HHV-6A Infection and Systemic Sclerosis: Clues of a Possible Association
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Clinical Samples
2.3. HHV-6 Serology
2.4. Analysis of KIR Type
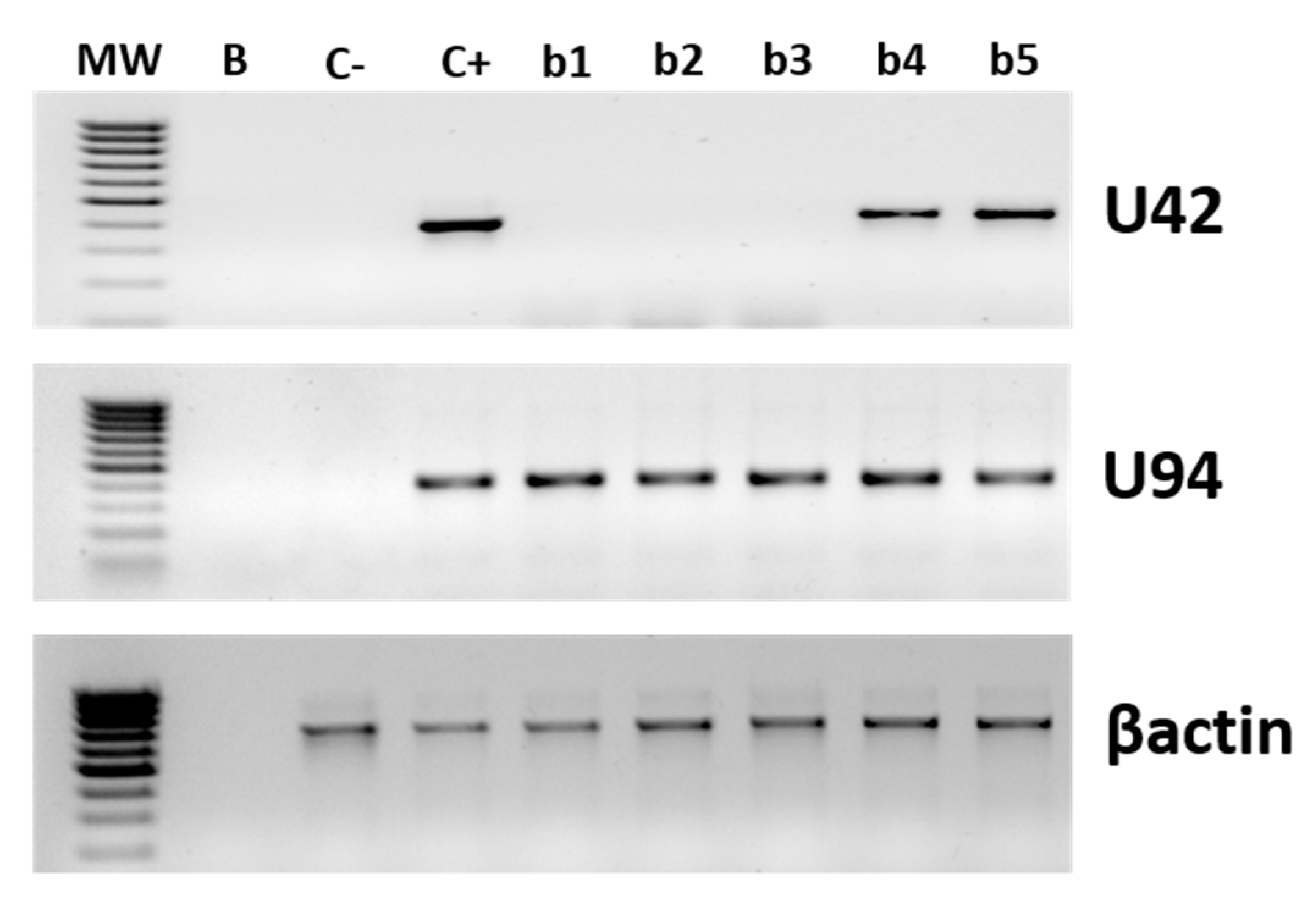
2.5. Virus DNA and RNA Analyses
2.6. Cells and Viruses for In Vitro Virus Infection
2.7. Endothelial Cell In Vitro Infection
2.8. Analysis of the Expression of Fibrosis-Associated Factors
2.9. Fluorospot Assay for Immune Cell Activation
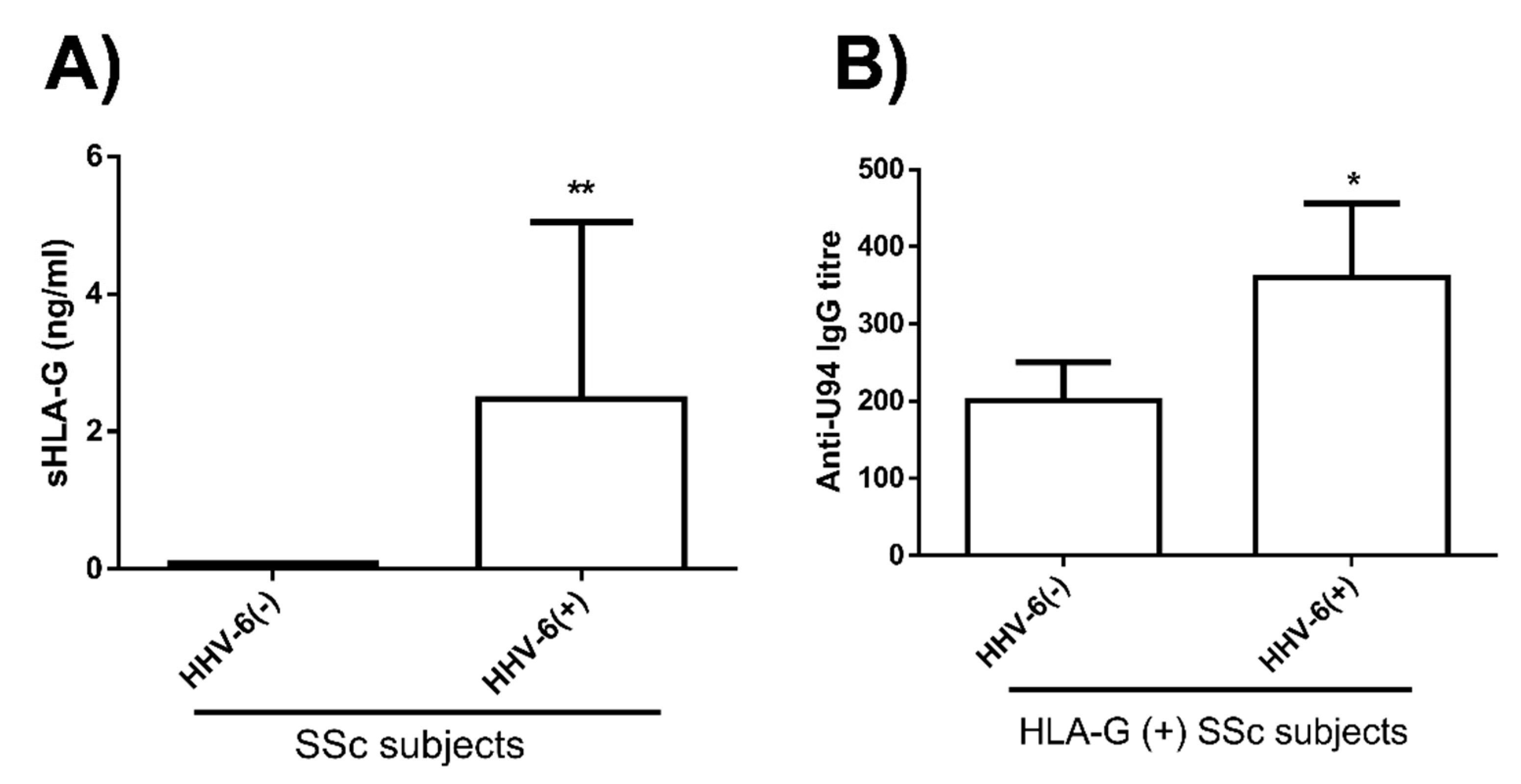
2.10. Analysis of HLA-G in Patients’ Blood
2.11. Statistical Analyses
3. Results
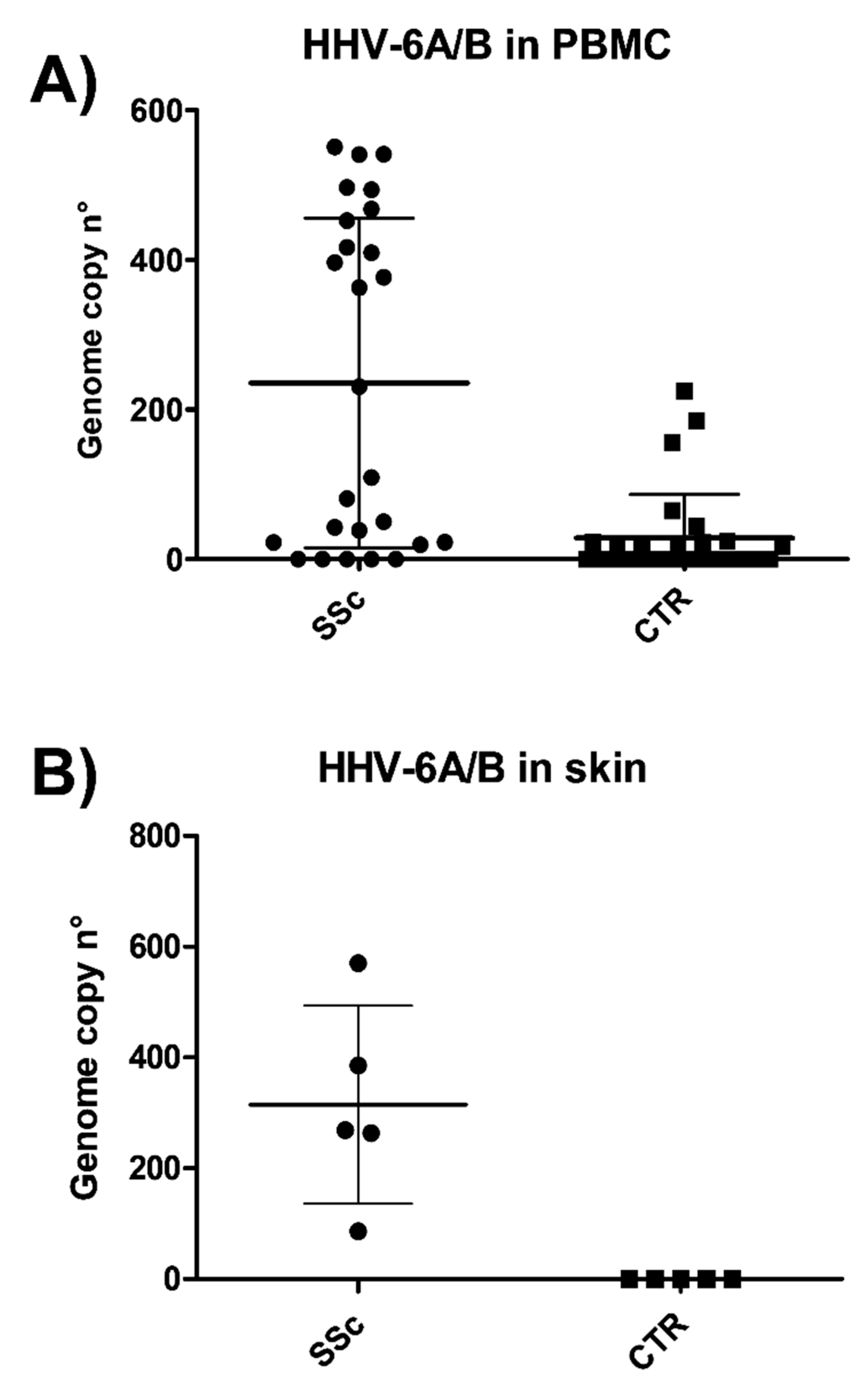
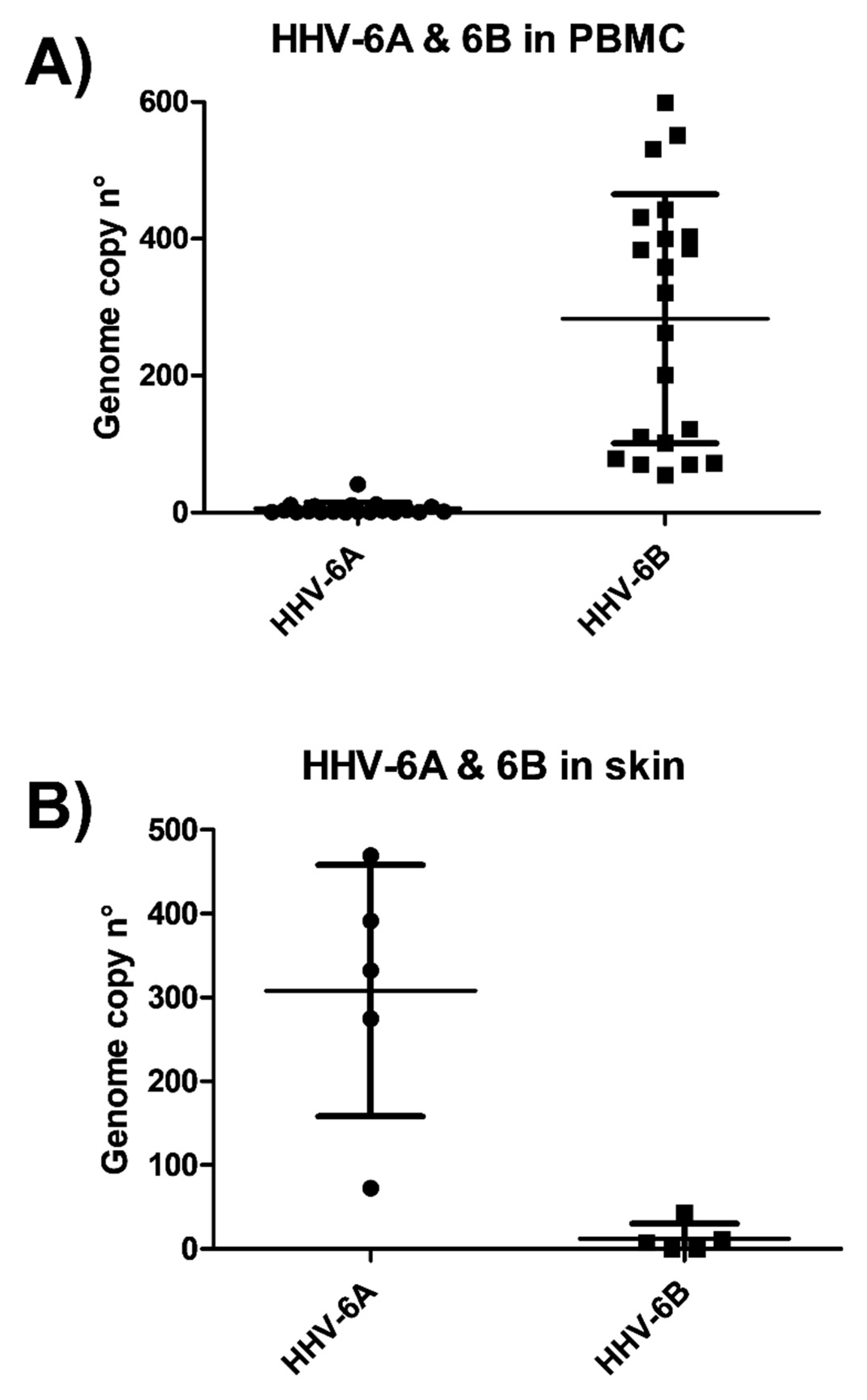
3.1. HHV-6 Detection in SSc Patients
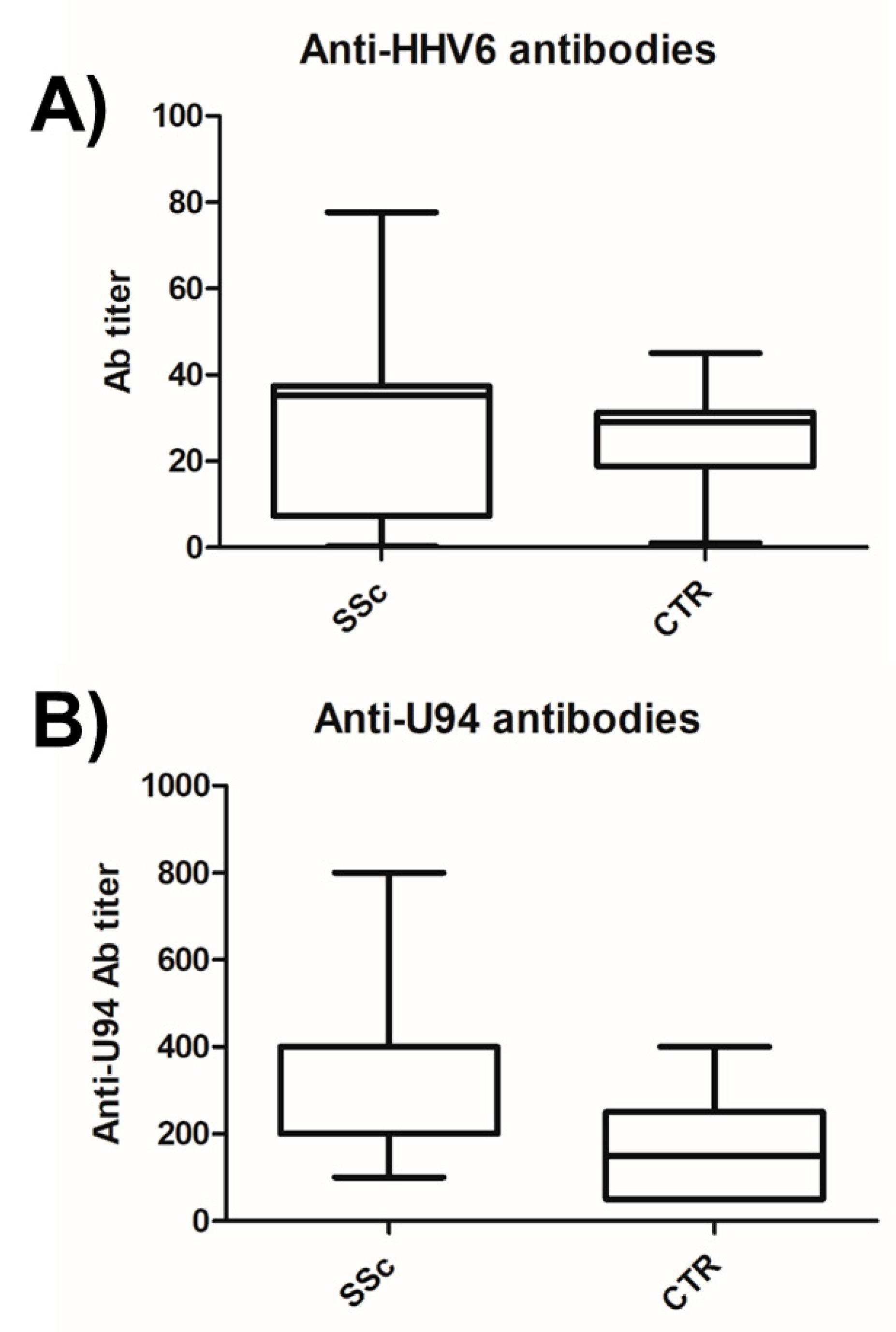
3.2. HHV-6 Serology of SSc Patients
3.3. KIR Typing in SSc Patients
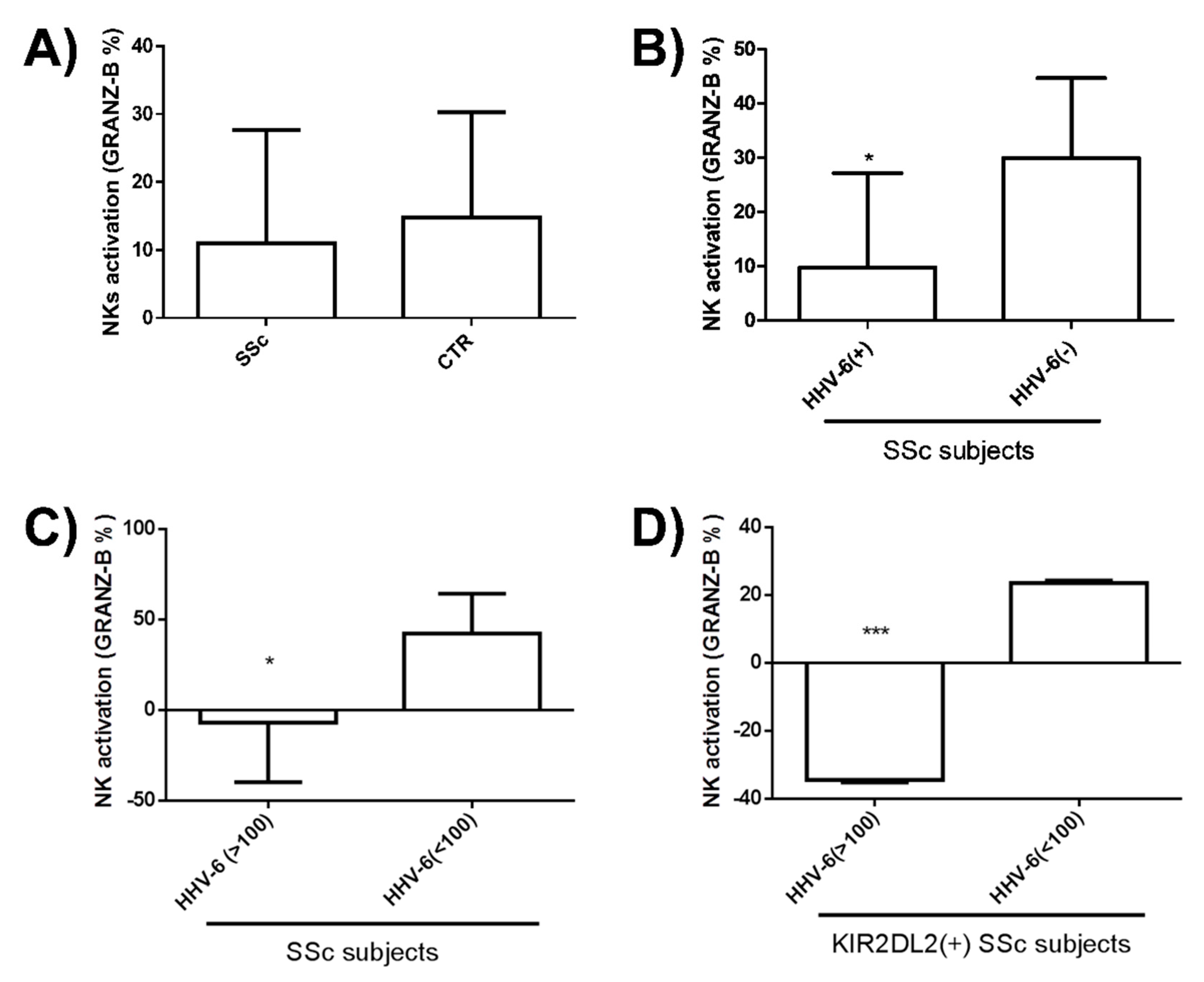
3.4. NK Response against HHV-6 in SSc Patients
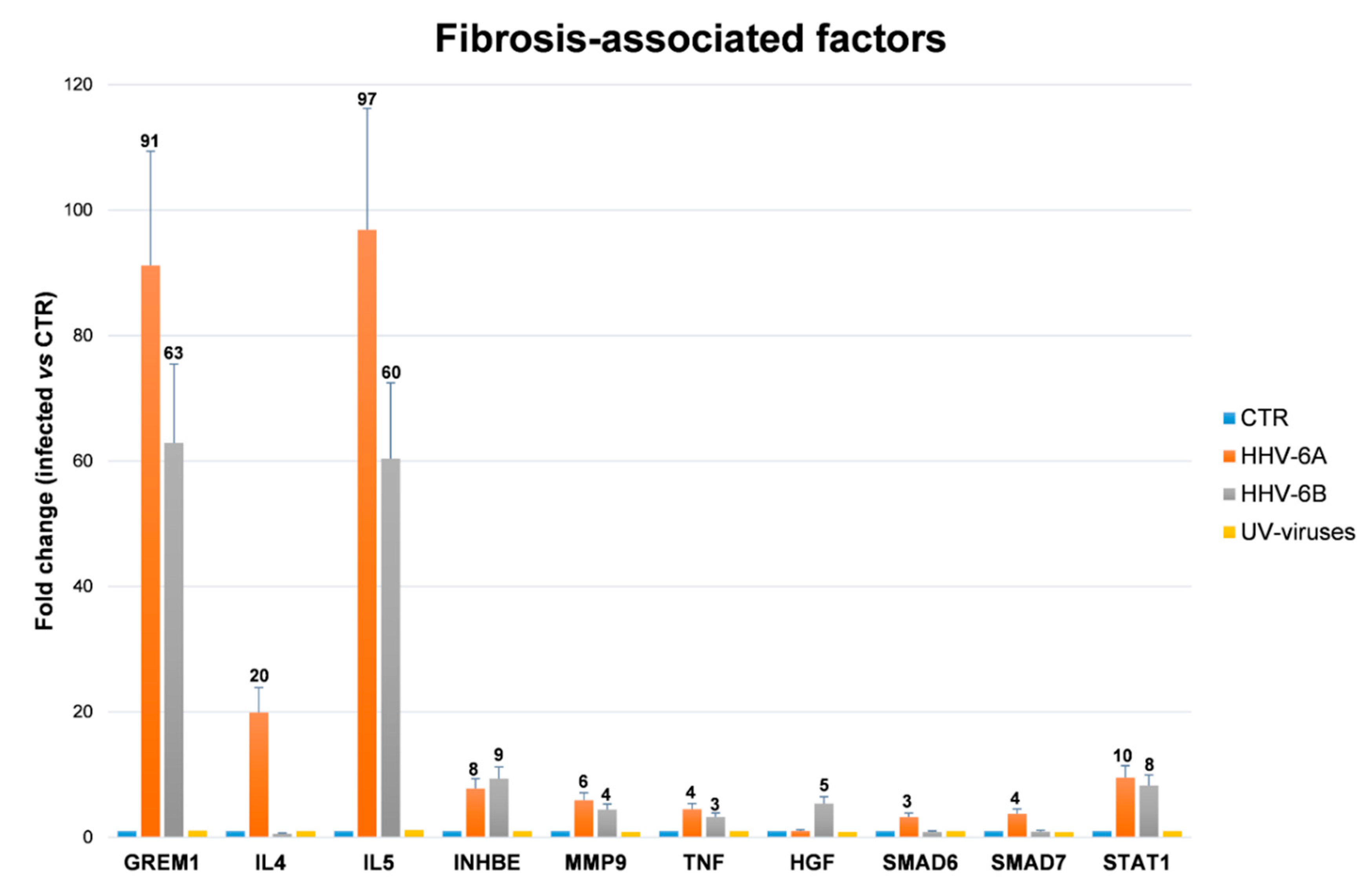
3.5. HHV-6 Induction of Fibrosis-Associated Factors in Endothelial Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ferri, C.; Valentini, G.; Cozzi, F.; Sebastiani, M.; Michelassi, C.; La Montagna, G.; Bullo, A.; Cazzato, M.; Tirri, E.; Storino, F.; et al. Systemic Sclerosis Study Group of the Italian Society of, R., Systemic sclerosis: Demographic, clinical, and serologic features and survival in 1.012 Italian patients. Med. Balt. 2002, 81, 139–153. [Google Scholar] [CrossRef] [PubMed]
- Sierra-Sepulveda, A.; Esquinca-Gonzalez, A.; Benavides-Suarez, S.A.; Sordo-Lima, D.E.; Caballero-Islas, A.E.; Cabral-Castaneda, A.R.; Rodriguez-Reyna, T.S. Systemic Sclerosis Pathogenesis and Emerging Therapies, beyond the Fibroblast. BioMed Res. Int. 2019, 2019, 4569826. [Google Scholar] [CrossRef] [PubMed]
- Ferri, C.; Sebastiani, M.; Lo Monaco, A.; Iudici, M.; Giuggioli, D.; Furini, F.; Manfredi, A.; Cuomo, G.; Spinella, A.; Colaci, M.; et al. Systemic sclerosis evolution of disease pathomorphosis and survival. Our experience on Italian patients’ population and review of the literature. Autoimmun. Rev. 2014, 13, 1026–1034. [Google Scholar] [CrossRef] [PubMed]
- Asano, Y. Systemic sclerosis. J. Derm. 2018, 45, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Farina, A.; Farina, G.A. Fresh Insights into Disease Etiology and the Role of Microbial Pathogens. Curr. Rheumatol. Rep. 2016, 18, 1. [Google Scholar] [CrossRef]
- Caselli, E.; Di Luca, D. Molecular biology and clinical associations of Roseoloviruses human herpesvirus 6 and human herpesvirus 7. New Microbiol. 2007, 30, 173–187. [Google Scholar]
- Ablashi, D.; Agut, H.; Alvarez-Lafuente, R.; Clark, D.A.; Dewhurst, S.; DiLuca, D.; Flamand, L.; Frenkel, N.; Gallo, R.; Gompels, U.A.; et al. Classification of HHV-6A and HHV-6B as distinct viruses. Arch. Virol. 2014, 159, 863–870. [Google Scholar] [CrossRef]
