCT00284687 | No results |
| Papillomaviridae | HPV | Artesunate | NCT03100045 | Ongoing |
| NCT04098744 | Ongoing | |||
| Flaviviridae | HCV | Hydroxychloroquine | NCT01833845 | Terminated due to failure to recruit subjects |
| NCT01272310 | Unknown | |||
| DENV | Chloroquine | NCT00849602 | Reduction in pain but not in length of disease [36] | |
| CHIKV | Chloroquine | NCT003913131 | No difference between CQ- and placebo-treated groups [37] | |
| Orthomyxoviridae | IAV | Chloroquine | NCT01078779 | No prevention of IAV infection [38] |
| Retroviridae | HIV | Hydroxychloroquine | [39,40] | Reduction in HIV-1 RNA load in plasma |
| Hydroxychloroquine | ISRCTN30019040 | Increased HIV-1 replication and decreased CD4 numbers [41] | ||
| Chloroquine | NCT00819390 | Modest reduction in immune activation [42] | ||
| Chloroquine | NCT02004314 | Patients did not experience any improvement after CQ treatment [43] | ||
| Chloroquine | NCT01650558 | Terminated, awaiting results [44,45] |
| Case Reports | |||
|---|---|---|---|
| HIV Status | Treatment | Outcome | References |
| HIV negative | Mefloquine | No PML progression | [72,73,74,77,78,79,81,82,83,84,86,87] |
| Fatal outcome | [75,76,80,85,88] | ||
| Mefloquine + mirtazapine | PML resolution with claimed effects | [86,87,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105] | |
| PML resolution due to other factors | [96,106,107,108,109] | ||
| Fatal outcome | [87,96,110,111,112,113,114,115,116] | ||
| Therapy suspension due to side effects | [117] | ||
| Mefloquine + mirtazapine + third partner drug | PML resolution | [118] | |
| Fatal outcome | [119] | ||
| Mefloquine + risperidone | Fatal outcome | [75] | |
| Mefloquine + risperidone + cytarabine | PML resolution | [120] | |
| Mefloquine + cidofovir | PML resolution due to other factors | [121] | |
| HIV positive | Mefloquine | No PML progression | [122,123,124,125] |
| Fatal outcome | [126] | ||
| Mefloquine + mirtazapine | Premature fatal outcome | [127,128] | |
| Clinical Trial | |||
| HIV negative versus HIV positive patients | Standard of care (SOC) versus SOC + mefloquine | Lack of differences: study ended prematurely | [129] |
| Virus Family | Virus Species | Drug | Type of Study (Model) | References |
|---|---|---|---|---|
| Herpesviridae | HSV-1 | Quinine sulfate | in vivo | [68,69] |
| EBV | Artesunate | in vitro | [50] | |
| Sulfonamides | in vitro | [182] | ||
| HCMV | Artemisinin | in vitro | [16] | |
| Artesunate | in vitro | [10,11,16,17,24,25,26,27,28,29,30,31,32,33,34] | ||
| Arthemeter | in vitro | [61,62] | ||
| HHV-6 | Artesunate | in vitro | [51,53] | |
| HHV-6B | Artesunate | in vitro | [52] | |
| KSHV | Doxycycline | in vitro | [183] | |
| HSE | Artemisinin | in vivo (mouse) | [35] | |
| Dihydroartemisinin | in vitro | [16] | ||
| KSHV | Sulfonamides | in vitro | [183] | |
| RCMV | Artesunate | in vivo (rat) | [11] | |
| Polyomaviridae | BKPyV | Artesunate | in vitro | [55] |
| JCPyV | Artesunate | in vitro | [54] | |
| Mefloquine | in vitro | [71] | ||
| Hepadnaviridae | HBV | Artemisinin, artesunate | in vitro | [17] |
| Papillomaviridae | HPV | Artemisinin | in vitro | [18] |
| Dihydroartemisinin | in vivo (dog) | [19] | ||
| Doxycycline | in vitro | [177] | ||
| Flaviviridae | BVDV(surrogate HCV) | Artemisinin | in vitro | [187,188] |
| Dihydroartemisinin | in vitro | [63] | ||
| HCV | Artemisinin | in vitro | [20,21,22] | |
| Artesunate | in vitro | [57] | ||