- Thomas, D.; Liakos, V.; Michou, V.; Kapranos, N.; Kaltsas, G.; Tsilivakos, V.; Tsatsoulis, A. Detection of herpes virus DNA in post-operative thyroid tissue specimens of patients with autoimmune thyroid disease. Exp. Clin. Endocrinol. Diabetes 2008, 116, 35–39. [Google Scholar] [CrossRef]
- Caruso, A.; Rotola, A.; Comar, M.; Favilli, F.; Galvan, M.; Tosetti, M.; Campello, C.; Caselli, E.; Alessandri, G.; Grassi, M.; et al. HHV-6 infects human aortic and heart microvascular endothelial cells, increasing their ability to secrete proinflammatory chemokines. J. Med. Virol. 2002, 67, 528–533. [Google Scholar] [CrossRef]
- Caruso, A.; Favilli, F.; Rotola, A.; Comar, M.; Horejsh, D.; Alessandri, G.; Grassi, M.; Di Luca, D.; Fiorentini, S. Human herpesvirus-6 modulates RANTES production in primary human endothelial cell cultures. J. Med. Virol. 2003, 70, 451–458. [Google Scholar] [CrossRef]
- Caruso, A.; Caselli, E.; Fiorentini, S.; Rotola, A.; Prandini, A.; Garrafa, E.; Saba, E.; Alessandri, G.; Cassai, E.; Di Luca, D. U94 of human herpesvirus 6 inhibits in vitro angiogenesis and lymphangiogenesis. Proc. Natl. Acad. Sci. USA 2009, 106, 20446–20451. [Google Scholar] [CrossRef] [PubMed]
- Caselli, E.; Zatelli, M.C.; Rizzo, R.; Benedetti, S.; Martorelli, D.; Trasforini, G.; Cassai, E.; degli Uberti, E.C.; Di Luca, D.; Dolcetti, R. Virologic and immunologic evidence supporting an association between HHV-6 and Hashimoto’s thyroiditis. PLoS Pathog. 2012, 8, e1002951. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, R.; Soffritti, I.; D’Accolti, M.; Bortolotti, D.; Di Luca, D.; Caselli, E. HHV-6A/6B Infection of NK Cells Modulates the Expression of miRNAs and Transcription Factors Potentially Associated to Impaired NK Activity. Front. Microbiol. 2017, 8, 2143. [Google Scholar] [CrossRef] [PubMed]
- Caselli, E.; Bortolotti, D.; Marci, R.; Rotola, A.; Gentili, V.; Soffritti, I.; D’Accolti, M.; Lo Monte, G.; Sicolo, M.; Barao, I.; et al. HHV-6A Infection of Endometrial Epithelial Cells Induces Increased Endometrial NK Cell-Mediated Cytotoxicity. Front. Microbiol. 2017, 8, 2525. [Google Scholar] [CrossRef]
- Di Luca, D.; Dolcetti, R.; Mirandola, P.; De Re, V.; Secchiero, P.; Carbone, A.; Boiocchi, M.; Cassai, E. Human herpesvirus 6: A survey of presence and variant distribution in normal peripheral lymphocytes and lymphoproliferative disorders. J. Infect. Dis. 1994, 170, 211–215. [Google Scholar] [CrossRef]
- Kondo, K.; Kondo, T.; Okuno, T.; Takahashi, M.; Yamanishi, K. Latent human herpesvirus 6 infection of human monocytes/macrophages. J. Gen. Virol. 1991, 72, 1401–1408. [Google Scholar] [CrossRef]
- Rotola, A.; Ravaioli, T.; Gonelli, A.; Dewhurst, S.; Cassai, E.; Di Luca, D. U94 of human herpesvirus 6 is expressed in latently infected peripheral blood mononuclear cells and blocks viral gene expression in transformed lymphocytes in culture. Proc. Natl. Acad. Sci. USA 1998, 95, 13911–13916. [Google Scholar] [CrossRef]
- Caselli, E.; Bracci, A.; Galvan, M.; Boni, M.; Rotola, A.; Bergamini, C.; Cermelli, C.; Dal Monte, P.; Gompels, U.A.; Cassai, E.; et al. Human herpesvirus 6 (HHV-6) U94/REP protein inhibits betaherpesvirus replication. Virology 2006, 346, 402–414. [Google Scholar] [CrossRef]
- Tesini, B.L.; Epstein, L.G.; Caserta, M.T. Clinical impact of primary infection with roseoloviruses. Curr. Opin. Virol. 2014, 9, 91–96. [Google Scholar] [CrossRef]
- Scotet, E.; Peyrat, M.A.; Saulquin, X.; Retiere, C.; Couedel, C.; Davodeau, F.; Dulphy, N.; Toubert, A.; Bignon, J.D.; Lim, A.; et al. Frequent enrichment for CD8 T cells reactive against common herpes viruses in chronic inflammatory lesions: Towards a reassessment of the physiopathological significance of T cell clonal expansions found in autoimmune inflammatory processes. Eur. J. Immunol. 1999, 29, 973–985. [Google Scholar] [CrossRef]
- Broccolo, F.; Drago, F.; Paolino, S.; Cassina, G.; Gatto, F.; Fusetti, L.; Matteoli, B.; Zaccaria, E.; Parodi, A.; Lusso, P.; et al. Reactivation of human herpesvirus 6 (HHV-6) infection in patients with connective tissue diseases. J. Clin. Virol. 2009, 46, 43–46. [Google Scholar] [CrossRef] [PubMed]
- Broccolo, F.; Drago, F.; Cassina, G.; Fava, A.; Fusetti, L.; Matteoli, B.; Ceccherini-Nelli, L.; Sabbadini, M.G.; Lusso, P.; Parodi, A.; et al. Selective reactivation of human herpesvirus 6 in patients with autoimmune connective tissue diseases. J. Med. Virol. 2013, 85, 1925–1934. [Google Scholar] [CrossRef] [PubMed]
- Rotola, A.; Merlotti, I.; Caniatti, L.; Caselli, E.; Granieri, E.; Tola, M.R.; Di Luca, D.; Cassai, E. Human herpesvirus 6 infects the central nervous system of multiple sclerosis patients in the early stages of the disease. Mult. Scler. 2004, 10, 348–354. [Google Scholar] [CrossRef]
- Alvarez-Lafuente, R.; Martinez, A.; Garcia-Montojo, M.; Mas, A.; De Las Heras, V.; Dominguez-Mozo, M.I.; Maria Del Carmen, C.; Lopez-Cavanillas, M.; Bartolome, M.; Gomez de la Concha, E.; et al. MHC2TA rs4774C and HHV-6A active replication in multiple sclerosis patients. Eur. J. Neurol. 2010, 17, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, R.; Gentili, V.; Casetta, I.; Caselli, E.; De Gennaro, R.; Granieri, E.; Cassai, E.; Di Luca, D.; Rotola, A. Altered natural killer cells’ response to herpes virus infection in multiple sclerosis involves KIR2DL2 expression. J. Neuroimmunol. 2012, 251, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Fox, R.I.; Saito, I.; Chan, E.K.; Josephs, S.; Salahuddin, S.Z.; Ahlashi, D.V.; Staal, F.W.; Gallo, R.; Pei-Ping, H.; Le, C.S. Viral genomes in lymphomas of patients with Sjogren’s syndrome. J. Autoimmun. 1989, 2, 449–455. [Google Scholar] [CrossRef]
- Ranger-Rogez, S.; Vidal, E.; Liozon, F.; Denis, F. Primary Sjogren’s syndrome and antibodies to human herpesvirus type 6. Clin. Infect Dis. 1994, 19, 1159–1160. [Google Scholar] [CrossRef]
- Krueger, G.R.; Sander, C.; Hoffmann, A.; Barth, A.; Koch, B.; Braun, M. Isolation of human herpesvirus-6 (HHV-6) from patients with collagen vascular diseases. In Vivo 1991, 5, 217–225. [Google Scholar]