| Chloroquine | in vitro | [148,149,150] | ||
| ZIKV | Mefloquine | in vitro | [130,131] | |
| Chloroquine | in vitro | [140,141] | ||
| Amodiaquine | in vitro | [140] | ||
| Atovaquone | in vitro | [166] | ||
| DENV | Quinine sulfate | in vitro | [66] | |
| Mefloquine | in vitro | [131] | ||
| Halofantrine | in vitro | [134] | ||
| Doxycycline | in vitro | [168,178] | ||
| Amodiaquine | in vitro | [162] | ||
| CHIKV | Doxycycline | in vitro | [169] | |
| Chloroquine | in vitro | [37,138,139] | ||
| Atovaquone | in vitro | [166] | ||
| Togaviridae | SFV | Halofantrine | in vitro | [134] |
| Rhabdoviridae | VSV | Doxycycline | in vitro | [170] |
| Orthomyxoviridae | IAV | Quinine sulfate | in vitro | [70] |
| In vivo (mouse) | [67] | |||
| Mefloquine | in vitro | [70] | ||
| Doxycycline | in vivo (mouse) | [171] | ||
| Chloroquine | in vitro | [159] | ||
| Coronaviridae | CoV | Chloroquine | in vitro | [145,146,147] |
| Picornaviridae | Enteroviruses | Chloroquine | in vitro | [160] |
| Chloroquine | in vitro/in vivo (mouse) | [161] | ||
| Filoviridae | EBOV | Chloroquine | in vitro | [142,143] |
| in vivo | [142] | |||
| Artesunate, amodiaquine | in vivo | [58] | ||
| Mefloquine | in vitro | [132] | ||
| Retroviridae | HIV | Artemisinin | in vitro | [23] |
| Doxycycline | in vitro | [23] | ||
| Mefloquine, toremifene, posaconazole | in vitro | [132] | ||
| Chloroquine | in vitro | [151,157,158] | ||
| in vivo | [155] | |||
| Chloroquine, hydroxychloroquine | in vitro | [152,153,154,156] | ||
| Phenuiviridae | SFTSV | Amodiaquine | In vitro | [163] |
| Virus Family | Virus Species | Drug | References |
|---|---|---|---|
| Herpesviridae | HCMV | Artesunate | [12,13,46,47,48] |
| Artesunate-amodiaquine | [49] | ||
| Arthemeter-lumefantrine | [64] | ||
| HHV6 | Artesunate | [52] | |
| Polyomaviridae | JCPyV | Artesunate | [54,55] |
| Mefloquine | [72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,122,123,124,125,126] | ||
| Mefloquine and mirtazapine | [86,87,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,127,128] | ||
| Mefloquine, mirtazapine and a third drug | [118,119] | ||
| Mefloquine and risperidone | [75] | ||
| Mefloquine, risperidone and cytarabine | [120] | ||
| Mefloquine and cidofovir | [121] | ||
| Mefloquine and PML standard of care | [129] | ||
| Filoviridae | EBOV | Artesunate-amodiaquine | [58,60] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Alessandro, S.; Scaccabarozzi, D.; Signorini, L.; Perego, F.; Ilboudo, D.P.; Ferrante, P.; Delbue, S. The Use of Antimalarial Drugs against Viral Infection. Microorganisms 2020, 8, 85. https://doi.org/10.3390/microorganisms8010085
D’Alessandro S, Scaccabarozzi D, Signorini L, Perego F, Ilboudo DP, Ferrante P, Delbue S. The Use of Antimalarial Drugs against Viral Infection. Microorganisms. 2020; 8(1):85. https://doi.org/10.3390/microorganisms8010085
Chicago/Turabian StyleD’Alessandro, Sarah, Diletta Scaccabarozzi, Lucia Signorini, Federica Perego, Denise P. Ilboudo, Pasquale Ferrante, and Serena Delbue. 2020. "The Use of Antimalarial Drugs against Viral Infection" Microorganisms 8, no. 1: 85. https://doi.org/10.3390/microorganisms8010085
APA StyleD’Alessandro, S., Scaccabarozzi, D., Signorini, L., Perego, F., Ilboudo, D. P., Ferrante, P., & Delbue, S. (2020). The Use of Antimalarial Drugs against Viral Infection. Microorganisms, 8(1), 85. https://doi.org/10.3390/microorganisms8010085