- Alvarez-Lafuente, R.; Fernandez-Gutierrez, B.; de Miguel, S.; Jover, J.A.; Rollin, R.; Loza, E.; Clemente, D.; Lamas, J.R. Potential relationship between herpes viruses and rheumatoid arthritis: Analysis with quantitative real time polymerase chain reaction. Ann. Rheum. Dis. 2005, 64, 1357–1359. [Google Scholar] [CrossRef]
- Boccara, O.; Lesage, F.; Regnault, V.; Lasne, D.; Dupic, L.; Bourdon-Lanoy, E.; Pannier, S.; Fraitag, S.; Audat, F.; Lecompte, T.; et al. Nonbacterial purpura fulminans and severe autoimmune acquired protein S deficiency associated with human herpesvirus-6 active replication. Br. J. Dermatol. 2009, 161, 181–183. [Google Scholar] [CrossRef]
- Potenza, L.; Luppi, M.; Barozzi, P.; Rossi, G.; Cocchi, S.; Codeluppi, M.; Pecorari, M.; Masetti, M.; Di Benedetto, F.; Gennari, W.; et al. HHV-6A in syncytial giant-cell hepatitis. N. Engl. J. Med. 2008, 359, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Machado-Sulbaran, A.C.; Ramirez-Duenas, M.G.; Navarro-Zarza, J.E.; Munoz-Valle, J.F.; Mendoza-Carrera, F.; Banos-Hernandez, C.J.; Parra-Rojas, I.; Montoya-Buelna, M.; Sanchez-Hernandez, P.E. KIR/HLA Gene Profile Implication in Systemic Sclerosis Patients from Mexico. J. Immunol. Res. 2019, 2019, 6808061. [Google Scholar] [CrossRef] [PubMed]
- Caselli, E.; Rizzo, R.; Ingianni, A.; Contini, P.; Pompei, R.; Di Luca, D. High prevalence of HHV8 infection and specific killer cell immunoglobulin-like receptors allotypes in Sardinian patients with type 2 diabetes mellitus. J. Med. Virol. 2014, 86, 1745–1751. [Google Scholar] [CrossRef] [PubMed]
- Estefania, E.; Gomez-Lozano, N.; Portero, F.; de Pablo, R.; Solis, R.; Sepulveda, S.; Vaquero, M.; Gonzalez, M.A.; Suarez, E.; Roustan, G.; et al. Influence of KIR gene diversity on the course of HSV-1 infection: Resistance to the disease is associated with the absence of KIR2DL2 and KIR2DS2. Tissue Antigens 2007, 70, 34–41. [Google Scholar] [CrossRef]
- Moraru, M.; Cisneros, E.; Gomez-Lozano, N.; de Pablo, R.; Portero, F.; Canizares, M.; Vaquero, M.; Roustan, G.; Millan, I.; Lopez-Botet, M.; et al. Host genetic factors in susceptibility to herpes simplex type 1 virus infection: Contribution of polymorphic genes at the interface of innate and adaptive immunity. J. Immunol. 2012, 188, 4412–4420. [Google Scholar] [CrossRef]
- Caselli, E.; Campioni, D.; Cavazzini, F.; Gentili, V.; Bortolotti, D.; Cuneo, A.; Di Luca, D.; Rizzo, R. Acute human herpesvirus-6A infection of human mesothelial cells modulates HLA molecules. Arch. Virol. 2015, 160, 2141–2149. [Google Scholar] [CrossRef]
- Rizzo, R.; D’Accolti, M.; Bortolotti, D.; Caccuri, F.; Caruso, A.; Di Luca, D.; Caselli, E. Human Herpesvirus 6A and 6B inhibit in vitro angiogenesis by induction of Human Leukocyte Antigen, G. Sci. Rep. 2018, 8, 17683. [Google Scholar] [CrossRef]
- Wastowski, I.J.; Sampaio-Barros, P.D.; Amstalden, E.M.; Palomino, G.M.; Marques-Neto, J.F.; Crispim, J.C.; Biral, A.C.; Rassi, D.M.; Carosella, E.D.; Moreau, P.; et al. HLA-G expression in the skin of patients with systemic sclerosis. J. Rheumatol. 2009, 36, 1230–1234. [Google Scholar] [CrossRef]
- Favoino, E.; Favia, I.E.; Vettori, S.; Vicenti, C.; Prete, M.; Valentini, G.; Perosa, F. Clinical correlates of human leucocyte antigen (HLA)-G in systemic sclerosis. Clin. Exp. Immunol. 2015, 181, 100–109. [Google Scholar] [CrossRef]
- Van den Hoogen, F.; Khanna, D.; Fransen, J.; Johnson, S.R.; Baron, M.; Tyndall, A.; Matucci-Cerinic, M.; Naden, R.P.; Medsger, T.A., Jr.; Carreira, P.E.; et al. 2013 classification criteria for systemic sclerosis: An American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis Rheumatol. 2013, 65, 2737–2747. [Google Scholar] [CrossRef]
- Clements, P.; Lachenbruch, P.; Siebold, J.; White, B.; Weiner, S.; Martin, R.; Weinstein, A.; Weisman, M.; Mayes, M.; Collier, D.; et al. Inter and intraobserver variability of total skin thickness score (modified Rodnan TSS) in systemic sclerosis. J. Rheumatol. 1995, 22, 1281–1285. [Google Scholar]
- Khanna, D.; Furst, D.E.; Clements, P.J.; Allanore, Y.; Baron, M.; Czirjak, L.; Distler, O.; Foeldvari, I.; Kuwana, M.; Matucci-Cerinic, M.; et al. Standardization of the modified Rodnan skin score for use in clinical trials of systemic sclerosis. J. Scleroderma Relat. Disord. 2017, 2, 11–18. [Google Scholar] [CrossRef]
- Rotola, A.; Cassai, E.; Farina, R.; Caselli, E.; Gentili, V.; Lazzarotto, T.; Trombelli, L. Human herpesvirus 7, Epstein-Barr virus and human cytomegalovirus in periodontal tissues of periodontally diseased and healthy subjects. J. Clin. Periodontol. 2008, 35, 831–837. [Google Scholar] [CrossRef]
- Caselli, E.; D’Accolti, M.; Soffritti, I.; Zatelli, M.C.; Rossi, R.; Degli Uberti, E.; Di Luca, D. HHV-6A in vitro infection of thyrocytes and T cells alters the expression of miRNA associated to autoimmune thyroiditis. Virol. J. 2017, 14, 3. [Google Scholar] [CrossRef]
- Caselli, E.; Boni, M.; Bracci, A.; Rotola, A.; Cermelli, C.; Castellazzi, M.; Di Luca, D.; Cassai, E. Detection of antibodies directed against human herpesvirus 6 U94/REP in sera of patients affected by multiple sclerosis. J. Clin. Microbiol. 2002, 40, 4131–4137. [Google Scholar] [CrossRef][Green Version]
- Borghi, A.; D’Accolti, M.; Rizzo, R.; Virgili, A.; Di Luca, D.; Corazza, M.; Caselli, E. High prevalence of specific KIR types in patients with HHV-8 positive cutaneous vascular lesions: A possible predisposing factor? Arch. Dermatol. Res. 2016, 308, 373–377. [Google Scholar] [CrossRef]
- Sedlak, R.H.; Hill, J.A.; Nguyen, T.; Cho, M.; Levin, G.; Cook, L.; Huang, M.L.; Flamand, L.; Zerr, D.M.; Boeckh, M.; et al. Detection of Human Herpesvirus 6B (HHV-6B) Reactivation in Hematopoietic Cell Transplant Recipients with Inherited Chromosomally Integrated HHV-6A by Droplet Digital PCR. J. Clin. Microbiol. 2016, 54, 1223–1227. [Google Scholar] [CrossRef]
- Caselli, E.; Fiorentini, S.; Amici, C.; Di Luca, D.; Caruso, A.; Santoro, M.G. Human herpesvirus 8 acute infection of endothelial cells induces monocyte chemoattractant protein 1-dependent capillary-like structure formation: Role of the IKK/NF-kappaB pathway. Blood 2007, 109, 2718–2726. [Google Scholar] [CrossRef]
- Rizzo, R.; Lo Monte, G.; Bortolotti, D.; Graziano, A.; Gentili, V.; Di Luca, D.; Marci, R. Impact of soluble HLA-G levels and endometrial NK cells in uterine flushing samples from primary and secondary unexplained infertile women. Int. J. Mol. Sci. 2015, 16, 5510–5516. [Google Scholar] [CrossRef]
- Broccolo, F.; Fusetti, L.; Ceccherini-Nelli, L. Possible role of human herpesvirus 6 as a trigger of autoimmune disease. Sci. World J. 2013, 2013, 867389. [Google Scholar] [CrossRef]
- Rotola, A.; Di Luca, D.; Cassai, E.; Ricotta, D.; Giulio, A.; Turano, A.; Caruso, A.; Muneretto, C. Human herpesvirus 6 infects and replicates in aortic endothelium. J. Clin. Microbiol. 2000, 38, 3135–3136. [Google Scholar]
- Di Luca, D.; Mirandola, P.; Ravaioli, T.; Bigoni, B.; Cassai, E. Distribution of HHV-6 variants in human tissues. Infect. Agents Dis. 1996, 5, 203–214. [Google Scholar]
- Campadelli-Fiume, G.; Mirandola, P.; Menotti, L. Human herpesvirus 6: An emerging pathogen. Emerg. Infect. Dis. 1999, 5, 353–366. [Google Scholar] [CrossRef]
- Trojanowska, M. Cellular and molecular aspects of vascular dysfunction in systemic sclerosis. Nat. Rev. Rheumatol. 2010, 6, 453–460. [Google Scholar] [CrossRef]
- Heron, M.; van Moorsel, C.H.; Grutters, J.C.; Huizinga, T.W.; van der Helm-van Mil, A.H.; Nagtegaal, M.M.; Ruven, H.J.; van den Bosch, J.M. Genetic variation in GREM1 is a risk factor for fibrosis in pulmonary sarcoidosis. Tissue Antigens 2011, 77, 112–117. [Google Scholar] [CrossRef]
- Church, R.H.; Ali, I.; Tate, M.; Lavin, D.; Krishnakumar, A.; Kok, H.M.; Hombrebueno, J.R.; Dunne, P.D.; Bingham, V.; Goldschmeding, R.; et al. Gremlin1 plays a key role in kidney development and renal fibrosis. Am. J. Physiol. Ren. Physiol. 2017, 312, F1141–F1157. [Google Scholar] [CrossRef]
- Staloch, D.; Gao, X.; Liu, K.; Xu, M.; Feng, X.; Aronson, J.F.; Falzon, M.; Greeley, G.H.; Rastellini, C.; Chao, C.; et al. Gremlin is a key pro-fibrogenic factor in chronic pancreatitis. J. Mol. Med. Berl. 2015, 93, 1085–1093. [Google Scholar] [CrossRef]
- Munz, B.; Smola, H.; Engelhardt, F.; Bleuel, K.; Brauchle, M.; Lein, I.; Evans, L.W.; Huylebroeck, D.; Balling, R.; Werner, S. Overexpression of activin A in the skin of transgenic mice reveals new activities of activin in epidermal morphogenesis, dermal fibrosis and wound repair. EMBO J. 1999, 18, 5205–5215. [Google Scholar] [CrossRef]
- Fertin, C.; Nicolas, J.F.; Gillery, P.; Kalis, B.; Banchereau, J.; Maquart, F.X. Interleukin-4 stimulates collagen synthesis by normal and scleroderma fibroblasts in dermal equivalents. Cell. Mol. Biol. 1991, 37, 823–829. [Google Scholar]
- Borthwick, L.A.; Wynn, T.A.; Fisher, A.J. Cytokine mediated tissue fibrosis. Biochim. Biophys. Acta 2013, 1832, 1049–1060. [Google Scholar] [CrossRef]
- Groves, A.M.; Johnston, C.J.; Misra, R.S.; Williams, J.P.; Finkelstein, J.N. Effects of IL-4 on pulmonary fibrosis and the accumulation and phenotype of macrophage subpopulations following thoracic irradiation. Int. J. Radiat. Biol. 2016, 92, 754–765. [Google Scholar] [CrossRef]
- Wynn, T.A. Integrating mechanisms of pulmonary fibrosis. J. Exp. Med. 2011, 208, 1339–1350. [Google Scholar] [CrossRef]
- Lam, G.K.; Hummers, L.K.; Woods, A.; Wigley, F.M. Efficacy and safety of etanercept in the treatment of scleroderma-associated joint disease. J. Rheumatol. 2007, 34, 1636–1637. [Google Scholar]
- Bosello, S.; De Santis, M.; Tolusso, B.; Zoli, A.; Ferraccioli, G. Tumor necrosis factor-alpha inhibitor therapy in erosive polyarthritis secondary to systemic sclerosis. Ann. Intern. Med. 2005, 143, 918–920. [Google Scholar] [CrossRef]
- Distler, J.H.; Schett, G.; Gay, S.; Distler, O. The controversial role of tumor necrosis factor alpha in fibrotic diseases. Arthritis Rheumatol. 2008, 58, 2228–2235. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, Y.; Tan, R.; Xiong, M.; He, W.; Fang, L.; Wen, P.; Jiang, L.; Yang, J. Mice lacking the matrix metalloproteinase-9 gene reduce renal interstitial fibrosis in obstructive nephropathy. Am. J. Physiol. Renal. Physiol. 2010, 299, F973–F982. [Google Scholar] [CrossRef]
- Feng, M.; Ding, J.; Wang, M.; Zhang, J.; Zhu, X.; Guan, W. Kupffer-derived matrix metalloproteinase-9 contributes to liver fibrosis resolution. Int. J. Biol. Sci. 2018, 14, 1033–1040. [Google Scholar] [CrossRef]
- Del Papa, N.; Pignataro, F. The Role of Endothelial Progenitors in the Repair of Vascular Damage in Systemic Sclerosis. Front. Immunol. 2018, 9, 1383. [Google Scholar] [CrossRef]
- Arcangeletti, M.C.; Maccari, C.; Vescovini, R.; Volpi, R.; Giuggioli, D.; Sighinolfi, G.; De Conto, F.; Chezzi, C.; Calderaro, A.; Ferri, C. A Paradigmatic Interplay between Human Cytomegalovirus and Host Immune System: Possible Involvement of Viral Antigen-Driven CD8+ T Cell Responses in Systemic Sclerosis. Viruses 2018, 10, 508. [Google Scholar] [CrossRef]
- Zakrzewska, K.; Arvia, R.; Torcia, M.G.; Clemente, A.M.; Tanturli, M.; Castronovo, G.; Sighinolfi, G.; Giuggioli, D.; Ferri, C. Effects of Parvovirus B19 In Vitro Infection on Monocytes from Patients with Systemic Sclerosis: Enhanced Inflammatory Pathways by Caspase-1 Activation and Cytokine Production. J. Investig. Dermatol. 2019, 139, 2125–2133.e1. [Google Scholar] [CrossRef]
- Ferri, C.; Artoni, E.; Sighinolfi, G.L.; Luppi, F.; Zelent, G.; Colaci, M.; Giuggioli, D. High serum levels of silica nanoparticles in systemic sclerosis patients with occupational exposure: Possible pathogenetic role in disease phenotypes. Semin. Arthritis Rheum. 2018, 48, 475–481. [Google Scholar] [CrossRef]
- Ferri, C.; Cazzato, M.; Giuggioli, D.; Sebastiani, M.; Magro, C. Systemic sclerosis following human cytomegalovirus infection. Ann. Rheum. Dis. 2002, 61, 937–938. [Google Scholar] [CrossRef]
- Ferri, C.; Zakrzewska, K.; Longombardo, G.; Giuggioli, D.; Storino, F.A.; Pasero, G.; Azzi, A. Parvovirus B19 infection of bone marrow in systemic sclerosis patients. Clin. Exp. Rheumatol. 1999, 17, 718–720. [Google Scholar]
| Parameters | SSc Patients | Controls |
|---|---|---|
| Number | 26 | 30 |
| Age (median, range) | 56 (37–74) | 52 (38–65) |
| Gender | ||
| Male (n, %) | 4 (15.4%) | 5 (16.6%) |
| Female (n, %) | 22 (84.6%) | 25 (83.3%) |
| HHV-6 Ab (n, %) | 20 (76.9%) | 22 (73.3%) |
| Disease duration (median years, range) | 5 (1–20) | - |
| mRSS (median, range) | 7 (0–33) | - |
| Cutaneous subgroups | ||
| Diffuse cutaneous SSc | 5 (19.2%) | - |
| Limited cutaneous SSc | 21 (80.7%) | - |
| Clinical signs (n, %) | ||
| Raynaud’s phenomenon | 26 (100%) | |
| Digital ulcer | 10 (38.5%) | - |
| Puffy fingers | 14 (53.8%) | - |
| Pitting | 8 (30.7%) | - |
| Telangiectasia | 13 (50%) | - |
| Arthralgia | 13 (50%) | - |
| Interstitial lung disease | 15 (57.7%) | - |
| Heart involvement | 1 (3.8%) | - |
| Esophageal dysfunction | 13 (50%) | - |
| Autoantibodies (n, %) | ||
| Positive ANA | 25 (96.1%) | - |
| Positive ACA | 9 (34.6%) | - |
| Positive anti-Scl70 | 9 (34.6%) | - |
| Treatments (n, %) * | ||
| Steroids | 3 (11.5%) | - |
| Immunosuppressors | 3 (11.5%) | - |
| Prostanoids | 19 (73.1%) | - |
| Bosentan | 7 (26.9%) | - |
| Calcium antagonist | 18 (69.2%) | - |
| KIR Type | SSc Patients (n = 26) | Controls (n = 30) |
|---|---|---|
| DL2/DL2 | 1 (3.8%) | 1 (3.3%) |
| DL2/DL3 | 8 (30.8%) | 11 (33.7%) |
| DL3/DL3 | 17 (65.4%) | 18 (60%) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caselli, E.; Soffritti, I.; D’Accolti, M.; Bortolotti, D.; Rizzo, R.; Sighinolfi, G.; Giuggioli, D.; Ferri, C. HHV-6A Infection and Systemic Sclerosis: Clues of a Possible Association. Microorganisms 2020, 8, 39. https://doi.org/10.3390/microorganisms8010039
Caselli E, Soffritti I, D’Accolti M, Bortolotti D, Rizzo R, Sighinolfi G, Giuggioli D, Ferri C. HHV-6A Infection and Systemic Sclerosis: Clues of a Possible Association. Microorganisms. 2020; 8(1):39. https://doi.org/10.3390/microorganisms8010039
Chicago/Turabian StyleCaselli, Elisabetta, Irene Soffritti, Maria D’Accolti, Daria Bortolotti, Roberta Rizzo, Gianluca Sighinolfi, Dilia Giuggioli, and Clodoveo Ferri. 2020. "HHV-6A Infection and Systemic Sclerosis: Clues of a Possible Association" Microorganisms 8, no. 1: 39. https://doi.org/10.3390/microorganisms8010039
APA StyleCaselli, E., Soffritti, I., D’Accolti, M., Bortolotti, D., Rizzo, R., Sighinolfi, G., Giuggioli, D., & Ferri, C. (2020). HHV-6A Infection and Systemic Sclerosis: Clues of a Possible Association. Microorganisms, 8(1), 39. https://doi.org/10.3390/microorganisms8010039








